Abstract
To attempt to introduce genetic information of disease resistance from Musa acuminata cv. Mas (AA) to Musa silk cv. Guoshanxiang (AAB) and obtain somatic hybrids, we developed an asymmetric protoplast fusion with 20% (w/v) polyethylene glycol (PEG). The protoplasts derived from embryogenic suspension cultural cells of cv. Guoshanxiang (AAB) and cv. Mas (AA) were, respectively treated with 1.5 mM iodoacetamide (IOA) and with ultraviolet light (UV) at an intensity of 50 W/m2 for 120 s. A total of 47 regenerated green plants were obtained and eight of which were survived in greenhouse. Six of the survived plants were identified as hybrids by RAPD analysis and only three hybrids were retained vigorously in field. The hybrid nature of the three plants was further confirmed according to their ISSR (inter-simple sequence repeat) patterns and the results indicated that they were true somatic hybrids. Chromosome analysis revealed that the three hybrids possessed an aneuploid chromosome number (2n = 34).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Banana (Musa spp.) is one of the most important fruit crops planted widely in tropical and subtropical countries. However, the production and quality of banana are being threatened by the spread of viral and fungal diseases. The application of classical breeding methods has limited success in genetically improving this crop, due to the high sterility and polyploidy of most cultivated bananas. For example, the disease-resistant varieties exist particularly in non-cultivated diploids bananas (AA), but the transfer of these characters to cultivated triploid varieties (AAB or AAA) is extremely difficult by using conventional breeding methods (Matsumoto et al. 2002).
The current transgenic approach through genetic transformation, which is capable of transferring specific gene(s) from any sources into cultivated crop species, provides a powerful tool to enrich the gene pool of commercial cultivars. However, many characters of agricultural interests are multigenic or ill-defined, and until now many resistance genes in banana have not been identified. From this point of view, somatic hybridization by protoplast fusion is a promising alternative strategy to improve banana. Gene transfer by means of somatic hybridization has a number of advantages over genetic engineering: (1) no prerequisite for identification of the genes; (2) a capacity to introduce quantitative traits by transferring a large number of genes; (3) transfer of both nuclear and cytoplasmic genome (Yan et al. 2004).
Since protoplast regeneration systems in several banana cultivars had already been established (Megia et al. 1993; Panis et al. 1993; Matsumoto and Oka 1998; Assani et al. 2001; Assani et al. 2002; Assani et al. 2006; Xiao et al. 2007; Xiao et al. 2008), the use of cell fusion techniques in banana breeding becomes a realizable objective. At present, very limited successes of somatic hybridization of banana have been reported though Matsumoto et al. (2002) firstly reported symmetric somatic hybridization between non-treated cv. ‘Maçã’ (AAB) and non-treated cv. ‘Lidi’ (AA) by electrofusion, and Assani et al. (2005) recently obtained somatic hybrids by symmetric fusions between cv. Gros Michel (AAA) and cv. SF265 (AA) either using PEG (polyethylene glycol) method and electrofusion.
It has been shown that somatic hybrids recovered from symmetric fusion combinations are often sterile and uncontrolled genomic instabilities with parts of one or both genomes being lost during the in vitro passage (Bauer-Weston et al. 1993; Spangenberg et al. 1994; Vlahova et al. 1997; Kisaka et al. 1998). This disadvantage hampers the use of somatic cell fusion for increasing nuclear genetic variability in plants. An alternative fusion method is asymmetric fusion (recipient–donor fusion). In this method, a nucleus-damaged protoplast from a donor species is fused to a cell division-inhibited recipient protoplast, thus allowing the introduction of chromosome fragments or a few chromosomes from the donor to recipient cell (Rasmussen et al. 1997). Successful asymmetric fusions have been reported in several important crops such as wheat (Xia et al. 2003), rice (Yan et al. 2004) and rapeseed (Wang et al. 2003). Until now, there was no report about asymmetric somatic hybridization in banana.
Both of Musa acuminata cv. Mas (AA; 2n = 2x = 22) and Musa silk cv. Guoshanxiang (AAB; 2n = 3x = 33) are popular commercial variety planted in South China. Banana of cv. Guoshanxiang (AAB) is susceptible to Fusarium oxysporun f. sp. Cubense race 1 (Huang et al. 2005); and cv. Mas (AA) has high resistance to this disease (Morpurgo et al. 1994). The aim of this study was to attempt to develop an asymmetric protoplast fusion and obtain somatic hybrids which may increase the resistance to this disease by the introduction of chromosome fragments or a few chromosomes from cv. Mas (AA; donor protoplasts) to cv. Guoshanxiang (AAB; recipient protoplasts) through the fusion. RAPD and ISSR analysis were used to identify the somatic hybrids.
Materials and methods
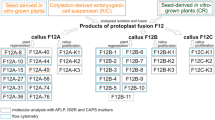
The main procedures of protoplast fusion and somatic hybrids selection as indicated in Fig. 1.
Plant materials and protoplast isolation
Embryogenic cell suspension (ECS) of cv. Guoshanxiang (AAB) and cv. Mas (AA) were used as the source of protoplasts. The ECS were initiated and maintained as we previously reported (Wei et al. 2005a, b). Suspensions were initiated and maintained in M2 medium (Côte et al. 1996) which consisted of basic MS medium (Murashige and Skoog 1962) supplemented with 4.1 μM biotin, 4.5 μM 2,4-D, 680 μM glutamine, 100 mg l−1 malt extract and 130 mM sucrose (pH 5.3). They were sub-cultured weekly on a gyratory shaker at 100 rpm and maintained at 27°C in the dark.
ECS sub-cultured in M2 medium for 4–6 days were harvested and passed through a 200 μm sieve to remove the large cell clumps, the sieved ECS were used for protoplast isolation. The isolation and purification of protoplasts were described by our previously report (Xiao et al. 2007).
Culture media and preparation of feeder-layer
Medium-B (Xiao et al. 2007) used for culture of protoplasts and fusion products consisted of basic MS medium, with 4.1 μΜ biotin, 4.5 μΜ 2,4-D, 680 μΜ glutamine, 100 mg l−1 malt extract, 117 mM sucrose, 0.4 M glucose and 0.5 mM 2-N-morpholino ethanesulfonic acid (MES), pH 5.8. M3 medium (Xiao et al. 2008) used for somatic embryogenesis consisted of basic MS medium with 4.1 μM biotin, 680 μΜ glutamine, 100 mg l−1 malt extract, 130 mM sucrose, 4.4 μΜ BA, 2.3 μΜ indole-3-acetic (IAA), 3 g l−1 gelrite, pH 5.8. Preparation of feeder-layer and the methods for culture of protoplasts and fusion products were based on the protocols reported by Xiao et al. (2007), ECS of cv. Mas (AA) were used as nurse cells.
Inactivation treatment and protoplast fusion
Protoplasts of cv. Guoshanxiang (AAB), as recipient protoplasts, were suspended in medium-B at a density of 1 × 106 per milliliter and treated with concentrations of iodoacetamide (IOA, dissolved in medium-B) ranging from 0.07 to 2.5 mM for 15 min at room temperature. Then, protoplasts were washed three times and resuspended with medium-B. Protoplasts of cv. Mas (AA), as donor protoplasts, also were washed 1–2 times and resuspended in medium-B after being exposed to UV at the density of 50 W/m2 for 60, 120 and 180 s, respectively.
The IOA-treated recipient protoplasts and UV-irradiated donor protoplasts at a same density (1 × 105 protoplasts per milliliter) were then mixed in a ratio of 1:1 for fusion. Their fusion was induced with PEG according to the method of Xia and Chen (1996). PEG at 15, 20, 25, 30, 35, and 40% (w/v), respectively were used to obtain optimum concentration. The frequencies of binary- and multi-fusion were estimated under microscope (ECLIPSE E600; Nikon, Japan).
Plant regeneration
The fusion mixtures were resuspended in medium-B to a density of 1 × 105 protoplasts per milliliter and then cultured on feeder layer. After cultured for 20–30 days, fast-growing cell colonies about 0.5–1 mm in diameters were selected and sub-cultured every 10–15 days for plant regeneration on M3 medium. All cultures were incubated in the dark, at 27 ± 1°C. The frequency of plant regeneration was recorded as total number of normal plantlets per 1 × 105 protoplasts. Regenerated plantlets were transferred to basic MS medium containing 0.1% activated charcoal under 16-8 photoperiod to promote shoot and root growth. After 4–6 weeks of growth in vitro, plantlets were acclimated for 2–3 weeks before they were transplanted to pots containing a mixture of soil and sand (3:1) in a greenhouse for 2 months, and then transferred to field.
Total DNA extraction
The genomic DNA for the PCR reaction was extracted from young leaves of cv. Guoshanxiang (AAB), cv. Mas (AA), and regenerated plantlets using a modified CTAB method (Du et al. 2001).
RAPD analysis
Forty 10-Mer arbitrary primers (Operon Technology, USA) were used in a RAPD primer screening analysis with DNA isolated from cv. Guoshanxiang (AAB) and cv. Mas (AA). Primers giving one or more distinct donor-specific RAPD-DNA bands were selected and used for the analysis of all regenerated plants at least twice with the same primer.
The polymerase chain reaction (PCR) was performed in a PTC-100™ programmable thermal control (MJ research Inc., USA) in a 20 μl of reaction mixture containing 0.5 U Taq polymerase, 1× PCR buffer, 1.5 mM MgCl2, 0.4 μΜ primers, 50 μΜ of each dNTP, and 50 ng of DNA template. Reactions were started with a denaturation at 94°C for 5 min, followed by 44 cycles of 94°C for 1 min, 37°C for 1 min and 72°C for 2 min, with a final extension at 72°C for 7 min. Amplified products were analyzed by electrophoresis in a 1.0% (w/v) agarose–ethidium bromide gels.
ISSR analysis
For ISSR analysis, 12 primers designed by UBC [University of British Columbia Biotechnology Lab (UBCBL) primer set, Canada] were utilized, they were 834(AG)8YT, 835 (AG)8YC, 836 (AG)8YA, 841 (GA)8YC, 842 (GA)8YG, 844 (CT)8RC, 845 (CT)8RG, 847 (CA)8RC, 848 (CA)8RG, 850 (GT)8YC, 851 (GT)8YG, 854 (TC)8RG, 857 (AC)8YG, 880 (GGAGA)3, (Y = C, T). All primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd.
The polymerase chain reaction (PCR) was performed in a 20-μl of reaction mixture containing 0.5 U Taq polymerase, 1× PCR buffer, 1.5 mM MgCl2, 0.8 μΜ primers, 200 μΜ of each dNTP, and 30 ng of DNA template. Reactions were started with a denaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 0.5 min, 55°C for 1 min and 72°C for 2 min, with a final extension at 72°C for 8 min. Amplified products were analyzed by electrophoresis in a 2.0% (w/v) agarose–ethidium bromide gels.
Chromosome counts
For chromosome counts, root tips cut from the parents and the hybrids grown in field were pretreated with 0.2% colchicine for 2 h, and fixed in Carnoy’s solution (ethanol: glacial acetic acid = 3:1, v:v) for 24 h. The root tips were then hydrolyzed in 1 N HCl at 60°C for 10–12 min and stained with modified carbol fuchsin (Xia and Chen 1996). More then 50 metaphase cells of each sample were analyzed.
Data collection and statistics
The results were obtained in three independent experiments. Each experiment was repeated at least twice. Data represented average ± SE of three replicates. The frequencies of cell divisions, cell colony formation and somatic embryo formation were, respectively calculated in 14, 45, and 90 days after protoplast plating related to the viable protoplasts.
Results
Effects of IOA or UV treatment on inactivation of protoplasts
As Fig. 2 indicated, when the treated protoplasts were cultured on medium-B for 45 days, the extent of inactivation of cv. Guoshanxiang (AAB) protoplasts was IOA concentration-dependent. Treatment with 0.07 mM IOA was not sufficient to inactivate the protoplasts, because about 51.7% of the protoplasts could still form cell colonies. However, after treatment with 1.5 mM IOA only less than 5% of the protoplasts could form cell colony, and they did not develop further. Treatment with 2.5 mM IOA completely inhibited cell divisions of the protoplasts. Thus, 1.5 mM was the optimum IOA concentration for the pretreatment of the cv. Guoshanxiang (AAB) protoplasts before protoplast fusion.
When the protoplasts of cv. Mas (AA) were treated with UV, protoplast viability was affected in a dose-dependent manner (Table 1). The protoplasts treated with UV for 60 s began to divide after 7 days of the treatment and formed small cell colonies after cultured on feeder-layer for 1 month, but none somatic embryo formed from these colonies. The protoplasts treated with UV for 120 s began to divide after 10 days of the treatment but none cell colony was formed. It was worth nothing that the treatment with UV for 180 s resulted in complete inhibition of cell division of the protoplasts. UV treatments for 60, 120 and 180 s, respectively were chosen for protoplast treatment before protoplast fusion.
Effects of PEG concentration on protoplast fusion
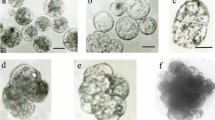
As showed in Fig. 3, when PEG solution was added dropwise, binary fusion (Fig. 4c–e) and multi fusion (more than three protoplasts involved) of the protoplasts were observed. Binary fusion frequency increased from 5.6 to 13.6% as the PEG concentration was increased from 15 to 20%, then decreased as PEG concentration was increased, while the multi fusion frequency still increased with the increasing of PEG concentration. The results indicated that 20% PEG was optimal for formation of binary fusion.
Plants regeneration from fusion products. a Protoplasts of cv. Guoshanxiang (AAB); bar 50 μm. b Protoplasts of cv. Mas (AA); bar 50 μm. c Two protoplast adhere; bar 25 μm. d–f The process of two protoplasts fusion; bar 15 μm. g Fusion products; bar 25 μm. h Cell colonies derived from fusion products; bar 35 μm. i Somatic embryos derived from fusion products; bar 5 mm. j Regenerated plants, bar 1.5 cm. k Regenerated plants in pots in greenhouse
Development of the fusion products
Visible cell colonies were observed at 20–25 days after protoplast fusion. When the cell colonies grew to a size of 0.5–1 mm in diameter, they were transferred to M3 medium for somatic embryo formation and germination. Although the highest frequency of embryo formation was obtained from combination cv. Guoshanxiang (AAB) + cv. Mas (AA) (UV 60 s), only 12 normal plantlets were regenerated from 1 × 105 protoplasts; The highest frequency of plant regeneration was obtained from fusion combination of cv. Guoshanxiang (AAB) + cv. Mas (AA) irradiated by UV for 120 s and 29 plantlets were regenerated from 105 protoplasts. However, only six plantlets were regenerated from 105 protoplasts when donor protoplasts were irradiated by UV for 180 s (Table 2). A total of 47 regenerated plantlets were obtained. Vigorously rooted plantlets were acclimated and transplanted into pots in greenhouse (Fig. 4k), eight of them survived. The eight survival plantlets were transplanted in field, finally three of them retained and grew vigorously.
RAPD analysis of the regenerated plants
Among the forty RAPD primers were tested for identification of the hybrids, six primers showed polymorphism between the fusion parents. The six primer sequences were: OPD-10 (GGTCTACACC), OPS-01 (CTACTGCGCT), OPU-03 (CTATGCCGAC), OPW-18 (TTCAGGGCAC), OPR-02 (CACAGCTGCC) and OPAC-04 (ACGGGACCTG). The eight plants, H3, H4, H5, H6, H7, H8, H9 and H11, survived in greenhouse and they were identified by RAPD analysis (Fig. 5 a–c). Plantlets H6, H7, H8, H9 were identified as hybrids by the presence of parent-specific bands inherited from both parents using OPAC-04 primer (line 4–7 in Fig. 5a), included three recipient-specific bands (white arrow indicated) and two donor-specific bands (black arrow indicated). Plantlet H11 and plantlet H5 were also identified as a hybrid by the amplified product of OPD-10 and OPR-02 primer, respectively. The plantlet H11 contained four recipient-specific, one donor-specific and a new RAPD bands (Fig. 5b) and plantlet H5 contained four recipient-specific and one donor-specific RAPD bands (Fig. 5c). The results of RAPD analyses revealed that bands specific to both fusion parents could be detected in the regenerated plants H5, H6, H7, H8, H9, H11, indicating genetic component of cv. Guoshanxiang (AAB) and cv. Mas (AA) has been incorporated into the plants. Therefore these plants could be confirmed as hybrids.
Determination of chromosome number
Chromosome number analysis of cv. Guoshanxiang (AAB) and cv. Mas (AA) indicated that their chromosome number are 33 and 22, respectively (Fig. 6a, b). This analysis also revealed that three retained plants possessed an aneuploid chromosome number (34) (Fig. 6c–e), which were fewer than the sum of the parental plants (55) and could be due to chromosome eliminations of one parent. However, their precise chromosomal constitution is not known. The chromosome number analysis further confirmed the hybrid nature of these regenerated plants.
Confirmation of hybrids by ISSR analysis
Among the twelve ISSR primers were tested for identification of the hybrids, two primers, 834(AG)8YT and 836 (AG)8YA (Y = C, T), showed polymorphism between the fusion parents and were selected to identify hybrids. The ISSR profiles obtained with 834(AG)8YT and 836 (AG)8YA (Y = C, T) suggested that three plants H5, H8, H9 retained in field posses a combination of both parental genomes (Fig. 7, arrow indicated), which were corroborated by RAPD analysis. These data suggested that whatever the ploidy level of the hybrid line was obtained, they were the result of recombination events in the nuclear genome.
ISSR analysis of plant DNA using the primers of 834 (AG)8YT and 836 (AG)8YA from cv. Guoshanxiang (AAB), cv. Mas (AA) and the putative somatic hybrids H5, H8 and H9. G: cv. Guoshanxiang (AAB); Mas: cv. Mas (AA); M: DNA molecular-weight marker. White arrow indicates band specific to cv. Guoshanxiang (AAB), black arrow indicates band specific to cv. Mas (AA)
Discussion
In this study, somatic hybrids were obtained from asymmetric protoplast fusion between recipient protoplasts of cv. Guoshanxiang (AAB) and donor protoplasts of cv. Mas (AA). RAPD and ISSR analysis revealed that bands specific to fusion parents could be detected in the somatic hybrids, indicating genetic component of cv. Guoshanxiang (AAB) and cv. Mas (AA) has been incorporated into the hybrids. To our knowledge, this is the first report of this kind of protoplast fusion in bananas.
To do asymmetric protoplast fusion, a common method is that recipient protoplasts are treated with IOA and donor protoplasts are irradiated with UV (Liu and Deng 1999; Varotto et al. 2001; Yamagishi et al. 2002; Yan et al. 2004). It has been showed that effects of UV on donor chromosome elimination and fragmentation are dose dependent (Forsberg et al. 1998; Zhou et al. 2002; Xiang et al. 2003). Wang et al. (1994) reported that UV irradiation inhibited protoplast division of Crambe abyssinica and high dosage of UV irradiation resulted in protoplast death. When UV dosage increased, the differentiation ability of colony formation and the frequency of plant regeneration decreased. It is also true for the protoplast fusion in banana. In this study, when donor protoplasts were treated with UV for 60 s, fusion products had a highest frequency of somatic embryo formation and relatively fewer plant regeneration. Similarly, fewer plantlets were regenerated from fusion products when donor protoplasts were irradiated by UV for 180 s. Whereas, the highest frequency of plantlet regeneration was derived from fusion products of which donor protoplasts were treated with UV for 120 s. These results further demonstrate that the UV treatment given to the donor protoplasts influenced the growth and development of fused products, suggesting that an optimal UV irradiation is a key factor for the asymmetric protoplast fusion.
By using UV-irradiation, the genome of the donor protoplasts may be largely inactivated and only a small portion of intact DNA will be transferred into the recipient protoplasts (Parokonny et al. 1994). In this study, chromosome counting of the hybrids revealed that the number of chromosomes in the hybrid cells was not simply the sum of parental sets, indicating that these hybrid lines are highly asymmetric. Three hybrids with 34 chromosomes might be due to a UV induced fragmentation of cv. Mas (AA) nucleus, the donor parent nucleus, and some genetic material from cv. Mas (AA) must have been integrated into the hybrid cells, through either chromosome recombination or chromosome substitution.
Through asymmetric protoplast fusion, many agronomic traits have been transferred into important crops. For example, Yue et al. (2001) transferred salt tolerance from Aeleuropus littorulis sinensis to wheat (Triticum aestivum L.); Wang et al. (2003) obtained rapeseed with high erucic acid content by asymmetric somatic hybridization between Brassica napus and Crambe abyssinica; Yan et al. (2004) transferred the bacterial blight resistance trait from Oryza meyeriana to O. sativa ssp. Japonica and obtained four high bacterial blight resistance hybrids. By using asymmetric protoplast fusion, the present study successfully obtained banana somatic hybrids between cv. Guoshanxiang (AAB) and cv. Mas (AA). It validates asymmetric protoplast fusion is a promising tool for transferring resistance gene between banana varieties and open a new window for banana variety improving. For this purpose, further study is needed to widen combination between banana varieties and then assess the agronomic characters of those obtained hybrids.
Abbreviations
- BA:
-
6-Benzylaminopurine
- 2, 4-D:
-
2,4-Dichlorophenoxyacetic acid
- ECS:
-
Embryogenic cell suspensions
- IOA:
-
Iodoacetamide
- ISSR:
-
Inter-simple sequence repeat
- MES:
-
2-N-morpholino ethanesulfonic acid
- NAA:
-
1-Naphthaleneacetic acid
- PEG:
-
Polyethylene glycol
- RAPD:
-
Random amplified polymorphism DNA
- UV:
-
Ultraviolet light
References
Assani A, Haïcour R, Wenzel G, Côte FX, Bakry F, Foroughi-Wehr B, Ducreux G, Aguillar ME, Grapin A (2001) Plant regeneration from protoplasts of dessert banana cv. Grande Naine (Musa spp., Cavendish sub-group AAA) via somatic embryogenesis. Plant Cell Rep 20:482–488. doi:10.1007/s002990100366
Assani A, Haïcour R, Wenzel G, Foroughi-Wehr B, Bakry F, Côte FX, Ducreux G, Ambroise A, Grapin A (2002) Influence of donor material and genotype on protoplast regeneration in banana and plantain cultivars (Musa spp.). Plant Sci 162:355–362. doi:10.1016/S0168-9452(01)00562-3
Assani A, Chabane D, Haicour R, Bakry F, Wenzel G, Foroughi-Wehr B (2005) Protoplast fusion in banana (Musa spp.): comparison of chemical (PEG: polyethylene glycol) and electrical procedure. Plant cell Tiss Org Cul 83:145–151
Assani A, Chabane D, Foroughi-Wehr B, Wenzel G (2006) An improved protocol for microcallus production and whole plant regeneration from recalcitrant banana protoplasts (Musa spp.). Plant Cell Tissue Organ Cult 85:257–264. doi:10.1007/s11240-005-9058-y
Bauer-Weston B, Keller W, Webb J (1993) Production and characterization of asymmetric somatic hybrids between Arabidopsis thaliana and Brassica napus. Theor Appl Genet 86:150–158. doi:10.1007/BF00222073
Côte FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from the male flower of Musa AAA cv. Grande Naine. Physiol Plant 97:285–290. doi:10.1034/j.1399-3054.1996.970211.x
Du DL, Su J, Zhou P, Zheng XQ, Huang BZ, Li FN (2001) RAPD analysis of 33 varieties of banana. Acta Bot Sin 43:1036–1042
Forsberg J, Dixelius C, Lagercrantz U, Glimelius K (1998) UV dose-dependent DNA elimination in asymmetric somatic hybrids between Brassica napus and Arabidopsis thaliana. Plant Sci 131:65–76. doi:10.1016/S0168-9452(97)00242-2
Huang BZ, Xu LB, Yang H, Tang XL, Wei YR, Qiu JS, Li GQ (2005) Preliminary results of field evaluation of banana germplasm resistant to Fusarium wilt disease. Guangdong Agric Sci 6:9–10 in Chinese with English abstract
Kisaka H, Kisaka M, Kanno A, Kameya T (1998) Intergeneric somatic hybridization of rice (Oryza sativa L.) and barley (Hordeum vulgare L.) by protoplast fusion. Plant Cell Rep 17:362–367. doi:10.1007/s002990050407
Liu JH, Deng XX (1999) Regeneration of hybrid calluses via donor-recipient fusion between Microcitrus papuana and Citrus sinensis. Plant Cell Tissue Organ Cult 59:81–87. doi:10.1023/A:1006399302843
Matsumoto K, Oka S (1998) Plant regeneration from protoplasts of a Brazilian dessert banana (Musa spp., AAB group). Acta Hortic 490:455–462
Matsumoto K, Vilarinhos AD, Oka S (2002) Somatic hybridization by electrofusion of banana protoplasts. Euphytica 125:317–324. doi:10.1023/A:1016071624090
Megia R, Hacour R, Tizroutine S, Bui Trang V, Rossignol L, Sihachakr D, Schwendiman J (1993) Plant regeneration from cultured protoplasts of the cooking banana cv. Bluggoe (Musa spp., ABB group). Plant Cell Rep 13:41–44. doi:10.1007/BF00232313
Morpurgo R, Lopato SV, Afza R, Novak FG (1994) Selection parameters for resistance to Fusarium oxysporum f.sp.Cubense race 1 and race 4 on diploid banana (Musa acuminata Colla). Euphytica 75:121–129. doi:10.1007/BF00024539
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Panis B, Wauwe AW, Swennen R (1993) Plant regeneration through direct somatic embryogenesis from protoplasts of banana (Musa spp.). Plant Cell Rep 12:403–407. doi:10.1007/BF00234701
Parokonny AS, Kenton A, Gleba YY, Bennett MD (1994) The fate of recombinant chromosomes and genome interaction in Nicotiana asymmetric somatic hybrids and their sexual progeny. Theor Appl Genet 89:488–497. doi:10.1007/BF00225385
Rasmussen JQ, Nepper JP, Rasmussen QS (1997) Regeneration and analysis of interspecific asymmetric potato-Solanum spp. hybrid plants selected by micromanipulation or fluorescence activated cell sorting (FACS). Theor Appl Genet 95:41–49. doi:10.1007/s001220050530
Spangenberg G, Valles MP, Wang ZY, Montavon P, Nagel J, Potrykus I (1994) Asymmetric somatic hybridization between tall fescue (Festuca arundinaceae Schreb) and irradiated Italian ryegrass (Lolium multiforum Lam) protoplast. Theor Appl Genet 88:509–519. doi:10.1007/BF01240911
Varotto S, Nenz E, Lucchin M, Parrini P (2001) Production of asymmetric somatic hybrid plants between Cichorium intybus L. and Helianthus annuus L. Theor Appl Genet 102:950–956. doi:10.1007/s001220000485
Vlahova M, Hinnisdales S, Frulleux F, Claeys M, Atanassov A, Jacobs M (1997) UV irradiation as a tool for obtaining asymmetric somatic hybrids between Nicotiana plumbaginigolia and Lycopersicon esculentum. Theor Appl Genet 94:184–191. doi:10.1007/s001220050398
Wang H, Xia GM, Chen HM (1994) Effect of UV on the protoplast division and chromosome variation of wheat. Acta Phytobiol Sin 4(1):69–72
Wang YP, Sonntag K, Rudloff E (2003) Development of rapeseed with high erucic acid content by asymmetric somatic hybridization between Brassica napus and Crambe abyssinica. Theor Appl Genet 106:1147–1155
Wei YR, Huang XL, Huang X, Li J, Xiao W, Li XJ (2005a) The induction of multiple buds and somatic embryogenesis of Musa silk Guoshanxiang (AAB). Act Hortic Sin 32:414–419 in Chinese with English abstract
Wei YR, Huang XL, Li J, Huang X, Li Z, Li XJ (2005b) Establishment of embryogenic cell suspension culture and plant regeneration of edible banana Musa acuminata cv. Mas (AA). Chin J Biotechnol 21:58–65 in Chinese with English abstract
Xia GM, Chen HM (1996) Plant regeneration from intergeneric somatic hybridization between Triticum aestivum L. and Leymmus chinensis (trin) Tzvel. Plant Sci 120:197–203. doi:10.1016/S0168-9452(96)04492-5
Xia GM, Xiang FN, Zhou AF, Wang H, Chen HM (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107:299–305. doi:10.1007/s00122-003-1247-7
Xiang FN, Xia GM, Chen HM (2003) Effect of UV dosage on somatic hybridization between common wheat (Triticum aestivum) and Avena sativa L. Plant Sci 164:697–707. doi:10.1016/S0168-9452(03)00021-9
Xiao W, Huang XL, Huang X, Chen YP, Dai XM, Zhao JT (2007) Plant regeneration from protoplasts of Musa acaminata cv. Mas (AA) via somatic embryogenesis. Plant cell Tiss Org Cult 90:191–200
Xiao W, Huang X, Wei YR, Zhao JT, Dai XM, Huang XL (2008) Plant regeneration from protoplast culture of Musa AAB Silk cv. Guoshanxiang. Act Hortic Sin 35(6):873–878 in Chinese with English abstract
Yamagishi H, Landgren M, Forsberg J, Glimelius K (2002) Production of asymmetric hybrids between Arabidopsis thaliana and Brassica nupus utilizing an efficient protoplast culture system. Theor Appl Genet 104:959–964. doi:10.1007/s00122-002-0881-9
Yan CQ, Qian KX, Yan QS, Zhang XQ, Xue GP, Huang WG, Wu YF, Zhao YZ, Xue ZY, Huang J, Xu GZ, Wu P (2004) Use of asymmetric somatic hybridization for transfer of the bacterial blight resistance trait from Oryza meyeriana L. to O. sativa L. ssp.japonica. Plant Cell Rep 22:569–575. doi:10.1007/s00299-003-0732-4
Yue W, Xia GM, Zhi DY, Chen HM (2001) Transfer of salt tolerance from Aeleuropus littorulis sinensis to wheat (Triticum aestivum L.) via asymmetric somatic hybridization. Plant Sci 161:259–266. doi:10.1016/S0168-9452(01)00382-X
Zhou AF, Chen XL, Xia GM, Chen HM (2002) Study of UV-fusion between common wheat and Haynaldia villosa. Acta Phytophysiol Sinica 28(4):305–310
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (30400287), Guangdong Natural Science Foundation of China (011126, 06023159), Programs for Science and Technology Development of Guangdong (2006B20101014) and Programs for Science and Technology Development of Guangdong (2006Z3-E0281).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, W., Huang, X., Gong, Q. et al. Somatic hybrids obtained by asymmetric protoplast fusion between Musa Silk cv. Guoshanxiang (AAB) and Musa acuminata cv. Mas (AA). Plant Cell Tiss Organ Cult 97, 313–321 (2009). https://doi.org/10.1007/s11240-009-9530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9530-1