Abstract
Suspension-derived protoplasts of Agropyron elongatum irradiated by ultra-violet light (UV) were fused with the suspension-derived protoplasts of Triticum astivum using PEG. Fertile intergeneric somatic hybrid plants were produced and various hybrid lines have been selected and propagated in successive generations. Their hybrid nature was confirmed by analysis of profiles of isozymes, RAPDs, and 5S rDNA spacer sequences, and via GISH analysis. By the procedure described, the phenotype and chromosome number of wheat could be maintained besides transfer of a few chromosomes and chromosomal fragments from the donor A. elongatum. The results above indicated that highly asymmetric fertile hybrid plants and hybrid progenies of wheat were produced via somatic hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic hybridization offers the possibility of circumventing barriers to sexual reproduction and allows for the improvement of a crop species by the transfer of agronomically relevant traits (Glimelius et al. 1991). As technical improvements have been made in hybrid induction, more and more interspecific and intergeneric fertile hybrids have been created (Waara and Glimelius 1995; Li et al. 1999), but it remains difficult to produce fertile hybrids involving widely separated Gramineae species. Thus, for example, in the combination Saccharum officinarum (Sugarcane) + Pennisetum americanum (pearl millet), only somatic embryos could be produced (Tabaeigadeh et al. 1986); while for Oryza sativa (rice) + Echinochloa (barnyard grass), only short-lived seedlings (1 cm in height) were recovered (Terada et al. 1987). Flowering festulolium (Festuca arundinacea + Lolium multiflorum) somatic hybrid plants represent the first example of mature hybrids within the Gramineae (Spangenberg et al. 1995). These plants were able to set seed following artificial pollination with L. multiflorum. Recently, fertile hybrids have also been obtained from the combination rice + barley (Kisaka et al. 1998), and rice + Zizania latifolia (Griseb) (Liu et al. 1999). For wheat, work carried out in our laboratory has generated intergeneric hybrid plants (Xia and Chen 1996; Zhou et al. 1996, 2001b) which could flower, but could not set seeds.
Agropyron elongatum (2n = 70) is a perennial forage grass characterized by interesting levels of tolerance to abiotic stress, resistance to some important diseases of wheat and a high seed protein content. Derivatives of sexual hybrids between wheat and A. elongatum have been used to generate high quality wheat cultivars (Li 1995). However the necessary chromosome engineering techniques are tedious and time consuming. The present report describes a somatic hybrid route to introgression, which will not only be more efficient than sexual crossing, but will also allow the exploitation of non-nuclear genes from both parents, which is not possible by the sexual route.
Materials and methods
Origin of wheat protoplasts
Protoplasts of common wheat (cv Jinan 177, 2n = 42) were prepared from cell suspensions which divided at a high frequency but were not capable of regenerating into whole plants. The methods for establishing suspensions and preparing protoplasts have been described previously (Xia et al. 1995; Xia and Chen 1996). The chromosome number of 90% cells varied from 2n = 30 to 2n = 38.
Origin and UV treatment of A. elongatum protoplasts
Young embryos of A. elongatum (2n = 70) were used for the establishment of calli. After 2 years of subculture, yellow/white, loose, fast-growing structures with a chromosome number of 50–60 were used as a source of protoplasts. The methods for callus and suspension establishment, and for protoplast preparation, were the same as for wheat. The calli and suspensions were non-regenerable, and the protoplasts did not divide under the conditions used in this experiment.
Newly prepared protoplasts were placed in small Petri dishes and care was taken to ensure that cell density was adjusted to permit the formation of a monolayer on the bottom of the Petri dish. These protoplasts were exposed to ultraviolet light at an intensity of 380 μW cm–2 for 0.5, 1, 3 and 5 min respectively, and washed twice with 0.6 M mannitol +0.5 M CaCl2.
Selection strategy
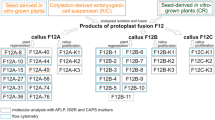
The procedure for hybrid production and selection was as follows (Fig. 1).
Fusion and culture procedure
For fusion, both the wheat and UV-irradiated A. elongatum protoplasts were adjusted to a density of 1 × 106 ml–1, mixed thoroughly in a ratio of 2:1 and fused using the PEG method (Lindsey 1991). The following fusion combinations were carried out: wheat protoplasts (T) + A. elongatum protoplasts (A), the latter receiving UV irradiation of 380 μW cm–2 for 0.5 min (I), 1 min (II), 3 min (III) and 5 min (IV). For each combination, the following controls were cultivated: T, A, T (+) A (mixture), T (+) T and A (+)A. The fusion products were cultured with P5 and then MB medium was developed for wheat protoplast culture (Xia et al. 1995). The former contains 1 mg·l–1 of 2,4-D, and the latter 1 mg·l–1 of 2,4-D and 0.5 mg·l–1 of zeatin. Fast-growing clones were transferred on to regeneration medium (MB containing 0.5 mg·l–1 each of indo-3-acetic acid and zeatin). To encourage rooting 2–3-cm-high plantlets were transferred to MB medium containing 1–3 mg·l–1 of pacclobutrozol and 0.5 mg·l–1 of naphthalene acetic acid, and cultured under fluorescent light at 2000 lx for 12 h per day at 4–12 °C before being potted into soil about 3–4 weeks later.
Culture of hybrid ovaries
Ovaries derived from hybrid plants were induced to form calli on MB medium containing 2 mg·l–1 of 2,4-D followed by subculture every 15–20 days. When the calli turned light yellow and granular about 2 months later, they were transferred to differentiation medium to regenerate plants (F0) and then transplanted into soil as described above.
Identification of putative hybrid calli and plants
Isozyme analysis
The soluble proteins of the regenerated calli and the leaves of hybrid plants and parents were extracted and separated on polyacrylamide gels. Esterase, peroxidase and malic dehydrogenase isozymes were analyzed as described elsewhere (Xia and Chen 1996).
RAPD analysis
Genomic DNA was isolated from young leaves of putative hybrid plants and parents according to Doyle and Doyle (1990). Informative RAPD primers were OPJ-04 (CCGAACACCG), OPJ-05 (CTCCATGGGG), OPJ-09 (TGAGCCTCAC), OPJ-11 (ACTCCTGCGA), OPJ-12 (GTCCCGTGGT), OPJ-13 (CCACACTACC), OPJ-14 (CACCCGGATG), OPB-08 (TGGACCGGTG) and OPB-09 (CTCACCGTCC). Methods for the production of RAPD profiles followed Xia et al. (1998).
5S rDNA spacer sequence analysis
Total DNA of F1 lines was isolated as above. Two pairs of primers (pI + pII; pIII + pIV) were used to amplify the spacer regions, following the methods of Zhou et al. (2001a). The sequences of these primers were: pI. 5′-GGTAGTACATCGATGGG; pII. 5′-GGAGTTCTGACGGGATCCGG; pIII. 5′-GGATGGGTGACCTCCCGGGAAGTCC and pIV. 5′-CGCTTAACTGCGGAGTTCTGATGGG.
Cytogenetical analysis of the hybrids and progenies
Chromosome analysis of the calli was performed according to Xia and Chen (1996). Root tips were excised, pretreated in water (4 °C) for 24 h, fixed in 3:1 ethanol:glacial acetic acid for 2 h at room temperature, rinsed in water for 15 min and incubated in an enzyme mixture [1.5% cellulase (Onozuka R-10), 0.1% Pectolyase Y-23] in 0.6 M mannitol, 5 mM CaCl2, pH 5.8 at 25 °C for 0.5–1 h. The partially digested root tips were gently rinsed in water for 15–30 min, fixed in 3:1 ethanol:glacial acetic acid for 15–30 min and then pressed for chromosome spreading on a glass slide. In situ hybridization was performed following the methods described by Zhou et al. (2001a).
Results
Fusion of wheat and A. elongatum protoplasts and culture of the fusion products
Wheat protoplasts appear transparent with dense cytoplasm, while A. elongatum ones contain abundant starch grains (Fig. 2a). About 4 h after fusion some hybrid cells could be recognized from their spherical shape (Fig. 2b). The cultures of combination I generally entered first division after 4–5 days. After 2 weeks in culture, some embryo-like structures (absent in the other combinations and controls) and many compact cell clumps were found, and some of these reached a size of 1–1.5 mm after 1 month of culture. Fourteen such clumps were transferred to a solid medium in the first lot, but only three regenerated normal green plantlets. Two clones produced albinos, one gave rise to short-lived seedlings, while others showed only green sports. Later lots of clones from combination I and the clones from combinations II and III grew slowly, and were all non-regenerable. No clones were observed from combination IV.
In all combinations, the control protoplasts showed either no sign of cell division or produced only small calli from which no plants differentiated. A total of 29 regenerated green plants were obtained in this experiment. Five of these survived and grew to the flowering stage, although more slowly than normal wheat. Stems and ears were wheat-like, but none were self-fertile. All dissected flowers had the normal complement of three stamens and a normal-looking pistil. It is possible that the sterility of these hybrids was due to the unfavorable climatic factors for flowering, rather than to any physiological or genetic defect.
Identification of hybridity of regenerated plants
Morphology
All regenerated plants were wheat-like, but showed traces of A. elongatum characteristics. The wheat ligule is long and broad, while that of A. elongatum is short with hairs on the top; the hybrid had no detectable ligule. The auricle of wheat is long with scattered hairs, while that of A. elongatum is small and sharp with thick hairs; the hybrid auricle is short, somewhat like A. elongatum but hair-less (Fig. 3). These characters were especially visible at the seedling stage.
Isozyme patterns
The hybrid nature of all the early formed clones (I1–I6) were identified by their esterase profiles, and the regenerated plants in addition by both malic acid dehydrogenase and peroxidase profiles (Fig. 4a, b). Only about 30% of the slowly growing calli showed esterase isozyme band(s) of both parents, but all of these were non-regenerable. This analysis indicated that only vigorous growing calli were hybrid in nature and had the capacity to regenerate whole plants.
RAPD analysis
Figure 5a, b, c show the RAPD profiles using the primers OPB-08, OPJ-04, -11, -12, -13, -14 for wheat, A. elongatum and hybrid plants. The results confirm that the hybrid plants carried DNA from both parents.
Chromosome counting
Over 90% of the chromosome numbers of the calli derived from wheat protoplasts were in the range of 30–38, while that in the root tips of the hybrids were 40–46 and chromosome fragments or small chromosomes could be observed in most of the hybrid cells.
From the above observations, and given that neither of the parental protoplasts were regenerable, we concluded that the plantlets obtained were asymmetric hybrids.
Hybrid progeny production and their characteristics
Fertile plant regeneration from hybrid ovaries
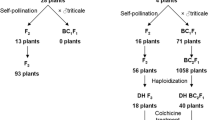
None of the regenerated plants set selfed seeds, although their flowers appeared to be morphologically normal. In order to obtain further generations, ovary culture was employed to induce plant regeneration. A total of 23 F0 seedlings from ovary derived clones were obtained, of which six grew to maturity. The plants grew slowly under natural spring conditions and were stunted in height (about 20 cm tall). Their phenotypes were largely wheat-like (Fig. 6a). Two plants (9%), originating from independent clones, set several seeds later than did the parent wheat. Hybrid seeds were smaller than those of common wheat, but were normal in shape and plumpness (Fig. 6b). These seeds gave rise to healthy mature F1 plants of varying phenotype.
Morphology of hybrid progeny
Two distinct phenotypes were observed among these progenies: type I had taller stems (75–85 cm) with large ears and grains (Fig. 7a), while type II had short stems (average 55 cm) with a strong tillering ability, smaller ears and grains (Fig. 7b). Through propagation and selection from F2 to F5, both hybrid lines were stabilized for morphology.
5S rDNA analysis
Variation in 5S rDNA spacer length was used to analyze nuclear DNA of the hybrid progenies at two specific loci. Hybrid DNA profiles consist of different combinations of fragments from each parent (Fig. 8a, b), demonstrating the hybridity of the progenies, which demonstrates the existence of parental DNA in the hybrid progenies.
GISH analysis of F2 and F5 hybrid plants
From GISH karyotypes of the different hybrid lines, variation in somatic chromosome number was in the range of 38–44, about 70% of which showed 2n = 42. This included 0–2 chromosomes or chromosomal fragments of A. elongatum, either as entire A. elongatum chromosomes and/or as translocated (intercalary or terminal) wheat/A. elongatum chromosomes (Fig. 9a, b).
GISH karyotypes of two hybrid progenies. a F2 II-1–4 hybrid plant showing two A. elongatum chromosomes and some translocations and insertions of chromosomal fragments of A. elongatum. b F5 I-1–5 showing translocations between parent chromosomes. ↑: chromosome or chromosomal fragment/arm of A. elongatum. (green–yellow colour indicative of A. elongatum origin)
Discussion
Selection and regeneration of asymmetric somatic hybrids of wheat × A. elongatum
The commonly used method for selection of asymmetric somatic hybrids involves irradiation (χ-, γ-ray or UV light) of the donor (Imamura et al. 1987; Famelaer et al. 1989; Vlahova et al. 1997; Forsberg et al. 1998) and chemical treatment (such as the metabolic inhibitor IOA) of the acceptor. However, such treatments inevitably damage the growth and development of some resulting hybrid cells (Hansen and Earle 1997; Zhou et al. 1996, 2001b; Xia and Chen 1996). An ideal selection system should involve minimum harm to hybrid cells. The restoration of morphogenetic potential in fusion products was a good selection system, which was demonstrated in Arabidopsis thaliana/Brassica campestris fusions (Gleba et al. 1984), and was also found in our wheat/A. elongatum hybrids. Somatic hybridization in Gramineae species has proven to be problematic, partly because of the difficulty in establishing embryogenic calli or cell suspensions as a source of protoplasts for fusion, and partly because it is hard to maintain embryogenic potential during cell subculture. Thus the strategy of using complementation of regeneration for the hybrid cells can also overcome a major block in hybrid plant production in wheat + grass combinations.
Effect of UV on chromosome fragmentation and elimination
Production of highly asymmetric somatic hybrids with a few chromosomes, or chromosomal fragments of the donor species, simultaneously overcomes both hybrid sterility (Dudits et al. 1987; Hinnisdales et al. 1991) and the introgression of too much exotic genetic material into the crop species. Beside the ionizing radiation, UV has also been used to induce production of asymmetric somatic hybrids. Fertile asymmetric hybrid plants were produced between Nicotiana plumbaginifolia and UV irradiated Lycopersicon esculentum (Vlahova et al. 1997). Forsberg et al. (1998) obtained highly asymmetric fertile hybrid plants between Brassica napus and UV-treated A. thaliana, and confirmed that DNA elimination was UV dose-dependent. Using GISH analysis in our experiments, it was clear that UV induced both donor chromosome elimination and fragmentation, and so could generate novel chromosomes, including intercalary translocations (Fig. 9a). Such chromotypes are particularly interesting in the context of alien introgression, since they can incorporate a minimal amount of alien chromatin. In contrast, introgression based on recombination or meiotic chromosome breakage tends to generate only single-breakpoint chromotypes, and a round of intercrossing overlapping introgression lines is required to generate intercalary introgressions.
Somatic hybridization in wheat breading
Many hybrids between common wheat and a number of its closely related grass species have been obtained by sexual hybridization, and some of these have been used as a basis for cultivar development (Prem and Ravindra 1999). However, poor fertility in early generations due to unbalanced chromosome number, and the absence of recombination between wheat and non-wheat chromosomes, makes this a cumbersome route for introgression. In contrast, somatic hybridization offers a quicker strategy for the introduction of novel traits (Yue et al. 2001). A further advantage of the somatic hybridization strategy is that it widens the range of donor species beyond those which are sexually compatible; as an example, we have reported the production of asymmetric hybrid plants between wheat and oat (Avena sativa L.) showing only one or two oat chromosomes, as well as lines only carrying oat chromosome fragments (Xiang et al. 2002). Following somatic hybridization, many hybrid plants with different genetic compositions can be obtained from one fusion experiment. Additionally, in contrast to sexual hybrids, somatic hybrids will include elements of both the nuclear and cytoplasmic genomes of the fusion parents, and this has the potential to derive novel variability. Some important traits are controlled by cytoplasmic genes, in particular cytoplasmic male sterility (CMS), and asymmetric somatic hybridization has been used successfully to transfer a wild abortive CMS gene into rice (Bijoya et al. 1999). Mixed-origin mitochondrial genes have been verified in the somatic hybrids of wheat + Haynaldia villosa (Zhou et al. 2001a) and other combinations of wheat + grass (unpublished data).
Phenotyping of the hybrid lines is underway, and preliminary data indicate that some lines show positive effects on flour quality and stress tolerance. For example, type I and type II hybrid lines we have described above show elevated seed protein content (about 17–22%) compared to that of the parent wheat (about 14%). The type II line appears to be of high quality for bread making (according to tests conducted by the Quality and Resource Test Centre of the Agricultural Science Academy of China), and type I has potential for enhanced yield and salt tolerance.
In conclusion, we have obtained highly asymmetric somatic hybrid plants and wheat genotypes with some specific traits via asymmetric hybridization, and we propose that asymmetric protoplast fusion between wheat and intergeneric grasses could be a promising tool for wheat introgression.
References
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dudits D, Maroy E, Praznovszky T, Olah Z, Gyorgyey J, Cella R (1987) Transfer of resistance traits from carrot into tobacco by asymmetric somatic hybridization: regeneration of fertile plants. Proc Natl Acad Sci USA 84:8434–8438
Bijoya B, Aniruddha PS, Hari SG (1999) Transfer of wild abortive cytoplasmic male sterility through protoplast fusion in rice. Mol Breed 5:319–327
Famelaer I, Gleba YY, Sidorov VA, Kaleda VA, Parakonny AS, Boryshuk NV, Cherep NN, Negrutiu I, Jacobs M (1989) Intergeneric asymmetric hybrids between Nicotiana plumbaginifolia and Nicotiana sylvestris obtained by 'gamma-fusion'. Plant Sci 61:105–117
Forsberg J, Dixelius C, Lagercrantz U, Glimelius K (1998) UV dose-dependent DNA elimination in asymmetric somatic hybrids between Brassica napus and Arabidopsis thaliana. Plant Sci 131:65–76
Gleba YY, Sytnik KM (eds) (1984) Protoplast fusion, genetic engineering in higher plants. Springer Verlag, Berlin Heidelberg
Glimelius K, Fahleson J, Landgren M, Sjodin C, Sundberg E (1991) Gene transfer via somatic hybridization in plants. Trends Biotechnol 9:24–30
Hansen LN, Earle ED (1997) Somatic hybrids between Brassica oleracea L. and Sinapis alba L. with resistance to Alternaria brassicae (Berk.) Sace. Theor Appl Genet 94:1078–1085
Hinnisdales S, Bariiller L, Mouras A (1991) Highly asymmetric intergeneric nuclear hybrids between Nicotiana and Petunia: evidence for recombinogenic and translocation events in somatic hybrid plants after "gamma"-fusion. Theor Appl Genet 82:609–614
Imamura J, Saul MW, Potrykus I (1987) X-ray irradiation promoted asymmetric somatic hybridization and molecular analysis of the products. Theor Appl Genet 74:445–450
Kisaka H, Kisaka M, Kanno A, Kameya T (1998) Intergeneric somatic hybridization of rice (Oryza sativa L.) and barley (Hordeum vulgare L.) by protoplast fusion. Plant Cell Rep 17:362–367
Li YG, Stoutjestijk PA, Larkin PJ (1999) Somatic hybridization for plant improvement. In: Soh W-Y, Bhojwani SS (eds) Morphogenesis in plant tissue cultures. Kluwer Academic Publishers. Dordrecht, pp 363–418
Li ZS (1995) Remote generic hybridization. In: Jin SB (ed) A study of Chinese wheat. China Agriculture Press. Beijing, pp 405–416
Lindsey K(1991) Plant tissue culture mannual, section D. Kluwer Academic Publishers Dordrecht, pp 1–17
Liu B, Liu ZL, Li XW (1999) Production of a highly asymmetric somatic hybrid between rice and Zizania latifolia (Griseb): evidence for inter-genomic exchange. Theor Appl Genet 98:1099–1103
Prem PJ, Ravindra NC (1999) Chromosome-mediated and direct gene transfer in wheat. Genome 42:570–583
Spangenberg G, Wang ZY, Legris G, Montavon P, Takamizo T, Perezvicente R, Valles MP, Nagel J, Potrykus I (1995) Intergeneric symmetric and asymmetric hybridization in Festuca and Lolium. Euphytica 85:235–245
Tabaeigadeh Z, Ferl JF, Vasil IK (1986) Somatic hybridization in the Gramineae: Saccharum officinarum L. (Sugarcane) and Pennisetum americanum (L.) K. Schum. Proc Natl Acad Sci USA 83:5616–5619
Terada R, Kyozuka J, Nishibayashi S, Shimamoto K (1987) Plantlet regeneration from somatic hybrids of rice (Oryza sativa L.) and barnyard grass (Echinochloa oryzicola Vasing). Mol Gen Genet 210:39–43
Vlahova M, Hinnisdales S, Frulleux F, Claeys M, Atanassov A, Jacobs M (1997) UV irradiation as a tool for obtaining asymmetric somatic hybrids between Nicotiana plumbaginifolia and Lycopersicon esculentum. Theor Appl Genet 94:184–191
Waara S, Glimelius K (1995) The potential of somatic hybridization in crop breeding, Euphytica 85:217–223
Xia GM, Chen HM (1996) Plant regeneration from intergeneric somatic hybridization between Triticum aestivum L. and Leymus chinensis (Trin.) Tzvel. Plant Sci 120:197–203
Xia GM, Li ZY, Zhou AF, Guo GQ, Chen HM (1995) Plant regeneration of wheat protoplasts prepared from different composition of suspension culture. Chinese J Biotech 11:289–293
Xia GM, Li ZY, Wang SL, Xiang FN, Chen PD, Liu DJ (1998) Asymmetric somatic hybridization between haploid wheat and UV irradiated Haynaldia villosa. Plant Sci 137:217–223
Xiang FL, Xia GM, Chen HM (2002) Somatic hybridization between Triticum aestivum and Avena sativa. Sci China (Series C) 32:299–305
Yue W, Xia GM, Zhi DY, Chen HM (2001) Transfer of salt tolerance from Aeleuropus littoralis Sinensis to wheat (Triticum aestivum L.) via asymmetric somatic hybridization. Plant Sci 161:259–263
Zhou AF, Xia GM, Chen HM (1996) Asymmetric somatic hybridization between Triticum aestivum L. and Haynaldia villosa Schur. Sci China (Series C) 39:617–626
Zhou AF, Xia GM, Zhang XQ, Chen HM, Hu H (2001a) Analysis of chromosomal and organellar DNA of somatic hybrids between Triticum aestivum and Haynaldia villosa Schur. Mol Gen Genet 265:387–393
Zhou AF, Xia GM, Chen HM (2001b) Comparative study of symmetric and asymmetric somatic hybridization between common wheat and Haynaldia villosa. Sci China (Series C) 44:617–626
Acknowledgements
This study was supported by the National Natural Science Foundation of China, No.30070397, the Trans-century Training Programme Foundation for Talents by the Ministry of Education in China and National 863 High Technology Research and Development Project No. 2001AA241032. We are grateful to Dr. Robert Koebner (John Innes Centre, UK) for language correction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Glimelius
Rights and permissions
About this article
Cite this article
Xia, G., Xiang, F., Zhou, A. et al. Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107, 299–305 (2003). https://doi.org/10.1007/s00122-003-1247-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-003-1247-7