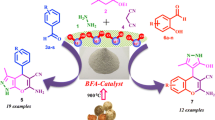
Abstract
A dry rind of Aegle marmelos (bael) fruit ash as a synergetic alternative material to an expensive, toxic and corrosive catalysts for the synthesis of biscoumarins and 2-amino-4H-chromenes at ambient temperature in water is reported. The spectroscopic evidence from EDX, FTIR, XRD and SEM analysis of bael fruit ash supports the presence of metal oxides, carbonates and hydroxides which are intensely responsible for the acceleration of the reactions. The striking features of this protocol are utilization of bio-waste, cost-effective, recyclable and biodegradable catalytic system, which provide good to excellent yields in a short reaction time.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One-pot multi-component reaction strategies offer eco-friendly and significant advantages over conventional linear-type syntheses by virtue of their convergence, productivity, reduction in work-up and purification steps, facile execution and high yield [1]. Because of these versatile advantages, recently, multi-component reactions have been widely used in the field of organic, medicinal and combinatorial chemistry.
The attractive features of 4H-chromenes motivated scientific community to design and develop natural product-derived analog of alkaloids, for example, Arisugacin A [2], Ophioglonin [3] and Hyperxanthone [4]. Similarly, some biscoumarin moieties have been found in nature including Bisosthenon (Citrus funadocao), Ismailin (Diospyros ismaili), Dicoumarol (Melilotus alba) and Gerberinol (Gerbera lanuginose) as shown in Fig. 1 [5].
Nowadays, the development of biscoumarins has received significant attention because they have potent biological [6, 7] and many pharmacological activities, such as antitumor [8], antioxidant [9], anti-inflammatory [10], cytotoxicity [11], anticancer [12], antiviral [13], antipyretic [14] and antimicrobial [15] properties. Some of the applied methodologies with the use of various catalysts such as water extract of waste onion peel ash [16], FeNi3–ILs [17], CuO–CeO2 [18], DBSA [19], molecular iodine [20], Zn(proline)2 [21], sodium dodecyl sulfate [22], W-doped ZnO nanocomposite [23], DBU [24], [bmim] [BF4] [25], ruthenium(III) chloride hydrate [26], SiO2Cl [27], TBAB [28] and Fe(SD)3 [29, 30] have been reported.
2-Amino-4H-chromenes and their derivatives exhibit a broad spectrum of biological and pharmaceutical activities such as antioxidant [31], anticancer [32], anti-HIV [33], anti-dyslipidemic [34], anti-hyperglycemic [35], xanthine oxidase inhibitory [36], antimicrobial [37], antiallergic [38], acetylcholinesterase, butyrylcholinesterase inhibitory [39] and Src kinase inhibitory activities [40]. For the synthesis of 2-amino-4H-chromenes from aryl aldehydes, 4-hydroxycoumarin and malononitrile with various catalysts such as polymer-supported sulfanilic acid [41], sodium dodecyl sulfate [42], ionic liquid [43], Fe3O4@SiO2–imid–PMAn [44], ammonium metavanadate [45], DBU [46], TBAB [47], N(Et)3 [48], MgO [49], starch [50], aqueous K2CO3 [51], H6P2W18O62.18H2O [52] and piperidine [53] have been used.
Water is the universal solvent that plays a significant role in all chemical reactions of life [54, 55]. Water as a reaction media came from the studies on ‘Hydrophobic effects on simple organic reactions in the water’ by Ronald Breslow in the 1980s [56]. Recently, Sharpless and co-workers have defined ‘on water’ conditions using water as a solvent for the reaction of water-insoluble reactants without the use of organic cosolvent [57]. Another study done by Engberts has shown that the Diels–Alder cycloaddition reaction accelerated very rapidly in water [58]. Notably, the use of water as a solvent has attracted much interest in recent years. Indeed, water offers many advantages because it is a cheap, readily available, non-toxic and nonflammable solvent, thus being very attractive from both an economic and environmental point of view [59].
Sustainability is a broad discipline which has become a watchword and guiding principle for modern society, and a growing appreciation is that the anthropogenic ‘bio-waste,’ in all its manifold forms can offer a valuable source of energy, chemicals, construction materials and high-value functional products. In the context of organic transformations, the waste material provides an alternative renewable natural feedstock as well as a good resource to create catalysts. The catalytic system developed from waste material serves to improve the overall energy and atom efficiency of existing as well as novel chemical processes [60].
In view of these data and in continuation of our research program concerning the development of catalyst from natural feedstock [61,62,63,64], herein, we report first time bio-waste-derived rind of bael fruit ash (BFA) as a green catalyst for the facile synthesis of biscoumarins and 2-amino-4H-chromenes using aryl aldehyde, 4-hydroxycoumarin and malononitrile via a tandem Knoevenagel–Michael reaction under aqueous medium (Scheme 1). Bael (Aegle marmelos) is a sacred tree of the Hindus, origin from India and known from ancient times. It is found growing along foothills of Himalaya in India. It is also grown in Egyptian gardens in Trinidad and Surinam as well as other countries [65, 66]. All parts of bael tree are medicinally useful, like leaves, flower, stem bark, roots, fruits, seeds, etc., and are used extensively in the Indian traditional system of medicine, the Ayurved [67, 68]. In the literature, it has been well studied and reported [69] that bael fruit ash contains K and Ca as major constituents along with host of other trace elements like Mn, Fe, Cu, Zn, Na and Mg. It is believed that metal oxides, carbonates and hydroxides may act as a promoter to catalyze the reaction.
The BFA catalyst was found to be cost-effective, highly efficient, innocuous, biodegradable and renewable resource material, which is an attractive alternative to other conventional reagents. Recently, bio-waste has been successfully employed as a catalyst by many researchers in organic transformation [70,71,72]. Therefore, we strongly believed that our developed catalytic system is the best and accomplished with excellent yield for a broad range of substrates that are highly attractive for industrial applications in the near future.
Experimental
Materials and methods
Except bael fruit rinds, all other chemicals used in the study were purchased from Sigma-Aldrich and used without any purification. The completion of reaction and purity of products were checked by TLC on Merck silica gel (60 F254) plates. The melting points were recorded using DBK programmable melting point apparatus by capillary methods and uncorrected. FTIR spectrum was recorded in ATR technique on a Bruker ALPHA FTIR spectrometer. Powder XRD patterns were collected on the Bruker AXS Analytical Instruments Pvt. Ltd. Germany (Model: D2 phaser diffractometer) by using Cu Kα radiation (λ = 1.5406 Å) at 10 mA, 30 kV, a scan step time 0.5 per sec, and a start position 10.0184 and end position 90.00 [2° Th.]. Scanning electron microscope images were obtained on FEI, NOVA, NanoSem 450 equipment. Quanta 200 3D FEI scanning electron microscope was used for energy-dispersive X-ray spectroscopy analysis. 1H and 13C NMR spectra were recorded with an Avance 300 instrument in CDCl3 and DMSO as a solvent with TMS as an internal standard. Chemical shifts (δ) are expressed in ppm.
Preparation of BFA catalyst
The dry rinds of bael fruits (Fig. 2a) were obtained from the local area, and species were authenticated by the department of botany. The collected dry rinds of bael fruit were washed with distilled water and then dried in the oven at 100 °C. The dried rinds were manually broken into smaller pieces (Fig. 2b) and then thermally treated for 2 h at 900 °C in a muffle furnace at a heating rate of 2 °C/min, and this temperature was maintained till no evolution of smoke, which resulted in fine soft ash. The resulting solid ash (Fig. 2c) was denominated as bael fruit ash (BFA) catalyst.
Typical procedure for the synthesis of 4-hydroxy-3-((4-hydroxy-2-oxo-2H-chromen-3-yl)(4-methoxyphenyl)methyl)-2H-chromen-2-one (3a)
In a 25-mL reaction flask, 4-methoxybenzaldehyde (1.0 mmol, 136 mg), 4-hydroxycoumarin (2.0 mmol, 324 mg), BFA (10 wt%, 13.6 mg) and H2O (3 mL) were placed. A resulting reaction mixture was stirred at ambient temperature for 15 min. The progress of the reaction was monitored by TLC (n-hexane/EtOAc, 7:3). After completion of the reaction, the product 3a was extracted with EtOAc (2 × 10 mL) from the reaction mixture. The combined organic phase was washed with H2O and dried (Na2SO4), and the solvent was removed under reduced pressure to afford a crude product. The pure product was obtained in a quantitative yield by recrystallization from 96% EtOH. All other derivatives were synthesized by employing the same procedure, and their structures were confirmed on the basis of spectral techniques (ESI). The physical and spectroscopic data are in consistent with the proposed structures and are in harmony with the literature values.
Typical procedure for the synthesis of 2-amino-4,5-dihydro-4-(4-methoxyphenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5c)
In a 25-mL reaction flask, 4-methoxybenzaldehyde (1.0 mmol, 136 mg), 4-hydroxycoumarin (1.0 mmol, 162 mg), malononitrile (1.0 mmol, 66 mg), BFA (10 wt%, 13.6 mg) and H2O (3 mL) were placed. A resulting reaction mixture was stirred at ambient temperature for 10 min. The progress of the reaction was monitored by TLC (n-hexane/EtOAc 7:3). After completion of the reaction, the product 5c was extracted with EtOAc (2x10 mL) from the reaction mixture. The combined organic phase was washed with H2O and dried (Na2SO4), and the solvent was removed under reduced pressure to afford a crude product. The pure product was obtained in a quantitative yield by recrystallization from 96% EtOH. All other derivatives were synthesized by employing the same procedure, and their structures were confirmed on the basis of spectral techniques (ESI). The physical and spectroscopic data are in consistent with the proposed structures and are in harmony with the literature values.
Results and discussion
Catalyst characterization
The literature reports of BFA reveal that the presence of metal oxides, carbonates and hydroxides along with transition elements has been studied and characterized by using various analytical techniques like EDX, FTIR, XRD and SEM analyses.
Energy-dispersive X-ray spectroscopy (EDX)
The distribution of elements as based on the EDX analysis of the BFA is shown in Fig. 3. The report reveals the distribution of the oxides of K, Ca, Mn, Fe, Cu, Zn, Na and Mg. It is believed that oxides of these metals react with reaction medium water to produce hydroxides, which provides basic sites for tandem Knoevenagel–Michael reaction pathway.
X-ray diffraction pattern (XRD)
The X-ray diffraction analysis of the BFA catalyst (Fig. 4) showed the characteristic peaks of metal oxides and carbonates present in the catalyst. The peaks at 2θ = 26.35, 28.37, 29.70, 30.68, 31.86, 32.99, 33.39, 35.81, 40.55, 41.80, 43.18, 50.17 62.31, 66.23 and 73.75 attributed to metal oxides and carbonates. Another peaks were also observed at 2θ = 13.38 and 19.40 due to metal hydroxides. The crystallite size of BFA was found to be 58 nm calculated using the Scherrer equation. Therefore, this active phase of the BFA catalyst was utilized for the conversion of reactants into the desired products.
Fourier transform infrared (FTIR) spectroscopy
The FTIR analysis was performed to investigate the functional groups present in BFA catalyst (Fig. 5). The characteristics absorption bands at 602, 1006, 1499 and 1660 cm−1 provide strong revelations about the presence of potassium oxides (K–O–K stretching), other metal oxides (CaO, MgO) and metal carbonates (C=O stretching), respectively. Also, the observation of broad and sharp absorption bands at 3336 cm−1 and 3740 cm−1 due to the small concentration of the hydroxyl group (–OH) in the spectrum supports the formation of metal hydroxides due to the absorption of moisture from the environment.
Scanning electron microscope (SEM)
The apparent morphology of BFA catalyst was examined by SEM analysis and is shown in Fig. 6. As observed from SEM micrographs, BFA appears porous in nature which provides a smooth and soft surface area for catalyzing the reaction.
Catalytic performance of BFA catalyst
The catalytic efficiency of BFA was explored in the one-pot three-component reaction, which involves 4-methoxybenzaldehyde and 4-hydroxycoumarin as a model precursor under different reaction conditions to afford 4-hydroxy-3-((4-hydroxy-2-oxo-2H-chromen-3-yl)(4-methoxyphenyl)methyl)-2H-chromen-2-one 3a. The success of the model reaction was examined in various reaction parameters with BFA (wt%) as a catalyst (Table 1). Initially, the model reaction was examined under catalyst-free and solvent-free conditions, and after 2 h, the formation of corresponding product was not observed on TLC (Table 1, entry 1).

As the reaction requires a catalyst, we examine the effect of BFA catalyst on this transformation; the model reaction was performed using various amounts of BFA catalyst (1–20 wt%) in H2O (3 mL) (Table 1, entries 2–5). The results show that excellent yield (94%) of desired product was obtained in 15 min when 10 wt% of BFA was employed in 3 mL H2O as a solvent (Table 1, entry 4). As reaction requires solvent, we also examined model reaction in different conventional organic solvents like EtOH, iso-PrOH, THF, DCM, CH3CN CHCl3 and toluene. The results reveal that polar protic and nonpolar solvent afforded moderate yields (Table 1, entries 6–8), while pure EtOH and H2O/EtOH (1:1) solvent system shows equally good results for the present synthesis (Table 1, entry 6 and 9). Also, we perform model reaction in water extract of bael fruit ash (3 mL) which shows equally good results after 15 min (Table 1, entry 10). For comparison purposes, we also performed the model reaction in the presence of parent bael fruit (PBF) powder and it was observed that no product was obtained (Table 1, entry 11) even after 2 h.
To determine the influence of the ashing temperature for preparation of BFA, bael fruit rinds were thermally treated at different temperatures between 200 and 900 °C and the resulted ash was tested for the model reaction to produce desired product (Table 2). The results show that BFA obtained after 900 °C temperature gave better result than BFA obtained at lower temperatures of thermal treatment (Table 2 entry 5).
By reacting a variety of differently substituted aryl aldehydes (1a–l) with a 4-hydroxycoumarin (2) under optimized reaction conditions, we managed to prepare a range of biscoumarin derivatives (3a–l) and the results are shown in Table 3. As shown, aryl aldehydes with substituents carrying either electron-withdrawing or electron-donating groups reacted successfully and gave the expected products in stipulated time period.
Although we did not investigate reaction mechanism, the proposed mechanism for the biscoumarins formation is depicted in Scheme 2. The BFA catalyst containing the mixture of active metal oxides and carbonates along with transition metals is responsible for its basicity, which is soluble to some extent in H2O which provides a number of Lewis basic sites (O2−and OH) along with Lewis acid sites (M2+) for the activation of reactants in the proper direction. At first, the aryl aldehyde 1 is activated by Lewis acidic sites of catalyst. The nucleophilic attack of 4-hydroxycoumarin 2 on activated aldehyde is followed by elimination of water molecules to generate Knoevenagel intermediate (I), which is further activated by Lewis basic and acidic sites of catalyst. The activated intermediate (I) undergoes Michael addition with second molecule of 4-hydroxycoumarin to afford the targeted biscoumarin product 3 through tautomerism of intermediate (II). As a result, the overall effect of our catalyst is a rate enhancement of the reaction.
Next, we identified the recovery and reusability of catalyst which is important for scaling up practical and industrial applications. A recycling experiment was conducted using the model reaction. After completion of the reaction, the product 3a was extracted with EtOAc (2 × 10 mL) from the reaction mixture. The combined organic phase was washed with H2O and dried (Na2SO4), as well as the solvent was removed under reduced pressure to afford a crude product. The recovered aqueous layer containing BFA catalyst was dried under vacuum and directly used for the next cycle with fresh reactants in H2O. The result obtained in our experiment (Fig. 7) was confirmed that the BFA could be reused up to fifth run with only negligible loss of activity. The presence of active metal oxides responsible for its basicity in recycled catalytic system was evidenced by the EDX analysis (Fig. 8), which revealed no appreciable change in the chemical composition of catalyst even after the fifth cycle.
The successful application of BFA as catalyst in the synthesis of biscoumarins encouraged us to explore its compatibility in synthesis for 2-amino-4H-chromene derivatives under optimized reaction conditions, and the results are summarized in Table 4. The results indicate that aldehydes with electron-withdrawing substituent were found to be suitable for this transformation as the corresponding products were obtained in high yields than aldehyde with electron-donating substituent.
We compared the efficiency of our method for the synthesis of biscoumarin 3a with other reported works (Table 5, entry 1–6). Each of these methods has its own advantages, but some of them suffer from disadvantages such as the employment of expensive catalysts, long reaction time, poor yield and none from natural sources. So the present method (Table 5, entry 7) furnishes the use of renewable feedstock, a green reaction medium, biodegradable and reusability of catalyst, with a shorter reaction time, while a small quantity of this inexpensive and readily available catalyst is sufficient to obtain good yield of the expected product.
Conclusion
In conclusion, we have demonstrated an efficient and green method for the synthesis of biscoumarin and 2-amino-4H-chromene derivatives using BFA in water under air atmosphere at RT. We utilized bio-waste-derived catalyst that acts as synergetic material for the tandem Knoevenagel–Michael reaction. This protocol avoids chromatographic separation, use of expensive base, toxic solvents and high temperature. BFA catalyst is ecologically benign which excludes the possibility of any imminent disposal problem. The ability to scale up the protocol also makes it a competitive and convenient catalyst for industrially important organic transformations.
References
L. Weber, Drug Discov. Today 7, 143 (2002)
Y. Mehellou, E.D. Clercq, J. Med. Chem. 53, 521 (2010)
M. Makino, Y. Fujimoto, Phytochemistry 50, 273 (1999)
S. Medda, S. Mukhopadhyay, M.K. Basu, J. Antimicrob. Chemother. 44, 791 (1999)
R.D.H. Murray, Fortchemie 35, 67 (1991)
Z. Karimi-Jaberi, M.R. Nazarifar, B. Pooladian, Chin. Chem. Lett. 23, 781 (2012)
L. Manolov, M.M. Caecilina, N. Danchev, Eur. J. Med. Chem. 14, 882 (2006)
H. Zhou, F. Dong, X. Du, Z. Zhou, H. Huo, W. Wang, H. Zhan, Y. Dai, J. Meng, Y. Sui, J. Li, F. Sui, Y. Zhai, Bioorg. Med. Chem. Lett. 26, 3876 (2016)
M.A. Musa, J.S. Cooperwood, M.F. Khan, Curr. Med. Chem. 15, 2664 (2008)
K.V. Sashidhara, M. Kumar, R.K. Modukuri, R. Sonkar, G. Bhatia, A.K. Khanna, S. Rai, R. Shukla, Bioorg. Med. Chem. Lett. 21, 4480 (2011)
M.I. Choudhary, N. Fatima, K.M. Khan, S. Jalil, S.A. Iqbal, Bioorg. Chem. 14, 8066 (2006)
A. Maresca, A. Scozzafava, C.T. Supuran, Bioorg. Med. Chem. Lett. 20, 7255 (2010)
C.X. Su, J.F. Mouscadet, C.C. Chiang, H.J. Tsai, L.Y. Hsu, Chem. Pharm. Bull. 54, 682 (2006)
A. Saeed, F.A. Larik, Chem. Heterocycl. Compd. 52, 450 (2016)
L.K. Singh, V.P. Singh, D. Katiyar, Med. Chem. 11, 128 (2015)
P.W. Chia, B.S. Lim, F.S.J. Yong, S.C. Poh, S.Y. Kan, Environ. Chem. Lett. 16(4), 1493 (2018)
J. Safaei-Ghomi, F. Eshteghal, H. Shahbazi-Alavi, Polycycl. Aromat. Comp. 40, 13 (2017)
J. Albadi, A. Mansournezhad, S. Salehnasab, Res. Chem. Intermed. 41(8), 5713 (2015)
B. Pawar, V. Shinde, A. Chaskar, Green. Sustain. Chem. 3, 56 (2013)
M. Kidwai, V. Bansal, P. Mothsra, S. Saxena, R.K. Somvanshi, S. Dey, T.P. Singh, J. Mol. Catal. A: Chem. 268, 76 (2007)
Z.N. Siddiqui, F. Farooq, Cat. Sci. Technol. 1, 810 (2011)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7, 890 (2010)
F. Shirini, M. Abedini, S. Zamani, H.F. Moafi, J. Nanostruct. Chem. 5, 123 (2015)
H. Hagiwara, N. Fujimoto, T. Suzuki, M. Ando, Heterocycles 53, 549 (2000)
J.M. Khurana, S. Kumar, Monatsh. Chem. 141, 561 (2010)
K. Tabatabaeian, H. Heidari, A. Khorshidi, M. Mamaghani, N.O. Mahmoodi, J. Serb. Chem. Soc. 77, 407 (2012)
R. Karimian, F. Piri, A.A. Safari, S.J. Davarpanah, J. Nanostruct. Chem. 3, 52 (2013)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
N.O. Mahmoodi, Z. Jalalifard, G.P. Fathanbari, J. Chin. Chem. Soc. 67, 172 (2020)
N.O. Mahmoodi, F.G. Pirbasti, Z. Jalalifard, J. Chin. Chem. Soc. 65, 383 (2018)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Chem. Lett. 19, 1139 (2009)
N.R. Emmadi, K. Atmakur, G.K. Chityal, S. Pombala, J.B. Nanubolu, Bioorg. Med. Chem. Lett. 22, 7261 (2012)
S.S. Mansoor, K. Logaiya, K. Aswin, P.N. Sudhan, J. Taibah Univ. Sci. 9, 213 (2015)
A. Kumar, R.A. Maurya, S.A. Sharma, P. Ahmad, A.B. Singh, G. Bhatia, A.K. Srivastava, Bioorg. Med. Chem. Lett. 19, 6447 (2009)
H.J. Wang, J. Lu, Z.H. Zhang, Monatsh. Chem. 141, 1107 (2010)
R. Kaur, F. Naaz, P.M.S. Bedi, S. Sharma, K. Nepali, S. Mehndiratta, M.K. Gupta, Med. Chem. Res. 24, 3334 (2015)
J.A. Makawana, M.P. Patel, R.G. Patel, Arch. Pharm. Chem. Life Sci. 345, 314 (2012)
P. Coudert, J.M. Coyquelet, J. Bastide, Y. Marion, J. Fialip, Ann. Pharm. Fr. 46, 91 (1988)
M. Khoobi, M. Alipour, A.H. Sakhteman, A. Moradi, M. Ghandi, S. Emami, H. Nadri, A. Foroumadi, A. Shafiee, Eur. J. Med. Chem. 68, 260 (2013)
A. Rafinejad, A. Fallah-Tafti, R. Tiwari, A.N. Shirazi, D. Mandal, K. Parang, A. Foroumadi, T. Akbarzadeh, A. Shafiee, Daru. J. Pharm. Sci. 20, 100 (2012)
J.P. Patel, J.R. Avalani, D.K. Raval, J. Chem. Sci. 125(3), 531 (2013)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7(4), 890 (2010)
D.S. Patel, J.R. Avalani, D.K. Raval, J. Saudi Chem. Soc. 20, S401 (2013)
M. Esmaeilpour, J. Javidi, F. Dehghani, F.N. Dodeji, RSC Adv. 5, 26625 (2015)
B.V. Shitole, N.V. Shitole, M.S. Shingare, G.K. Kakde, Curr. Chem. Lett. 5, 137 (2016)
J.M. Khurana, B. Nand, P. Saluja, Tetrahedron 66, 5637 (2010)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50(28), 4125 (2009)
A.M. Shestopalov, Y.M. Emelianova, V.N. Nesterov, Russ. Chem. Bull. 51, 2238 (2002)
N. Ramireddy, S. Abbaraju, C.G. Zhao, Tetrahedron Lett. 52, 6792 (2011)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 391 (2014)
M. Kidwai, S. Saxena, R.K.M. Khalilur, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4292 (2005)
M.M. Heravi, B. AlimadadiJani, F. Derikvand, F.F. Bamoharram, H.A. Oskooie, Catal. Commun. 10, 272 (2008)
S. Irani, M.T. Maghsoodlou, N. Hazeri, Indian J. Chem. 56B, 649 (2017)
R. Ludwig, Angew. Chem. Int. Ed. 40, 1808 (2001)
P. Ball, Chem. Phys. Chem. 9, 2677 (2008)
R. Breslow, Acc. Chem. Res. 24, 159 (1991)
S. Narayan, J. Muldoon, M.G. Finn, V.V. Fokin, H.C. Kolb, K.B. Sharpless, Angew. Chem. Int. Ed. 44, 3275 (2005)
S. Otto, J.B.F.N. Engberts, J.C.T. Kwak, J. Am. Chem. Soc. 120, 9517 (1998)
M. Simon, C. Li, Chem. Soc. Rev. 41, 1415 (2012)
J. Bennett, K. Wilson, A.F. Lee, J. Mater. Chem. A4, 3617 (2016)
R.C. Patil, U.P. Patil, A.A. Jagdale, S.K. Shinde, S.S. Patil, Res. Chem. Intermed. 46, 3527 (2020)
U.P. Patil, R.C. Patil, S.S. Patil, J. Heterocycl. Chem. 56, 1898 (2019)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. Intermed. 44(3), 1775 (2018)
S.K. Shinde, S.A. Damate, S.T. Morbale, M.U. Patil, S.S. Patil, RSC Adv. 7, 7315 (2017)
A.V.S.S. Sambamurthy, N.S. Subrahmanyam, Fruits and Nuts, A Text Book of Economic Botany, vol. 4 (Wiley Eastern Limited, New Delhi, 1989), p. 697
S.S. Purohit, S.P. Vyas, in Aegle marmelos Correa ex Roxb. (Bael), Medicinal plant cultivation-A Scientific Approach, Agrobios, Jodhpur, vol. 280 (2004)
A. Jhajhria, K. Kumar, Int. J. Pharm. Sci. Rev. Res. 36(2), 121 (2016)
P.K. Patel, J. Sahu, L. Sahu, N.K. Prajapati, B.K. Dubey, Int. J. Pharm. Phytopharmacol. Res. 1(5), 332 (2012)
C.S. Laddha, S.G. Kunjalwar, P.R. Itankar, M. Tauqeer, Asian J. Pharm. Clin. Res. 8(1), 76–78 (2015)
P.B. Hiremath, K. Kantharaju, ChemistrySelect 5, 1896 (2020)
Y. Sun, W. Jin, C. Liu, Molecules 24, 3838 (2019)
K. Rajkumari, D. Das, G. Pathak, L. Rokhum, New J. Chem. 43, 2134 (2018)
A.N. Nadaf, K. Shivashankar, J. Heterocycl. Chem. 55(8), 676 (2018)
B. Maleki, Org. Prep. Proc. Int. 48, 303 (2016)
Acknowledgements
One of the authors, Mr. Rupesh C. Patil, is grateful to the Chhatrapati Shahu Maharaj Research Training and Human Development Institute (SARTHI), Pune (Government of Maharashtra), for the award of fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, R.C., Shinde, S.K., Patil, U.P. et al. A synergetic role of Aegle marmelos fruit ash in the synthesis of biscoumarins and 2-amino-4H-chromenes. Res Chem Intermed 47, 1675–1691 (2021). https://doi.org/10.1007/s11164-020-04367-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04367-6