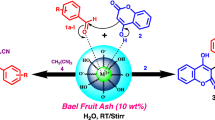
Abstract
Bisenols and its derivatives are attractive heterocyclic compounds exhibiting a wide range of biological properties, including anticancer, antipyretic and antimicrobial characteristics. Many catalytic systems have been reported to enhance the synthesis of bisenols, but these catalytic systems suffer from several drawbacks, such as the use of external metals, expensive and toxic chemicals. Thus, the development of a greener and efficient catalyst for the synthesis of bisenols is highly sought after. Herein, an improved protocol for the synthesis of bisenols in the water extract of burned-ash of onion peel waste (ash-water extract) as an efficient catalytic system is reported. Upon completion of the reaction, the crude mixture was extracted with ethyl acetate and the ash-water extract was successfully reused for several times in the synthesis of a variety of bisenols. Bisenols were obtained in good to excellent yields (62–94%) by using various benzaldehyde and 4-hydroxycoumarin catalyzed by the ash-water extract. Moreover, all pure products were obtained by precipitation without the need of column purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the search for simple and efficient green chemical processes has been the key driving force in both the industrial and academic communities (Simon and Li 2012). In this regard, efforts have been devoted on using environmentally acceptable reagents, solvents and catalysts, and enforce on the use of more eco-friendly protocols in accomplishing organic chemical processes (Yang et al. 2016a, b). Aqueous media, in particular, has attracted significant attention as they are environmentally friendly, cheap, safe and readily available (Li and Chen 2006; Wagare et al. 2017). Aqueous organic reactions have also been recognized as environmentally benign tools in the modification of biomolecules and in the synthesis of bioactive molecules (Wang et al. 2003; Yang et al. 2016a, b). Moreover, the use of water as solvent has been reported to enhance the rate of chemical reactions (Bhowmick et al. 2015; Jafari and Ghadami 2016; Miklós and Fülöp 2016; Vamisetti et al. 2017; Yorulmaz et al. 2017).
Lately, there has been a growing interest among chemists to use waste water and the water extracts of fruits and vegetables to perform organic reactions (Sarmah et al. 2017). The use of these biodegradable/recyclable media serves as a promising and attractive tool for industrial application in the near future. Among the organic reactions that were performed using these non-classical solvents include the Suzuki–Miyaura reaction (Boruah et al. 2015), Sonogashira reaction (Dewan et al. 2016), synthesis of 3-carboxycoumarins (Fiorito et al. 2016), Henry reaction (Surnenia et al. 2016), Dakin reaction (Saikia and Borah 2015) and several other examples. Likewise, bio-wastes are an appealing alternative to improve the “greenness” of organic chemical processes. Every month, million tons of bio-wastes were generated by eateries and food processing factories (Choi et al. 2015). The generation of food waste has increased as a result of the high demand for agricultural production to meet the ever-growing world population (Marshall and Farahbakhsh 2013). Majority of the bio-wastes are allocated to landfill, and this has seriously impacted our ecosystem, wildlife and the human health (Gao et al. 2015).
Onion (Allium cepa L.) is an ubiquitous crop grown not only for human consumption, but also for their therapeutic and other functional purposes (Nile and Park 2013). Every year, it is estimated that about 100,000–500,000 tons of onion waste were generated in the developed countries alone (Sharma et al. 2016). In this regard, here we would like to present a green and efficient method of synthesizing bisenols which was carried out by using the waste onion peel ash-water extract. The bisenol and its derivatives have demonstrated to possess a broad range of biological activities, including anticancer (Maresca et al. 2010), antiviral (human immunodeficiency virus) (Su et al. 2006), antipyretic (Saeed and Larik 2016), antimicrobial (Singh et al. 2015; Qu et al. 2014) and several other favorable properties. The conventional methods of synthesizing bisenols involved the condensation of 4-hydroxycoumarin and benzaldehydes in the presence of external metals, expensive and environmentally harmful catalysts, such as phosphotungstic acid (Singh et al. 2010), tetrabutylammonium bromide (Khurana and Kumar 2009), l-proline–zinc (Siddiqui and Farooq 2011), manganese (II) chloride (Sangshetti et al. 2009), sulphated titania (Karmakar et al. 2012) and triethylammonium hydrogen sulfate (Patil et al. 2017). The abundant onion peel waste generated across the world is an appealing resource, especially in regard with its application in organic synthesis, and thus aid in reducing the environmental pressure. In organic synthesis, the prospect of using an inexpensive and environmental benign natural feedstock extract to accomplish organic processes, and at the same time able to overcome the above-mentioned drawbacks, is promising.
Experimental
Materials and equipments
All chemicals and solvent used in this study including ethyl acetate, benzaldehyde, 2-chlorobenzaldehyde, 3-chlorobenzaldehyde, 4-chlorobenzaldehyde, 2-bromobenzaldehyde, 3-bromobenzaldehyde, 3-fluorobenzaldehyde, 4-isopropylbenzaldehyde, 4-methoxybenzaldehyde, chloroform-d (CDCl3), dimethyl sulfoxide-d6 (DMSO-d6) and silica gel 60 (0.063–0.200 mm) were purchased from Sigma-Aldrich and were used without further purification. The proton (1H) and carbon-13 (13C) nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 solvent on Bruker Avance III 400 MHz spectrometer. The chemical shifts are quoted in δ (parts per million, ppm), and coupling constants (J) are quoted in hertz (Hz). The following abbreviations correlate with the multiplicity of NMR signals: s = singlet, d = doublet, t = triplet, m = multiplet and br.s = broad singlet. In addition, the units of measure abbreviation are provided as follows: min = minute, h = hour, mmol = milimole, g = gram, °C = degree celcius, % = percent, mg/L = milligram per liter and mL = milliliter. The chemical abbreviations used in this manuscript were included as follows: H = proton, Cl = chlorine, Br = bromine, F = fluorine, MeO = methoxy and Me = methyl. The gas chromatography mass spectrometry (GC–MS) analyses were performed using Shimadzu QP2010SE equipped with a Supelco fused silica capillary column (30 m × 0.25 mm i.d., 0.25 mm film thickness). The mass spectra values are quoted in unit of mass-to-charge ratio (m/z). The determination of metal content was performed using Varian Vista Pro inductive coupled plasma-optical emission spectroscopy (ICP-OES).
Preparation of the ash-water extract
The onion waste was collected at a local restaurant. The onion peel was separated from the bulb and was thoroughly washed with distilled water. Subsequently, the onion peel was sun-dried for two days. The onion peel ash-water extract was prepared according to a previous literature (Boruah et al. 2015). Firstly, the dried onion peels (2.5 g) were cut into small pieces and were burned to ash in a furnace at 500 °C for 1 h (h). About 1.0 g of the ash was transferred to a 250-mL conical flasks suspended with 30 mL of distilled water, and the mixture was magnetically stirred for 1 h. The resulting mixture was then filtered and the filtrate is termed as the ash-water extract.
Metal content analysis on the ash-water extract
0.1 g of onion peel ash was weighed and put into a 50-mL polypropylene centrifuge tube. After the addition of deionized water (30 mL), the tube was capped and shaken for 3 min to allow thorough mixing of the onion peel ash and extraction solution. The onion peel ash was filtered, and the filtrate was transferred into a 50-mL beaker. The filtrate was heated on a hot plate until a final volume of 5 mL was obtained for simultaneous metal determination by ICP-OES analysis. The blank solution was prepared using 5 mL of deionized water. The sample was analyzed in triplicates. The ICP-OES data on the ash-water extract were shown as mean value ± standard deviation (SD) in parts per billion (ppb). The ICP-OES working standards ranged from 0.01 to 10 mg/L were prepared daily from the multiple-element stock solutions (Inorganic Venture, IV-inductive coupled plasma mass spectrometry-71A). An internal standard solution, namely the scandium, was used to reduce the spectral interference and matrix effects. A set of known concentration of standard solutions were treated as a quality control for measuring the unknown concentrations, and the analytical accuracy of the ICP-OES analysis was within 5% for all measured elements.
General procedure for the synthesis of bisenols
The ash-water extract (1 mL) was added into a 25-mL round bottom flask that was suspended with a mixture of 4-hydroxycoumarin (2 mmol) and benzaldehydes (1 mmol). The reaction mixture was stirred magnetically at 80 °C. The formation of bisenols was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the aqueous layer was extracted three times with 10 mL ethyl acetate (3 × 10 mL). The organic layer was then transferred into a small vial and a 20 mL of ice-cold water was added into the same vial. The pure product was obtained by precipitation without further column purification. The remaining ash-water extract catalytic system was reused for the synthesis of other bisenols under the same condition. In addition, experiments such as the optimization study and synthesis of each bisenols were carried out twice and the yields were shown as mean value ± standard deviation (SD).
Spectroscopic data of selected bisenols
3,3′-(Phenylmethylene)bis(4-hydroxy-2H-chromen-2-one) 3a
1H-NMR (400 MHz, CDCl3): δ 6.11 (s, 1H); 7.22 (m, 2H); 7.26 (m, 1H); 7.34(m, 2H); 7.38(m, 2H); 7.42 (m, 2H); 7.63(m, 2H); 8.00(d, 1H, J = 7.1 Hz); 8.08 (d, 1H, J = 7.1 Hz); 13C NMR (100 MHz, CDCl3) δ 36.14, 103.91, 105.61, 116.64, 116.70, 124.38, 124.80, 126.58, 126.88, 128.64, 132.82, 135.20, 152.30, 152.50, 164.61, 165.80, 166.89, 169.30; GC–MS: C25H16O6, m/z 412.09.
3,3′-((4-Chlorophenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) 3d
1H-NMR (400 MHz, DMSO-d6): δ 6.34 (s, 1H); 7.17 (d, 2H, J = 8.3 Hz); 7.33–7.45 (m, 6H); 7.62 (t, 2H); 7.95 (d, 2H, J = 7.5 Hz); 13C NMR (100 MHz, DMSO-d6) δ 35.80, 103.94, 116.02, 117.80, 118.70, 123.84, 123.90, 129.20, 130.90, 132.10, 139.50, 152.20, 164.80, 165.21 GC–MS: C25H 3515 ClO6, m/z 446.06; C25H 3715 ClO6, m/z 448.04.
3,3′-((3-Bromophenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) 3f
1H-NMR (400 MHz, DMSO-d6): δ 6.38 (s, 1H); 7.22–7.26 (m, 2H); 7.34–7.40 (m, 6H); 7.64 (t, 2H); 7.95 (d, 2H, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6) δ 35.98, 103.63, 115.96, 117.80, 121.57, 123.70, 123.95, 126.04, 128.50, 129.32, 130.19, 131.91, 143.50, 152.30, 164.56, 165.58; GC–MS: C25H 7915 BrO6, m/z 490.01; C25H 8115 BrO6, m/z 492.00.
3,3′-((3-Fluoroyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) 3g
1H-NMR (400 MHz CDCl3): δ 6.06 (s, 1H); 7.02 (t, 2H); 7.19 (m, 2H); 7.42 (d, 4H); 7.65 (t, 2H); 8.02 (m, 2H); 11.34(s, 1H); 13C NMR (100 MHz, CDCl3) δ 35.59, 103,81, 105.51, 115.42, 115.66, 116.37, 116.68, 124.36, 124.98, 128.09, 128.22, 130.77, 130.78, 133.06, 152.28; 152.50; 160.11; 163.34; 164.56; 165.89; 166.76; 169.21; GC–MS: C25H15FO6, m/z 430.69.
3,3′-((4-Methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) 3h
1H-NMR (400 MHz DMSO-d6): δ 3.88 (s, 3H); 6.51 (s, 1H); 7.00 (d, 2H, J = 8.6 Hz); 7.26 (d, 2H, J = 8.4 Hz); 7.54(t, 2H); 7.55(d, 2H, J = 7.9 Hz); 7.81 (t, 2H); 8.10(d, 2H, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6,) δ 35.41, 55.18, 104,72, 113.75, 16.21, 117. 54, 123.98, 124.10, 127.95, 130.89, 132.28, 152.27, 157.62, 157.62, 164.69, 165.08; GC–MS: C26H15O7, m/z 442.11.
3,3′-(p-Tolylmethylene)bis(4-hydroxy-2H-chromen-2-one) 3i
1H-NMR (400 MHz DMSO-d6): δ 2.28 (s, 3H); 6.41 (s, 1H); 7.08 (s, 4H); 7.38 (t, 2H); 7.43(d, 2H, J = 8.5 Hz); 7.66(t, 2H, J = 7.9 Hz); 7.97 (d, 2H, J = 7.9 Hz); 12.18(br.s, 2H); 13C NMR (100 MHz, DMSO-d6,) δ 20.54, 35.58, 104,45, 116.12, 117.37, 123.86, 124.00, 126.66, 128.81, 132.18, 134.74, 136.00, 152.11, 164.65, 164.98; GC–MS: C26H18O6, m/z 426.11.
3,3′-((Phenyl)methylene)bis(4-hydroxy-6-methyl-2H-chromen-2-one) 3j
1H-NMR (400 MHz DMSO-d6): δ 2.35 (s, 6H); 6.27 (s, 1H); 7.04–7.68 (m, 11H); 12.48 (s, 2H); 13C NMR (100 MHz, DMSO-d6,) δ 20.68, 36.35, 103.71, 115.78, 116.38, 123.08, 125.13, 126.87, 127.87, 131.85, 132.07, 142.77, 150.87, 165.02, 165.89; GC–MS: C27H20O6, m/z 440.13.
Results and discussion
For the optimization study, benzaldehyde (1 mmol) and 4-hydroxycoumarin (2 mmol) were selected as the model starting materials. These chemicals were added into a 25-mL round bottom flask containing the ash-water extract. The reaction mixture was magnetically stirred, and the progress of the reaction was monitored by TLC. Initially, 0.2 mL of ash-water extract was added to the round bottom flask containing the model starting materials and the reaction mixture was heated at 40 °C. After 20 min, the yield of 3a was found to be poor (40%) (Table 1, entry 2). It is noteworthy that the yield of 3a increased by increasing the temperature and the amount of ash-water extract used (Table 1, entry 4). The yield of 3a was found to be excellent (94% yield), when 1.0 mL of ash-water extract was used as solvent and the reaction mixture was heated at 80 °C for 40 min (Table 1, entry 5). On the contrary, prolonging the reaction time and the use of a higher temperature (more than 80 °C) did not give rise to a better result (Table 1, entry 6–8). In this study, a control experiment was also performed to synthesize 3a by using model starting materials in the presence of water as solvent, but only 30% yield was recorded (Table 1, entry 1). Based on these results, the best reaction condition was determined (Table 1, entry 5) and this optimized reaction condition was extended for downstream studies.
Next, the general applicability of the ash-water extract catalytic system was examined by employing different aldehydes to synthesize a variety of bisenol derivatives 3a–j under the optimized condition. In all the cases, the bisenols were obtained by precipitation without the need of column purification (62–94% yield). As shown in Table 2, all the reactions afforded the corresponding bisenols 3a–j in good to excellent yields (Table 2, entries 1–10). In particular, benzaldehydes bearing the electron-withdrawing groups afforded the bisenols 3a–g in good to excellent yields within 40-min reaction time (Table 2, entries 1–7). The current protocol was also found to be efficient, when substituting the 4-hydroxycoumarin with 4-hydroxy-6-methylcoumarin, the corresponding product 3j was afforded in good yields (Table 2, entry 10). The catalytic performance of the ash-water extract was regarded as unprecedented due to the fact that no external bases and additive as catalyst were involved. In addition, all reactions were completed in a short reaction time. The synthesized bisenols 3a–j were characterized by NMR (1H and 13C) and GC–MS methods.

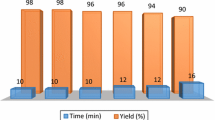
Furthermore, the reusability of the ash-water extract catalytic system was also investigated. It was observed that the ash-water extract catalytic system could be reused in subsequent reactions for up to five times, without significant loss of catalytic activity. Upon the completion of a reaction, the crude mixture was first extracted with ethyl acetate and the ash-water extract was reused for the synthesis of 3a. The yield of 3a was found to be excellent (92.8 ± 0.75%) after five successive synthetic reactions by using the recycled ash-water extract catalytic system (Fig. 1). The successive reuse of the catalytic system without significant loss of activity after each recovery indicates that this catalytic system may achieve the goal of green chemistry (Liu et al. 2014).
Recyclability test of ash-water extract for the synthesis of 3a. Reaction conditions: 1 mmol of benzaldehydes, 2 mmol of 4-hydroxycoumarin, 1 mL of ash-water extract, 80 °C. The ash-water extract can be recycled and reused for up to five times, without significant loss of activity. The recyclability result indicates that the ash-water extract is a recyclable and efficient catalytic media
The metal content and chemical constituents of the onion peel may vary according to the geographical location, seasonal harvesting and processing. As such, we have characterized the metal content of the ash-water extract by using ICP-OES, which resulted in the following output (mean value ± SD) in parts per billion (ppb): potassium (11749.00 ± 227.00), sodium (92.00 ± 0.21), magnesium (528.00 ± 40.00), phosphorus (87.00 ± 5.00), yttrium (33.00 ± 0.02) and boron (32.00 ± 3.00). Magnesium was found to be one of the major metals in this study, and also a common mineral identified in the onion peel of other geographical region (Ariyama et al. 2007). Based on the laboratory result, we found that under identical optimized reaction condition, by substituting the ash-water extract with magnesium oxide (1.0 mmol) in 5 mL of water, the formation of 3a was found to be in satisfactory yields (64%), which is in agreement with a previous study reported by Safaei-Ghomi et al. (2014) on the role of magnesium oxide as nanocatalyst. Thus, we hypothesized that the alkaline oxides, in particular, the magnesium oxide, resulted from the ash-water extract act as catalysts in the formation of bisenols. In order to show the merit of the current protocol, the previous protocols and their yields for the synthesis of 3a are summarized in Table 3. Most of the reported methods employed for the synthesis of bisenols involved the condensation of 4-hydroxycoumarin and benzaldehydes in the presence of external metals, non-recyclable catalysts, expensive reagents or environmentally harmful catalysts. In the current work, we have demonstrated that the waste peel of onion could be transformed into valuable and eco-friendly catalytic system that benefits not only to the mankind, but also reducing the impact caused by the disposal of this bio-waste to our environment. Furthermore, the current improved protocol is capable of minimizing the use of toxic chemicals and, at the same, time provides an alternative way of onion waste management.
Conclusion
In summary, we have demonstrated an efficient catalytic system for the synthesis of bisenols by using the waste onion peel ash-water extract. Under the improved protocol, it offers several advantages over the previous methods, which includes the elimination of toxic chemicals, cheap and the end products were obtained in good to excellent yields (62–94%). Furthermore, the ash-water extract catalytic system could be reused up to five times without the significant loss of activity. In addition, all pure products were obtained by precipitation without the need of column purification. This current improved protocol is capable of minimizing the use of toxic chemicals that is of scientifically significant and at the same time provides an alternative way of bio-waste management. We anticipate that the current protocol will provide a great utility in the synthesis of other heterocyclic compounds in the near future.
References
Ariyama K, Aoyama Y, Mochizuki A, Homura Y, Kadokura M, Yasui A (2007) Determination of the geographic origin of onions between three main production areas in Japan and other countries by mineral composition. J Agric Food Chem 55:347–354. https://doi.org/10.1021/jf062613m
Bhowmick S, Mondal A, Ghosh A, Bhowmick KC (2015) Water: the most versatile and nature’s friendly media in asymmetric organocatalyzed direct aldol reactions. Tetrahedron Assym 26:1215–1244. https://doi.org/10.1016/j.tetasy.2015.09.009
Boruah PR, Ali AA, Chetia M, Saikia B, Sarma D (2015) Pd(OAc)2 in WERSA: a novel green catalytic system for Suzuki–Miyaura cross-coupling reactions at room temperature. Chem Commun 51:11489–11492. https://doi.org/10.1039/C5CC04561D
Choi IS, Cho EJ, Moon JH, Bae HJ (2015) Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem 188:537–541. https://doi.org/10.1016/j.foodchem.2015.05.028
Dewan A, Sarmah M, Bora U, Thakur AJ (2016) A green protocol for ligand, copper and base free Sonogashira cross-coupling reaction. Tetrahedron Lett 57:3760–3763. https://doi.org/10.1016/j.tetlet.2016.07.021
Fiorito S, Taddeo VA, Genovese S, Epifano F (2016) A green chemical synthesis of coumarin-3-carboxylic and cinnamic acids using crop-derived products and waste waters as solvents. Tetrahedron Lett 57:4795–4798. https://doi.org/10.1016/j.tetlet.2016.09.023
Gao S, Li L, Geng K, Wei X, Zhang S (2015) Recycling the biowaste to produce nitrogen and sulfur self-doped porous carbon as an efficient catalyst for oxygen reduction reaction. Nano Energy 16:408–418. https://doi.org/10.1016/j.nanoen.2015.07.009
Jafari AA, Ghadami M (2016) Efficient synthesis of α, β-unsaturated ketones with trans-selective Horner–Wadsworth–Emmons reaction in water. Environ Chem Lett 14:223–228. https://doi.org/10.1007/s10311-016-0552-8
Karmakar B, Nayak A, Banerji J (2012) Sulfated titania catalyzed water mediated efficient synthesis of dicoumarols—a green approach. Tetrahedron Lett 53:4343–4346. https://doi.org/10.1016/j.tetlet.2012.06.024
Khurana JM, Kumar S (2009) Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett 50:4125–4127. https://doi.org/10.1016/j.tetlet.2009.04.125
Li CJ, Chen L (2006) Organic chemistry in water. Chem Soc Rev 35:68–82. https://doi.org/10.1039/B507207G
Liu DX, Li FL, Li HX, Gong WJ, Gao J, Lang JP (2014) Efficient and reusable CuI/1, 10-phenanthroline-catalyzed oxidative decarboxylative homocoupling of arylpropiolic acids in aqueous DMF. Eur J Org Chem 2014:4817–4822. https://doi.org/10.1002/ejoc.201402416
Maresca A, Scozzafava A, Supuran CT (2010) 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 20:7255–7258. https://doi.org/10.1016/j.bmcl.2010.10.094
Marshall RE, Farahbakhsh K (2013) Systems approaches to integrated solid waste management in developing countries. Waste Manag 33:988–1003. https://doi.org/10.1016/j.wasman.2012.12.023
Miklós F, Fülöp F (2016) A simple green protocol for the condensation of anthranilic hydrazide with cyclohexanone and N-benzylpiperidinone in water. J Heterocycl Chem 53:32–37. https://doi.org/10.1002/jhet.1844
Nile SH, Park SW (2013) Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front Life Sci 7:224–228. https://doi.org/10.1080/21553769.2014.901926
Patil SK, Awale DV, Vadiyar MM, Patil SA, Bhise SC, Kolekar SS (2017) Simple protic ionic liquid [Et3NH][HSO4] as a proficient catalyst for facile synthesis of biscoumarins. Res Chem Intermed 43:5365–5376. https://doi.org/10.1007/s11164-017-2932-5
Qu D, Li J, Yang XH, Zhang ZD, Luo XX, Li MK, Li X (2014) New biscoumarin derivatives: synthesis, crystal structure, theoretical study and antibacterial activity against Staphylococcus aureus. Molecules 19:19868–19879. https://doi.org/10.3390/molecules191219868
Saeed A, Larik FA (2016) Metal-free synthesis of isocoumarins (microreview). Chem Heterocycl Compd 52:450–452. https://doi.org/10.1007/s10593-016-1911-x
Safaei-Ghomi J, Eshteghal F, Ghasemzadeh MA (2014) Solvent-free synthesis of dihydropyrano[3, 2-c]chromene and biscoumarin derivatives using magnesium oxide nanoparticles as a recyclable catalyst. Acta Chim Slov 61:703–708
Saikia B, Borah P (2015) A new avenue to the Dakin reaction in H2O2–WERSA. RSC Adv 5:105583–105586. https://doi.org/10.1039/c5ra20133k
Sangshetti JN, Kokare ND, Shinde DB (2009) Water mediated efficient one-pot synthesis of bis-(4-hydroxycoumarin)methanes. Green Chem Lett Rev 2:233–235. https://doi.org/10.1080/17518250903393874
Sarmah M, Mondal M, Utpal B (2017) Agro-waste extract based solvents: emergence of novel green solvent for the design of sustainable processes in catalysis and organic chemistry. ChemistrySelect 2:5180–5188. https://doi.org/10.1002/slct.201700580
Sharma K, Mahato N, Nile SH, Leeb ET, Lee YR (2016) Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct 7:3354–3369. https://doi.org/10.1039/C6FO00251J
Siddiqui ZN, Farooq F (2011) Zn(Proline)2: a novel catalyst for the synthesis of dicoumarols. Catal Sci Technol 1:810–816. https://doi.org/10.1039/c1cy00110h
Simon MO, Li CJ (2012) Green chemistry oriented organic synthesis in water. Chem Soc Rev 41:1415–1427. https://doi.org/10.1039/C1CS15222J
Singh P, Kumar P, Katyal A, Kalra R, Dass SK, Prakash S, Chandra R (2010) Phosphotungstic acid: an efficient catalyst for the aqueous phase synthesis of bis-(4-hydroxycoumarin-3-yl)methanes. Catal Lett 134:303–308. https://doi.org/10.1007/s10562-009-0239-x
Singh LK, Priyanka Singh V, Katiyar D (2015) Design, synthesis and biological evaluation of some new coumarin derivatives as potential antimicrobial agents. Med Chem 11:128–134. https://doi.org/10.2174/1573406410666140902110452
Su CX, Mouscadet JF, Chiang CC, Tsai HJ, Hsu LY (2006) HIV-1 integrase inhibition of biscoumarin analogues. Chem Pharm Bull 54:682–686. https://doi.org/10.1248/cpb.54.682
Surnenia N, Baruaa NC, Saikia B (2016) Application of natural feedstock extract: the Henry reaction. Tetrahedron Lett 57:2814–2817. https://doi.org/10.1016/j.tetlet.2016.05.048
Vamisetti GB, Chowdhury R, Kumar M, Ghosh SK (2017) ‘On water’ organocatalyzed enantioselective synthesis of highly functionalized cyclohexanones with an all-carbon quaternary centre from allylidene malononitriles and enones. Tetrahedron Assym 28:317–323. https://doi.org/10.1016/j.tetasy.2016.12.012
Wagare DS, Netankar PD, Shaikh M, Farooqui M, Durrani A (2017) Highly efficient microwave-assisted one-pot synthesis of 4-aryl-2-aminothiazoles in aqueous medium. Environ Chem Lett 15:475–479. https://doi.org/10.1007/s10311-017-0619-1
Wang Q, Chan Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG (2003) Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc 125:3192–3193. https://doi.org/10.1021/ja021381e
Yang C, Su W-Q, Xu D-Z (2016a) Ionic liquid [Dabco-H][AcO] as a highly efficient and recyclable catalyst for the synthesis of various bisenol derivatives via domino Knoevenagel–Michael reaction in aqueous media. RSC Adv 6:99656–99663. https://doi.org/10.1039/C6RA23018K
Yang Y, Zhang S, Tang L, Hu Y, Zha Z, Wang Z (2016b) Catalyst free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water. Green Chem 18:2609–2613. https://doi.org/10.1039/C6GC00313C
Yorulmaz T, Aydogan F, Yolacan C (2017) New and effective proline-based catalysts for asymmetric aldol reaction in water. Synth Commun 47:78–85. https://doi.org/10.1080/00397911.2016.1252988
Acknowledgements
The author would like to acknowledge the talent and publication enhancement-research Grant (TAPE-RG) Universiti Malaysia Terengganu for its research Grant (Vot. No. 55111) and the Universiti Malaysia Terengganu for its research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chia, P.W., Lim, B.S., Yong, F.S.J. et al. An efficient synthesis of bisenols in water extract of waste onion peel ash. Environ Chem Lett 16, 1493–1499 (2018). https://doi.org/10.1007/s10311-018-0764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0764-1