Abstract
A series of 4-aryl/heteroaryl-4H-fused pyrans was synthesized via multicomponent reaction in a microwave synthesizer. All the pyrans were evaluated for in vitro xanthine oxidase inhibition. Structure–activity relationship was also established. Among the series of 108 compounds, Compound 5n was the most potent displaying remarkable inhibition against the enzyme with an IC50 value of 0.59 μM. Enzyme kinetic study was carried out for the compound 5n to determine the type of inhibition. The study revealed that the compound 5n was a mixed-type inhibitor. Molecular modelling studies were also performed to figure out the interactions of both the enantiomers of 5n with the amino acid residues of the enzyme.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative hydroxylation of hypoxanthine and xanthine catalysed by xanthine oxidase to produce uric acid and reactive oxygen species leads to many diseases such as gout and at least symptoms of diseases such as oxidative damage to the tissue (Sharma et al., 2014; Stockert et al., 2002; Borges et al., 2002; Hille, 2006). Therefore, the selective inhibition of XO may result in broad-spectrum chemotherapeutic for gout, cancer, inflammation and oxidative damage (Borges et al., 2002; Hille, 2006; Pacher et al., 2006). Allopurinol (Hille, 2006; Pacher et al., 2006), 2-alkyl hypoxanthines (Biagi et al., 2001; Robins et al., 1985), pterin and 6-formylpterin (Oettl and Reibneggar, 1999) represent the class of purine-based xanthine oxidase inhibitors. All these inhibitors have been successfully utilized and have proved their inhibitory potential towards the enzyme. However, these purine-based inhibitors have been reported to be associated with Steven–Johnson syndrome and worsening of renal function induced in some of the patients (Borges et al., 2002; Hille, 2006; Pacher et al., 2006). Keeping in view these side effects, our research group has been actively involved in the design of some non-purine xanthine oxidase inhibitors in the recent past such as azaflavones (Nepali et al. 2011a, b), n-acetyl pyrazolines (Nepali et al. 2011a, b), β-acetamido compounds (Dhiman et al., 2012), naphthopyrans (Sharma et al., 2014) and 4,6-diaryl/heteroarylpyrimidin-2(1H)-ones (Shukla et al., 2014).
Polyfunctionalized 4H-pyrans have a unique role in medicinal chemistry due to their wide range of biological and pharmacological activities (Elnagdi et al., 1983; Goldmann and Stoltefus, 1991). These compounds have been utilized as anticancer agents, anticoagulants, spasmolytics and antianaphylactics (Andreani and Lapi, 1960; Bonsignore et al., 1993). 4H-Pyran derivatives containing heterocyclic rings are extensively used for their pharmacological activities (Green et al., 1995; Sanchez et al., 2012). Fused pyran derivatives also exhibit a wide spectrum of pharmacological activities and biological activities, such as insecticidal (Uher et al., 1994), antiviral and antileishmanial (Perez-Perez et al., 1995; Fan et al., 2010), anticonvulsant and antimicrobial activities (Aytemir et al., 2004). Also, many of them are non-peptide human immunodeficiency virus (HIV) protease inhibitors (Wang et al., 1996; Pochet et al., 1996; Mazumder et al., 1996). Pyrans are also an important structural motif in number of non-purine xanthine oxidase inhibitors (Nepali et al. 2011a, b; Star and Marby, 1971; Cos et al., 1998). Coumarins and flavonoids represent the class of fused pyrans as non-purine xanthine oxidase inhibitors (Nepali et al. 2011a, b; Cos et al., 1998; Da-Silva et al., 2004; Lin et al., 2002). Both the classes have been extensively explored for their xanthine oxidase inhibitory potential and insights about the structure–activity relationship, and their interactions with the amino acid residues of the enzyme have also been figured out. Recently working on similar lines, our research group synthesized and evaluated a series of naphthopyrans for in vitro xanthine oxidase inhibition in view of some of the potent non-purine xanthine oxidase inhibitors possessing benzopyran skeleton. The potent inhibitory potential of some naphthopyrans was attributed to the interactions of pyran ring as indicated by molecular modelling studies (Sharma et al., 2014).
In continuation of our search for non-purine-based xanthine oxidase inhibitors (Dhiman et al., 2012; Nepali et al. 2011a, b; Sharma et al., 2014; Singh et al., 2014; Shukla et al., 2014; Virdi et al., 2014) and motivated by the promising xanthine oxidase inhibitory potential of naphthopyrans, the present study screens a library of fused pyrans in diverse scaffolds for xanthine oxidase inhibition. A library of 4-aryl/heteroaryl-4H-fused pyrans was synthesized and evaluated against the enzyme. The type of inhibition and the interactions of the most potent inhibitor with the amino acid residues of the enzyme have also been figured out.
Results and discussion
Synthesis
A library of 4H-pyrans was synthesized as shown in Scheme 1. The compounds were synthesized by exposing a mixture of aromatic aldehyde, malononitrile, C–H-activated acidic compound and catalytic amount of DMAP to microwave radiation in a microwave synthesizer operating at 150 °C with the maximum microwave power of 400 W (Scheme 1). The structures of the synthesized compounds were elucidated by 1H NMR and 13C NMR. All spectral data were in accordance with assumed structures.
In vitro xanthine oxidase assay
In vitro screening of the pyrans using bovine milk xanthine oxidase (grade 1, ammonium sulphate suspension) enzymatic assay was performed as described in the literature (Escribano et al., 1988; Takano et al., 2005). Allopurinol (Pacher et al., 2006) was employed as reference inhibitor. The molecules exhibiting % age inhibition of more than 80 % at 50 µM were further tested in triplicate for the xanthine oxidase inhibitory activity to calculate the IC50 values. Among a series of 108 compounds, 41 compounds were found to display a % age inhibition of >80 % and were tested at different concentration against xanthine oxidase (Table 1; Fig. 1). Compounds 5m and 5n displayed significant inhibitory potential with IC50 values, 0.9 and 0.59 μM, respectively (IC50 value of allopurinol = 8.29 μM). Figure 2 shows interesting structure–activity relationship for the inhibitory effects against the enzyme. Careful observation of the IC50 values of the compounds indicates that nature of Ring A and Ring C remarkably influences the activity. Few generalizations about the structure–activity relationship are as follows: (1) compounds with nitro- and halo-substituted Ring C (1g, 1j, 1s, 2s, 3g, 3j, 3s, 4g, 4j, 4s, 5g, 5j, 5s, 6g, 6j, 6s, 7g, 7j, 7s, 8g, 8j, 8s) exhibited significant inhibition, whereas compounds with methoxy- and hydroxy-substituted phenyl rings (1e, 1h, 1i, 1k, 1p, 1q, 2e, 2f, 2h, 2i, 2k, 2q, 3c, 3f, 3h, 3i, 3k, 3q, 3r, 4h, 4i, 5h, 5i, 5k, 5p, 5q, 6e, 6h, 6i, 6k, 6q, 7h, 7i, 8h, 8i) did not qualify for the evaluation at different concentration (i.e. % age inhibition <80). (2) The influence of placement of substitution was also evident from Fig. 1 as compounds with para substituted phenyl rings (halo and nitro groups) were the only one to pass the initial screening at 50 μM by displaying % age inhibition of >80 (1g, 1j, 1s, 2s, 3g, 3j, 3s, 4g, 4j, 4s, 5q, 5j, 5s, 6g, 6j, 6s, 7g, 7j, 7s, 8g, 8j, 8s). (3) The compounds with substituted phenyl rings (substitutions other than nitro and halo, Ring C) were even less active than compounds bearing unsubstituted phenyl ring (Ring C) (1a, 3a, 4a, 5a, 6a, 7a, 8a). (4) Replacement of phenyl rings with naphthyl ring (compare 1a, 3a, 5a with 1l, 3l, 5l) resulted in decline in the inhibitory potential of the compounds as naphthyl-substituted (Ring C) inhibitors displayed % age inhibition of <80 % and did not qualify for evaluation at different concentrations. (5) Replacement of phenyl rings with heteroaryl rings (Ring C) resulted in drastic improvement in the activity (compare 1a, 2a, 3a, 4a, 5a, 6a, 7a, 8a with 1m, 1n, 2n, 3m, 3n, 4n, 5m, 5n, 6n, 7n, 8m, 8n). (6) Among the heteroaryl-substituted compounds (Ring C), compounds with thiophenyl Ring C were more active than the compound with furanyl ring (Ring C) (compare 1n, 3n, 5n, 8n with 1m, 3m, 5m, 8m). This could be attributed to the higher aromatic character of thiophene ring as compared to Furan ring. Overall the preference order for Ring C is as follows: thiophene > furanyl > phenyl with halo (preferable chloro at para position) > phenyl with nitro (para substituted). (7) Ring A also displayed significant influence on the inhibitory potential. Coumarin-substituted (Ring A) compounds were found to be the most active (compare 5a with 1a, 2a, 3a, 4a, 6a, 7a, 8a). (8) Compounds with bicyclic ring (Ring A) also displayed a significant inhibitory potential higher than compounds with monocyclic rings (compare 5a, 6a, 8a with 1a, 2a, 3a, 4a, 7a). (9) Both 1-naphthyl- and 2-naphthyl-substituted pyrans (Ring A) displayed promising results; however, no competition was observed between the activity profiles of two. Overall the preference order for Ring A is as follows: coumarin > 1-naphthyl = 2-naphthyl > pyrazole > cyclohexanedione > thiobarbituric acid > barbituric acid > 5,5-dimethyl cyclohexanedione. Figure 2 represents the structure–activity relationship.
Compound 5n, the most active of the series with an IC50 value 0.59 μM, was further investigated for enzyme kinetics study and molecular modelling studies.
Enzyme kinetic study
Compound 5n was further investigated for the type of inhibition, and enzyme kinetics study was carried out. The Lineweaver–Burk plot (Fig. 3) revealed that the compound 5n was mixed-type XO inhibitor. The pattern of graph shows that it is a form of mixed inhibition scenario. The K m, V max and slope are all affected by concentration of the inhibitor. The inhibitor has increased the K m and slope (K m/V max) while decreasing the V max. Moreover, Figure 3 shows that intersecting lines on the graph converge to the left of the y-axis and above the x-axis which indicates that the value of α (a constant that defines the degree to which inhibitor binding affects the affinity of the enzyme for substrate) is >1. Mixed-type inhibitors are those which are capable of binding to both the free enzyme and the enzyme–substrate complex. However, keeping in view the pattern of intersecting lines on the graph, it can be assumed that the inhibitor preferentially binds to the free enzyme and not the enzyme–substrate complex (Copeland, 2005).
Molecular modelling study
Molecular docking study was performed to get structural insights into the binding behaviour of the potent compound 5n. A flexible docking study was performed using Gold Software (GOLD 2012). Compound 5n has a chiral centre; therefore, both R and S conformations of 5n were docked. The binding poses with highest fitness score were selected, and their binding interactions were studied.
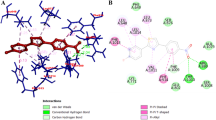
The docking study reveals that S-enantiomer of 5n fits well in the binding site, while R-enantiomer was not able to get in the cavity (Fig. 4). The binding interactions of S-enantiomer with highest score were studied. In binding pose, S-enantiomer of 5n fits well in the binding cavity and gets stabilized by various molecular interactions. The chromene ring gets sandwiched in Phe914 and Phe1009 showing “face-to-face” and “edge-to-face” pie-stacking, respectively. The carbonyl group of chromene ring was found to involve in hydrogen bonding with Thr1010. Another hydrogen bonding was observed between Glu802 and oxygen of 5n. The sulphur of thiophene ring was found to be involved in van der Waals interactions with Ser876. The above interactions provided an insight behind the inhibition of XO by 5n (Fig. 4).
Conclusion
Allopurinol, a well-known xanthine oxidase inhibitor, is a competitive inhibitor and has been employed as standard for the in vitro and in vivo studies over the years. However, its use has been associated with some complications. The competitive inhibitors are basically purine-based structures, and the interactions of purine analogue XO inhibitors with the activities of purine and pyrimidine metabolism enzymes such as guanine deaminase, HGPRT (hypoxanthine–guanine phosphoribosyltransferase), PNP (purine nucleoside phosphorylase), OPRT (orotate phosphoribosyltransferase) and OMPDC (orotidine-5-monophosphate decarboxylase) leading to the hypersensitivity (Steven–Johnson) a syndrome characterized by fever, skin rash, hepatitis, leukocytosis with eosinophilia and worsening renal function induced in some of the patients has basically encouraged us to focus on XO inhibitors with structurally diverse and novel non-purine isosteres. Moreover, the success of febuxostat has further motivated us to focus on non-purine isosters. Febuxostat is a non-purine selective inhibitor of xanthine oxidase. It works by non-competitively blocking the molybdenum pterin centre which is the active site on xanthine oxidase. Many long- and short-term clinical trials have proved the efficacy of febuxostat in the treatment of gout and lowering uric acid levels. In these studies, febuxostat was found to be superior to allopurinol in reducing the serum uric acid levels. Thus, all these reasons have collectively led us to investigate non-competitive chemical architectures for xanthine oxidase inhibition. Keeping in view the success of some non-purine xanthine oxidase inhibitors, a library of 4H-pyrans was designed in the present study and the compounds were evaluated for inhibitory effects against the enzyme xanthine oxidase. All the compounds were first screened at 50 µM, and the compounds displaying a % age inhibition of >80 were further evaluated at different concentrations. Structure–activity relationship revealed that Ring A as well as Ring C remarkably influences the inhibitory potential. The most potent compound 5n was investigated to explore the type of inhibition it was exerting, and thus, enzyme kinetics study was carried out on 5n. The Lineweaver–Burk plot revealed that compound 5n was a mixed-type inhibitor. The compound was studied for its interactions with the amino acid residues. The 3D structural coordinates of XO were obtained from protein databank (PDB ID: 1VDV) for the docking study. The docking study reveals that S-enantiomer of 5n fits well in the binding site, while R-enantiomer was not able to get in the cavity (Fig. 4). S-enantiomer of 5n fits well in the binding cavity and gets stabilized by various molecular interactions, i.e. “face-to-face” and “edge-to-face” pie-stacking and hydrogen bonding.
Experimental
The reagents were purchased from Sigma-Aldrich, Merck, CDH, Loba chem., Spectro chem., India, and used without further purification. All yields refer to isolated products after purification. Biotage Microwave Synthesizer (Model: Initiator) operating at 150 °C with the microwave power maximum level of 400 W. Products were characterized by spectral data. 1H NMR and 13C NMR spectra were recorded on Bruker Avance II 500 NMR Spectrometer and JEOL AL 300 NMR Spectrometer. The spectra were measured in DMSO-d6 relative to TMS (0.00 ppm). Melting points were determined in open capillaries and were uncorrected.
Experimental procedure for the synthesis of 4H-pyrans (1, 2, 3, 4, 5, 6, 7, 8)
A mixture of aromatic aldehyde (1 mmol), malononitrile (1 mmol), C–H-activated acidic compound (1 mmol) and catalytic amounts of DMAP (5 mol%) in a 50-ml conical flask was exposed to microwave radiation for 20 min in a microwave reactor operating at 150 °C with the maximum microwave power of 400 W. Cold methanol was added to the reaction mixture, and the solid precipitates were filtered off to obtain the desired product.
The structures of the synthesized compounds were elucidated by 1H NMR and 13C NMR. All spectral data were in accordance with assumed structures. In each occasion, the spectral data (1H and 13C NMR) of known compounds were compared with that reported in the literature.
2-Amino-5,6,7,8-tetrahydro-5-oxo-4-phenyl-4H-chromene-3-carbonitrile (1a) (Yu and Da-Ming, 2012; Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-fluorophenyl)-5-oxo-4H-chromene-3-carbonitrile (1b) (Yu and Da-Ming, 2012), 2-amino-5,6,7,8-tetrahydro-4-(4-bromophenyl)-5-oxo-4H-chromene-3-carbonitrile (1c) (Yu and Da-Ming, 2012), 2-amino-5,6,7,8-tetrahydro-4-(2-methoxyphenyl)-5-oxo-4H-chromene-3-carbonitrile (1h) (Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-methoxyphenyl)-5-oxo-4H-chromene-3-carbonitrile (1i) (Xu et al., 2011; Rostamnia and Morsali, 2014), 2-amino-5,6,7,8-tetrahydro-4-(4-nitrophenyl)-5-oxo-4H-chromene-3-carbonitrile (1j) (Yu and Da-Ming, 2012; Xu et al., 2011; Rostamnia and Morsali, 2014; Hosseini-Monfared et al., 2013), 2-amino-5,6,7,8-tetrahydro-4-(4-hydroxyphenyl)-5-oxo-4H-chromene-3-carbonitrile (1k) (Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(furan-2-yl)-5-oxo-4H-chromene-3-carbonitrile (1m) (Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(3,4-dimethoxyphenyl)-5-oxo-4H-chromene-3-carbonitrile (1q) (Yu and Da-Ming, 2012; Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-chlorophenyl)-5-oxo-4H-chromene-3-carbonitrile (1s) (Yu and Da-Ming, 2012; Rostamnia and Morsali, 2014; Hosseini-Monfared et al., 2013), 2-amino-5,6,7,8-tetrahydro-4-(3-nitrophenyl)-5-oxo-4H-chromene-3-carbonitrile (1v) (Xu et al., 2011), 2-amino-5,6,7,8-tetrahydro-7,7-dimethyl-5-oxo-4-phenyl-4H-chromene-3-carbonitrile (2a) (Kumar et al., 2009; Sadegh and Ali, 2014; Yu and Da-Ming, 2012; Gao et al., 2008; Hasaninejad et al., 2013; Jiang-Cheng et al., 2011; Bihani et al., 2013; Khaksar et al., 2012; Banerjee et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2b) (Sadegh and Ali, 2014; Gao et al., 2008; Khaksar et al., 2012), 2-amino-5,6,7,8-tetrahydro-4-(4-bromophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2g) (Sadegh and Ali, 2014; Khaksar et al., 2012), 2-amino-5,6,7,8-tetrahydro-4-(2-methoxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2h) (Yu and Da-Ming, 2012; Jiang-Cheng et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2i) (Kumar et al., 2009; Jiang-Cheng et al., 2011; Bihani et al., 2013; Khaksar et al., 2012; Banerjee et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2j) (Kumar et al., 2009; Sadegh and Ali, 2014; Yu and Da-Ming, 2012; Hasaninejad et al., 2013; Jiang-Cheng et al., 2011; Bihani et al., 2013; Khaksar et al., 2012; Banerjee et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-hydroxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2k) (Gao et al., 2008; Jiang-Cheng et al., 2011; Banerjee et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(thiophen-2-yl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2n) (Hasaninejad et al., 2013), 2-amino-5,6,7,8-tetrahydro-4-(3,4-methoxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2q) (Sadegh and Ali, 2014; Jiang-Cheng et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2s) (Sadegh and Ali, 2014; Yu and Da-Ming, 2012; Gao et al., 2008; Jiang-Cheng et al., 2011; Bihani et al., 2013; Khaksar et al., 2012; Banerjee et al., 2011), 2-amino-5,6,7,8-tetrahydro-4-(3-nitrophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (2v) (Kumar et al., 2009; Yu and Da-Ming, 2012; Jiang-Cheng et al., 2011; Bihani et al., 2013; Khaksar et al., 2012; Banerjee et al., 2011), 6-amino-2,4-dihydro-3-methyl-4-phenylpyrano[2,3-c]pyrazole-5-carbonitrile (3a) (Ali and El-Remaily, 2013; Paul et al., 2013; Bora et al., 2013; Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(4-fluorophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3b) (Ali and El-Remaily, 2013; Bora et al., 2013), 6-amino-2,4-dihydro-4-(3-hydroxyphenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3c) (Bora et al., 2013), 6-amino-2,4-dihydro-4-(3-chlorophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3d) (Ali and El-Remaily, 2013), 6-amino-2,4-dihydro-4-(2-hydroxyphenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3f) (Ali and El-Remaily, 2013), 6-amino-2,4-dihydro-4-(4-bromophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3g) (Ali and El-Remaily, 2013; Paul et al., 2013; Bora et al., 2013), 6-amino-2,4-dihydro-4-(4-methoxyphenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3i) (Ali and El-Remaily, 2013; Paul et al., 2013; Bora et al., 2013; Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(4-nitrophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3j) (Ali and El-Remaily, 2013; Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(4-hydroxyphenyl)-3-methylpyrano [2,3-c]pyrazole-5-carbonitrile (3k) (Ali and El-Remaily, 2013; Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(naphthalen-2-yl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3l) (Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(thiophen-2-yl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3n) (Paul et al., 2013), 6-amino-2,4-dihydro-4-(3,4-dimethoxyphenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3q) (Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(4-chlorophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3s) (Ali and El-Remaily, 2013; Bolligarla and Das, 2011; Bihani et al., 2013), 6-amino-2,4-dihydro-4-(pyridin-4-yl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (3t) (Colombo et al., 2003), 2-amino-4,5-dihydro-5-oxo-4-phenylpyrano[3,2-c]chromene-3-carbonitrile (5a) (Safaei et al., 2012; Jain et al., 2013; Kidwai and Sexena, 2006), 2-amino-4,5-dihydro-4-(4-bromophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5g) (Safaei et al., 2012; Jain et al., 2013), 2-amino-4,5-dihydro-4-(2-methoxyphenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5h) (Wang et al., 2010), 2-amino-4,5-dihydro-4-(4-methoxyphenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5i) (Jain et al., 2013), 2-amino-4,5-dihydro-4-(4-nitrophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5j) (Jain et al., 2013), 2-amino-4,5-dihydro-4-(4-hydroxyphenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5k) (Xiang-Shan et al., 2005; Kidwai and Sexena, 2006; Gong et al., 2009), 2-amino-4,5-dihydro-4-(furan-2-yl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5m) (Safaei et al., 2012; Jain et al., 2013), 2-amino-4,5-dihydro-4-(thiophen-2-yl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5n) (Jain et al., 2013), 2-amino-4,5-dihydro-4-(1H-indol-2-yl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5o) (Abd-El-Aziz et al., 2004), 2-amino-4,5-dihydro-4-(4-hydroxy-3-methoxyphenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5p) (Bihani et al., 2013), 2-amino-4,5-dihydro-4-(4-chlorophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5s) (Jain et al., 2013; Kidwai and Sexena, 2006), 2-amino-4,5-dihydro-4-(3-nitrophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5v) (Jain et al., 2013), 2-amino-4,5-dihydro-4-(2-bromophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5w) (Wang et al., 2010), 2-amino-4-phenyl-4H-benzo[h] chromene-3-carbonitrile (6a) (Bihani et al., 2013; Khurana et al., 2010), 2-amino-4-(4-fluorophenyl)-4H-benzo[h]chromene-3-carbonitrile (6b) (Khurana et al., 2010), 2-amino-4-(4-bromophenyl)-4H-benzo[h]chromene-3-carbonitrile (6g) (Khurana et al., 2010), 2-amino-4-(2-methoxyphenyl)-4H-benzo[h]chromene-3-carbonitrile (6h) (Maalej et al., 2012), 2-amino-4-(4-methoxyphenyl)-4H-benzo[h]chromene-3-carbonitrile (6i) (Bihani et al., 2013), 2-amino-4-(4-nitrophenyl)-4H-benzo[h]chromene-3-carbonitrile (6j) (Bihani et al., 2013; Khurana et al., 2010), 2-amino-4-(4-chlorophenyl)-4H-benzo[h] chromene-3-carbonitrile (6s) (Bihani et al., 2013; Khurana et al., 2010), 2-amino-4-(3-nitrophenyl)-4H-benzo[h]chromene-3-carbonitrile (6v) (Bihani et al., 2013; Khurana et al., 2010), 7-amino-2,3,4,5-tetrahydro-2,4-dioxo-5-phenyl-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7a) (Devi et al., 2003), 7-amino-5-(3-chlorophenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7d) (Xiang-Shan et al., 2005), 7-amino-5-(2-methoxyphenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7h) (Safaei et al., 2012), 7-amino-5-(4-nitrophenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7j) (Safaei et al., 2012), 7-amino-5-(4-chlorophenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7s) (Safaei et al., 2012), 7-amino-5-(3-nitrophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7v) (Safaei et al., 2012), 3-amino-1-phenyl-1H-benzo[f] chromene-2-carbonitrile (8a) (Bihani et al., 2013; Wang et al., 2008), 3-amino-1-(4-fluorophenyl)-1H-benzo[f]chromene-2-carbonitrile (8b) (Wang et al., 2008), 3-amino-1-(4-bromophenyl)-1H-benzo[f]chromene-2-carbonitrile (8g) (Wang et al., 2008), 3-amino-1-(4-methoxyphenyl)-1H-benzo[f]chromene-2-carbonitrile (8i) (Wang et al., 2008), 3-amino-1-(4-nitrophenyl)-1H-benzo[f]chromene-2-carbonitrile (8j) (Wang et al., 2008; Bihani et al., 2013), 3-amino-1-(furan-2-yl)-1H-benzo[f]chromene-2-carbonitrile (8m) (Wang et al., 2008), 3-amino-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carbonitrile (8s) (Wang et al., 2008; Bihani et al., 2013), 3-amino-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carbonitrile (8v) (Wang et al., 2008; Bihani et al., 2013).
The characterization data for the synthesized new compounds are given below:
2-Amino-4-(3-chlorophenyl)-5,6,7,8-tetrahydro-5-oxo-4H-chromene-3-carbonitrile (1d)
Yield 80 %; mp: 210–211 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.27–7.35 (2H, m), 7.12–7.18 (2H, m), 7.08 (2H, bs, D2O exchangeable protons), 4.22 (1H, s), 2.63 (2H, m), 2.30 (2H, t, J = 6 Hz), 1.93 (2H, m). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 20.22 (CH2), 26.94 (CH2), 35.74 (CH2), 36.73 (CH), 57.97 (C), 113.57 (C), 120.01 (CN), 126.45 (Ar–C), 127.07 (Ar–C), 127.47 (Ar–C), 130.75 (Ar–C), 133.36 (C–Cl), 147.74 (Ar–C), 158.97 (C–C), 165.33 (C–NH2), 196.37 (C=O). Anal. Calcd. for C16H13ClN2O2: C, 72.18; H, 3.94; Cl, 10.65; N, 8.42; Found: C, 72.33; H, 3.58; Cl, 10.95; N, 8.56.
2-Amino-4-(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1e)
Yield 60 %; mp: 200–201 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 6.86 (2H, s, D2O exchangeable protons), 6.61 (1H, d, J = 8.1 Hz,), 6.54 (1H, s), 6.41 (1H, d, J = 8.1 Hz), 4.01 (1H, s), 2.58 (2H, bs), 2.26 (2H, bs), 1.92 (2H, m). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 19.39 (CH2), 26.17 (CH2), 36.85 (CH2), 38.63 (CH), 58.19 (C), 113.96 (C), 120.27 (CN), 118.18 (Ar–C), 116.2 (Ar–C), 123.14 (Ar–C), 136.22 (Ar–C), 144.21 (C–OH), 147.27 (C–OH), 158.12 (C), 165.33 (C–NH2), 196.97 (C=O). Anal. Calcd. for C16H14N2O4: C, 64.42; H, 4.73; N, 9.39; Found: C, 64.24; H, 5.11; N, 9.55.
2-Amino-4-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1l)
Yield 83 %; mp: 210–211 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.39 (1H, d, J = 8.1 Hz), 7.90 (1H, d, J = 1.5 Hz), 7.77 (1H, d, J = 8.1 Hz), 7.57 (1H, d, J = 5.1 Hz), 7.41–7.54 (3H, m), 7.25 (1H, d, J = 6.9 Hz), 6.95 (2H, s, D2O exchangeable protons), 5.15 (1H, s), 2.71 (2H, m), 2.30 (2H, m), 1.98 (2H, m). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 20.31 (CH2), 26.74 (CH2), 35.69 (CH2), 36.78 (CH), 58.88 (C), 113.69 (C), 117.33(CN), 124.52 (Ar–C), 124.96 (Ar–C), 125.43 (Ar–C), 125.67 (Ar–C), 126.73 (Ar–C), 126.86 (Ar–C), 128.94 (Ar–C), 132.66 (Ar–C), 133.57 (Ar–C), 134.01 (Ar–C), 158.67 (C), 165.98 (C–NH2), 196.77 (C=O). Anal. Calcd. for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86; Found: C, 75.20; H, 5.30; N, 9.10.
2-Amino-4-(thiophen-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1n)
Yield 85 %; mp: 158–159 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.38 (1H, d, J = 4.8 Hz), 7.119 (2H, s, D2O exchangeable protons), 6.85–6.92 (2H, m), 4.53 (1H, s), 2.56 (2H, bs), 2.31 (2H, bs), 1.90 (2H, m). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 20.33 (CH2), 25.64 (CH), 26.96 (CH2), 36.87 (CH2), 58.44 (C), 113.74 (C), 120.22 (CN), 123.67 (Ar–C), 126.61 (Ar–C), 126.98 (Ar–C), 139.77 (Ar–C), 158.71 (C), 165.83 (C–NH2), 196.27 (C=O). Anal. Calcd. for C14H12N2O2S: C, 61.75; H, 4.44; N, 10.29; S, 11.77; Found: C, 61.90; H, 4.35; N, 10.45; S, 11.81.
2-Amino-4-(4-hydroxy-3-methoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1p)
Yield 76 %; mp: 200–201 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.87 (1H, s, D2O exchangeable proton), 6.91 (2H, bs, D2O exchangeable proton), 6.67 (2H, m), 6.51 (1H, d, J = 8.1 Hz), 4.09 (1H, s), 3.72 (3H, s), 2.59 (2H, bs), 2.27 (2H, bs), 1.91 (2H, m). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 20.42 (CH2), 26.80(CH2), 36.24(CH2), 37.37 (CH), 56.22 (OCH3), 58.78 (C), 113.11 (C), 114.22 (Ar–C), 116.17 (Ar–C), 120.99 (CN), 122.88 (Ar–C), 135.42 (Ar–C), 142.23 (C–OH), 151.39 (C–OCH3), 158.91 (C), 166.01 (C–NH2), 196.72 (C=O). Anal. Calcd. for C17H16N2O4: C, 65.38; H, 5.16; N, 8.97; Found: C, 64.80; H, 5.02; N, 8.93.
2-Amino-4-(3-methylthiophen-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1u)
Yield 78 %; mp: 150–151 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.19 (1H, d, J = 4.8 Hz), 7.01 (2H, s, D2O exchangeable protons), 6.74 (1H, d, J = 5.1 Hz), 4.57 (1H, s), 2.60 (2H, bs), 2.28 (2H, bs), 2.23 (3H, s), 1.96 (2H, bs). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 12.33 (CH3), 19.10 (CH2), 20.30 (CH), 26.44 (CH2), 36.22 (CH2), 58.65 (C), 113.99 (C), 120.27 (CN), 124.10 (Ar–C), 124.70 (Ar–C), 135.11 (Ar–C), 135.99 (Ar–C), 158.31 (C), 166.21 (C–NH2), 196.11 (C=O). Anal. Calcd. for C15H14N2O2S: C, 62.92; H, 4.93; N, 9.78; S, 11.20; Found: C, 63.21; H, 4.58; N, 9.94; S, 11.32.
2-Amino-4-(3,4-dihydroxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (2e)
Yield 57 %; mp: 185–186 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.78 (2H, bs, D2O exchangeable protons), 6.89 (2H, s, D2O exchangeable protons), 6.61 (1H, d, J = 8.1 Hz), 6.52 (1H, d, J = 1.8 Hz), 6.38 (1H, dd, J = 1.8 and 8.1 Hz), 3.97 (1H, s), 2.55 (2H, bs), 2.22 (1H, d, J = 16.2 Hz), 2.08 (1H, d, J = 16.2 Hz), 1.06 (3H, s), 0.92 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 27.23 (CH3), 28.95 (CH3), 32.25 (C), 35.27 (CH2), 50.52 (CH2), 59.30 (C), 113.79 (C), 115.12 (Ar–C), 115.74 (Ar–C), 118.36 (CN), 123.1 (Ar–C), 136.27 (Ar–C), 144.40 (C–OH), 145.39 (C–OH), 158.87 (C), 162.31 (C–NH2), 196.11 (C=O). Anal. Calcd. for C18H18N2O4: C, 66.25; H, 5.56; N, 8.58; Found: C, 65.98; H, 5.75; N, 8.32.
2-Amino-4-(2-hydroxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (2f)
Yield 68 %; mp: 80–81 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.72 (1H, s, D2O exchangeable proton), 6.32–6.89 (7H, m), 4.84 (1H, s), 2.55 (2H, bs), 2.22 (1H, d, J = 16.2 Hz), 2.08 (1H, d, J = 16.2 Hz), 1.06 (3H, s), 0.92 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 27.23 (CH3), 29.05 (CH3), 32.46 (C), 35.78 (C), 50.15 (CH2), 59.36 (C), 113.23 (C), 115.25 (Ar–C), 118.63 (CN), 121.22 (Ar–C), 122.36 (Ar–C), 127.78 (Ar–C), 130.20 (Ar–C), 147.41 (C), 158.68 (C–OH), 162.26 (C–NH2), 196.01 (C=O). Anal. Calcd. for C18H18N2O3: C, 72.95; H, 6.80; N, 9.45; Found: C, 72.17; H, 7.10; N, 9.64.
6-Amino-4-(2-methoxyphenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (3h)
Yield 84 %; mp: 170–171 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 12.01 (1H, s, D2O exchangeable proton), 7.19 (1H, m), 6.96–7.01 (2H, m), 6.90 (1H, m), 6.79 (2H, s, D2O exchangeable protons), 4.97 (1H, s), 3.78 (3H, s), 1.79 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 10.55 (CH3), 11.10 (CH), 54.45 (OCH3), 56.11 (C), 112.75 (Ar–C), 110.96 (Ar–C), 114.90 (C), 120.99 (Ar–C), 121.00 (CN), 126.80 (Ar–C), 130.01 (Ar–C), 136.82 (C), 142.27 (C–OCH3), 156.09 (C), 162.00 (C–NH2). Anal. Calcd. for C15H14N4O2: C, 63.82; H, 5.00; N, 19.85; Found: C, 64.09; H, 4.75; N, 19.76.
6-Amino-4-(furan-2-yl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (3m)
Yield 67 %; mp: 185–186 (DEC) °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 12.16 (1H, s, D2O exchangeable proton), 7.53 (1H, bs), 6.95 (2H, s, D2O exchangeable proton), 6.37 (1H, d, J = 1.8 Hz), 6.17 (1H, d, J = 2.7 Hz), 4.77 (1H, s), 1.97 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 10.03 (CH3), 30.28 (CH), 54.45 (C), 95.57 (C), 106.09 (Ar–C), 110.69 (Ar–C), 121.04 (CN), 136.28 (C), 142.72 (Ar–C), 155.27 (Ar–C), 156.18 (C), 161.94 (C–NH2). Anal. Calcd. for C12H10N4O2: C, 59.50; H, 4.16; N, 23.13; Found: C, 59.81; H, 3.97; N, 23.29.
6-Amino-4-(1H-indol-2-yl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (3o)
Yield 66 %; mp: 190–191 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.69 (1H, s, D2O exchangeable proton), 8.91 (1H, s, D2O exchangeable proton), 8.34 (1H, d, J = 7.2 Hz), 7.92 (1H, s), 7.48 (1H, d, J = 7.5 Hz), 7.21–7.29 (2H, m), 4.84 (1H, s), 1.76 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 10.88 (CH3), 30.02 (CH), 54.44 (C), 96.03 (C), 105.55 (Ar–C), 110.66 (Ar–C), 119.94 (Ar–C), 120.15 (CN), 120.88 (Ar–C), 122.22 (Ar–C), 128.04 (Ar–C), 135.20 (Ar–C), 136.55 (Ar–C), 142.05 (C), 162.27 (C–NH2). Anal. Calcd. for C16H13N5O: C, 65.97; H, 4.50; N, 24.04; Found: C, 65.66; H, 4.33; N, 24.13.
6-Amino-3-methyl-4-(2,3,4-trimethoxyphenyl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (3r)
Yield 79 %; mp: 196–197 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 12.10 (1H, s, D2O exchangeable protons), 6.87 (2H, D2O exchangeable protons), 6.43 (2H, bs), 4.58 (1H, s), 3.85 (6H, s), 3.84 (3H, s), 1.87 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 10.53 (CH3), 12.01 (CH), 54.28 (OCH3), 55.63 (C), 56.27 (OCH3), 56.36 (OCH3), 96.75 (C), 105.30 (Ar–C), 110.96 (Ar–C), 120.69 (CN), 123.36 (Ar–C), 136.42 (C), 139.91 (C–OCH3), 142.94 (C–OCH3), 147.71 (C–OCH3), 151.72 (C), 162.76 (C–NH2). Anal. Calcd. for C17H18N4O4: C, 59.64; H, 5.30; N, 16.37; Found: C, 59.78; H, 5.25; N, 16.55.
6-Amino-3-methyl-4-(3-methylthiophen-2-yl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (3u)
Yield 85 %; mp: 179–180 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 12.15 (1H, s, D2O exchangeable proton), 7.02(1H, d, J = 5.1 Hz), 6.86 (2H, bs, D2O exchangeable protons), 6.78 (1H, d, J = 5.1 Hz), 5.01 (1H, s), 2.16 (3H, s), 1.83 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 10.35 (CH3), 11.55 (CH3), 12.70 (CH), 54.90 (C), 110.76 (C), 120.07 (CN), 124.11 (Ar–C), 124.71 (Ar–C), 133.31 (Ar–C), 135.56 (Ar–C), 136.89 (C), 142.24 (C), 162.08 (C–NH2). Anal. Calcd. for C13H12N4OS: C, 57.34; H, 4.44; N, 20.57; S, 11.77; Found: C, 56.99; H, 4.71; N, 20.34; S, 11.98.
7-Amino-2-oxo-5-phenyl-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4a)
Yield 82 %; mp: 180–181 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.98 (2H, d, J = 7.5 Hz), 7.19 (2H, d, J = 7.5 Hz), 4.56 (1H, s), 3.89 (3H, s). 13C NMR (DMSO-d6, 500 MHz, v TMS = 0): 36.53 (CH), 59.63 (C), 94.62 (C), 115.17 (CN), 125.88 (Ar–C), 126.71 (Ar–C), 126.77 (Ar–C), 142.25 (Ar–C), 151.99 (C=O), 158.36 (C), 160.36 (C–NH2), 174.62 (C=S). Anal. Calcd. for C14H10N4O2S: C, 56.37; H, 3.38; N, 18.78; S, 10.75; Found: C, 56.60; H, 3.15; N, 18.92; S, 10.82.
7-Amino-5-(3-chlorophenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4d)
Yield 87 %; mp: 190–191 °C, 1H NMR (DMSO-d6, 300 MHz): δ, TMS = 0): 9.11 (1H, s), 10.91 (1H, s), 7.41 (1H, s), 7.24–7.33 (2H, m), 7.13 (1H, d, J = 8.3 Hz), 7.06 (1H, s), 4.59 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 36.86 (CH), 59.97 (C), 94.75 (C), 115.67 (CN), 125.61 (Ar–C), 127.63 (Ar–C), 128.94 (Ar–C), 130.26 (Ar–C), 134.43 (C–Cl), 143.34 (Ar–C), 151.69 (C=O), 158.24 (C), 160.56 (C–NH2), 174.67 (C=S). Anal. Calcd. for C14H9ClN4O2S: C, 50.53; H, 2.73; Cl, 10.65; N, 16.84; S, 9.64; Found: C, 50.80; H, 2.51; Cl, 10.76; N, 16.96; S, 9.38.
7-Amino-5-(4-bromophenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4g)
Yield 88 %; mp: 185–186 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.17 (1H, s, D2O exchangeable proton), 7.47 (2H, d, J = 8.1 Hz), 7.18 (2H, d, J = 8.4 Hz), 7.15 (2H, bs, D2O exchangeable proton), 4.23 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.86 (CH), 59.97 (C), 94.75 (C), 115.67 (CN), 120.55 (C–Br), 131.25 (Ar–C), 131.32 (Ar–C), 131.51 (Ar–C), 131.82 (Ar–C), 141.24 (Ar–C), 151.37 (C=O), 158.59 (C), 160.86 (C–NH2), 174.78 (C=S). Anal. Calcd. for C14H9BrN4O2S: C, 44.58; H, 2.40; Br, 21.18; N, 14.85; S, 8.50; Found: C, 44.81; H, 2.12; Br, 21.36; N, 14.52; S, 8.39.
7-Amino-5-(2-methoxyphenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4h)
Yield 80 %; mp: 180–181 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 10.88 (1H, s), 9.01 (1H, s), 7.13 (1H, d, J = 8.0 Hz), 6.87–6.89 (3H, m), 6.84–6.88 (2H, m), 4.29 (1H, s), 3.64 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.42 (CH), 56.31 (OCH3), 59.78 (C), 94.79 (C), 114.41 (Ar–C), 115.99 (CN), 121.58 (Ar–C), 121.89 (Ar–C), 126.88 (Ar–C), 130.12 (Ar–C), 151.98 (C=O), 157.66 (C), 158.37 (C–OCH3), 160.00 (C–NH2), 174.28 (C=S). Anal. Calcd. for C15H12N4O3S: C, 54.87; H, 3.68; N, 17.06; S, 9.77; Found: C, 54.44; H, 3.92; N, 17.32; S, 9.49.
7-Amino-5-(4-methoxyphenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4i)
Yield 76 %; mp: 200–201 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.98 (2H, d, J = 7.5 Hz), 7.19 (2H, d, J = 7.5 Hz), 4.56 (1H, s), 3.89 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.35 (CH), 56.42 (OCH3), 59.36 (C), 94.26 (C), 113.96 (Ar–C), 114.13 (Ar–C), 115.71 (CN), 129.00 (Ar–C), 129.54 (Ar–C), 136.08 (Ar–C), 151.90 (C=O), 157.76 (C), 158.63 (C–OCH3), 160.63 (C–NH2), 174.26 (C=S). Anal. Calcd. for C15H12N4O3S: C, 54.87; H, 3.68; N, 17.06; S, 9.77; Found: C, 54.51; H, 3.88; N, 17.34; S, 9.82.
7-Amino-5-(4-nitrophenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4j)
Yield 84 %; mp: 268–269 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 10.93 (1H, s), 9.41 (1H, s), 8.13 (2H, d, J = 8.5 Hz), 7.49 (2H, d, J = 8.5 Hz), 7.08 (2H, s), 3.29 (1H, s). 13C NMR (DMSO, 500 MHz, δ, TMS = 0): 36.59 (CH), 59.23 (C), 94.28 (C), 115.58 (CN), 121.57 (Ar–C), 121.89 (Ar–C), 130.28 (Ar–C), 130.85 (Ar–C), 145.14 (Ar–C), 148.95 (C=O), 151.39 (Ar–C), 158.27 (C), 160.33 (C–NH2), 174.68 (C=S). Anal. Calcd. for C14H9N5O4S: C, 48.98; H, 2.64; N, 20.40; S, 9.34; Found: C, 49.12; 2.36; N, 20.54; S, 9.60.
7-Amino-2-oxo-5-(thiophen-2-yl)-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4n)
Yield 81 %; mp: 212–213 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 12.09 (1H, bs), 11.42 (1H, bs), 7.38 (2H, bs), 7.02 (1H, d), 6.57 (1H, m), 6.49 (1H, d), 4.21 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 34.33 (CH), 59.65 (C), 94.56 (C), 115.69 (CN), 123.65 (Ar–C), 126.71 (Ar–C), 126.99 (Ar–C), 139.77 (Ar–C), 151.91 (C=O), 158.79 (C), 160.45 (C–NH2), 174.85 (C=S). Anal. Calcd. for C12H8N4O2S2: C, 47.36; H, 2.65; N, 18.41; S, 21.07; Found: C, 47.56; H, 2.45; N, 18.19; S, 21.29.
7-Amino-5-(4-chlorophenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4s)
Yield 85 %; mp: 194–195 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.1 (1H, s), 9.2 (1H, s), 7.73 (2H, d, J = 8.0 Hz), 7.17 (2H, d, J = 8.0 Hz), 6.97 (2H, s), 4.37 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.97 (CH), 59.60 (C), 94.50 (C), 115.11 (CN), 128.48 (Ar–C), 128.84 (Ar–C), 130.01 (Ar–C), 130.10 (Ar–C), 131.53 (C–Cl), 140.30 (Ar–C), 151.97 (C=O), 158.15 (C), 160.17 (C–NH2), 174.19 (C=S). Anal. Calcd. for C14H9ClN4O2S: C, 50.53; H, 2.73; Cl, 10.65; N, 16.84; S, 9.64; Found: C, 50.81; H, 2.39; Cl, 10.54; N, 16.78; S, 9.78.
7-Amino-5-(3-methylthiophen-2-yl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4u)
Yield 80 %; mp: 228–229 °C, 1H NMR (DMSO-d6, 300, δ, TMS = 0): 12.04 (1H, bs), 11.02 (1H, bs), 7.26 (2H, bs), 6.48 (1H, d), 6.62 (1H, m), 3.99 (1H, s), 2.20 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 13.13 (CH3), 33.03 (CH), 59.32 (C), 94.43 (C), 115.54 (CN), 124.11 (Ar–C), 124.17 (Ar–C), 133.95 (Ar–C), 135.09 (Ar–C), 151.15 (C=O), 158.59 (C), 160.19 (C–NH2), 174.91 (C=S). Anal. Calcd. for C13H10N4O2S2: C, 51.65; H, 3.33; N, 18.53; S, 10.61; Found: C, 51.39; H, 3.61; N, 18.72; S, 10.31.
7-Amino-5-(3-nitrophenyl)-2-oxo-4-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4v)
Yield 80 %; mp: 200–201 °C, 1H NMR (DMSO-d6, 300, δ, TMS = 0): 11.13 (1H, bs), 9.15 (1H, s), 8.16 (1H, s), 8.01 (1H, d, J = 8.0 Hz), 7.56–7.63 (2H, m), 4.33 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.99 (CH), 59.95 (C), 94.55 (C), 115.16 (CN), 118.28 (Ar–C), 124.34 (Ar–C), 129.29 (Ar–C), 135.45 (Ar–C), 143.53 (Ar–C), 150.60 (C–NO2), 151.06 (C=O), 158.84 (C), 160.48 (C–NH2), 174.88 (C=S). Calculated Anal. Calcd. for C14H9N5O4S: C, 48.98; H, 2.64; N, 20.40; S, 9.34; Found: C, 49.10; H, 2.34; N, 20.68; S, 9.02.
2-Amino-4,5 dihydro-4-(4-fluorophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (5b)
Yield 84 %; mp: 190–191 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.83 (1H, d, J = 8.1 Hz), 7.65 (1H, dd, J = 8.1 and 7.1 Hz), 7.44 (1H, m), 7.32 (1H, d, J = 8.4 Hz), 7.21 (2H, d, J = 7.9 Hz), 7.12 (2H, d, J = 8.0 Hz), 4.31 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 37.81 (CH), 58.14 (C), 105.24 (C), 115.21 (Ar–C), 115.41 (Ar–C), 115.45 (Ar–C), 116.40 (Ar–C), 119.14 (CN), 123.32 (Ar–C), 125.40 (Ar–C), 128.34 (Ar–C), 130.58 (Ar–C), 130.60 (Ar–C), 139.12 (Ar–C), 152.50 (Ar–C), 159.10 (C–NH2), 159.90 (C–F), 160.12 (C), 161.90 (C=O). Anal. Calcd. for C19H11FN2O3: C, 68.26; H, 3.32; F, 5.68; N, 8.38; Found: C, 68.32; H, 3.28; F, 5.70; N, 8.44.
2-Amino-4-(naphthalene-2-yl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5l)
Yield 88 %; mp: 210–211 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.43 (1H, d, J = 7.8 Hz), 7.94–7.99 (2H, m), 7.82 (1H, d, J = 8.1 Hz), 7.72 (1H, m), 7.44–7.62 (5H, m),7.32–7.41 (3H, m), 5.47 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 36.87 (CH), 58.16 (C), 104.03 (C), 117.30 (Ar–C), 119.66 (CN), 121.55 (Ar–C), 125.66 (Ar–C), 125.79 (Ar–C), 126.15 (Ar–C), 126.71 (Ar–C), 127.32 (Ar–C), 127.43 (Ar–C), 127.76 (Ar–C), 127.89 (Ar–C), 131.65 (Ar–C), 133.94 (Ar–C), 135.35 (Ar–C), 152.56 (Ar–C), 153.49 (C–NH2), 158.98 (Ar–C), 160.10 (C=O). Anal. Calcd. for C23H14N2O3: C, 75.40; H, 3.85; N, 7.65; Found: C, 75.26; H, 4.00; N, 7.35.
2-Amino-4-(3,4-dimethoxyphenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile (5q)
Yield 76 %; mp: 170–171 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.92 (1H, d, J = 8.1 Hz), 7.66 (1H, dd, J = 8.1 Hz and 7.2 Hz), 7.44 (1H, m), 7.37 (1H, d, J = 9 Hz), 6.83–6.86 (2H, m), 6.76 (1H, d, J = 8.7 Hz), 4.43 (1H, s), 3.73 (3H, s), 3.76 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 36.98 (CH), 55.97 (OCH3), 56.02 (OCH3), 58.61 (C), 104.59 (C), 112.11 (Ar–C), 112.36 (Ar–C), 113.51 (Ar–C), 117.03 (Ar–C), 119.76 (CN), 120.14 (Ar–C), 122.93 (Ar–C), 125.10 (Ar–C), 133.31 (Ar–C), 136.31 (Ar–C), 148.42 (Ar–C), 148.99 (Ar–C), 152.58 (C), 153.64 (C–NH2), 158.39 (C), 160.00 (C=O). Anal. Calcd. for C21H16N2O5: C, 67.02; H, 4.28; N, 7.44; Found: C, 67.26; H, 3.98; N, 7.64.
2-Amino-4-(3-chlorophenyl)-4H-benzo[h]chromene-3-carbonitrile (6d)
Yield 82 %; mp: 176–177 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.25 (1H, d, J = 8.1 Hz), 7.89 (1H, d, J = 7.8 Hz), 7.56–7.67 (3H, m), 7.12 (1H, d, J = 8.4 Hz), 4.97 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 29.33 (CH), 56.10 (C), 117.67 (CN), 121.20 (Ar–C), 124.56 (Ar–C), 126.49 (Ar–C), 126.97 (Ar–C), 127.23 (Ar–C), 127.47 (Ar–C), 127.86 (Ar–C), 128.18 (Ar–C), 131.20 (Ar–C), 133.25 (Ar–C), 133.73 (C–Cl), 143.25 (Ar–C), 148.61 (Ar–C), 160.74 (C–NH2). Anal. Calcd. for C20H13ClN2O: C, 72.18; H, 3.94; Cl, 10.65; N, 8.42; Found: C, 71.94; H, 3.63; Cl, 10.86; 8.22.
2-Amino-4-(3,4-dihydroxyphenyl)-4H-benzo[h]chromene-3-carbonitrile (6e)
Yield 71 %; mp: 150–151 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.30–8.39 (2H, m), 7.83–7.97 (2H, m), 7.18–7.73 (4H, m), 6.92 (1H, d, J = 8.4 Hz), 7.18 (2H, s, D2O exchangeable protons), 5.80 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 30.10 (CH), 59.22 (C), 115.36 (Ar–C), 117.76 (CN), 117.96 (Ar–C), 120.23 (Ar–C), 120.93 (Ar–C), 121.10 (Ar–C), 122.35 (Ar–C), 125.71 (Ar–C), 125.74 (Ar–C), 127.48 (Ar–C), 132.77 (Ar–C), 134.44 (Ar–C), 143.05 (Ar–C), 144.20 (C–OH), 147.55 (C–OH), 160.31 (C–NH2). Anal. Calcd. for C20H14N2O3: C, 72.72; H, 4.27; N, 8.48; Found: C, 72.99; H, 3.98; N, 8.72.
2-Amino-4-(4-hydroxyphenyl)-4H-benzo[h]chromene-3-carbonitrile (6k)
Yield 85 %; mp: 228–229 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 9.35 (1H, s, D2O exchangeable protons), 8.23 (1H, d, J = 8.1 Hz), 7.87 (1H, d, J = 8.1 Hz), 7.57–7.63 (3H, m), 7.03–7.11 (5H, m), 6.69(2H, d, J = 8.1 Hz), 4.77 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 29.88 (CH), 57.24 (C), 115.83 (Ar–C), 118.92 (CN), 121.14 (Ar–C), 123.23 (Ar–C), 124.84 (Ar–C), 126.80 (Ar–C), 127.07 (Ar–C), 129.14 (Ar–C), 133.06 (Ar–C), 136.64 (Ar–C), 143.02 (Ar–C), 151.74 (C–OH), 160.43 (C–NH2). Anal. Calcd. for C20H14N2O2: C, 76.42; H, 4.49; N, 8.91; Found: C, 76.22; H, 4.64; N, 9.02.
2-Amino-4-(thiophen-2-yl)-4H-benzo[h]chromene-3-carbonitrile (6n)
Yield 83 %; mp: 231–232 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.22 (1H, d, J = 8.1 Hz), 7.89 (1H, d, J = 7.8 Hz), 7.55–7.65 (3H, m), 7.36 (1H, d, J = 4.5 Hz), 7.24–7.28 (3H, m), 7.08 (1H, bs), 6.93 (1H, bs), 5.26 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 30.53 (CH), 59.02 (C), 119.84 (CN), 120.44 (Ar–C), 120.97 (Ar–C), 121.12 (Ar–C), 123.67 (Ar–C), 125.84 (Ar–C), 125.98 (Ar–C), 126.37 (Ar–C), 126.63 (Ar–C), 126.74 (Ar–C), 132.77 (Ar–C), 139.44 (Ar–C), 143.50 (Ar–C), 160.77 (C–NH2). Anal. Calcd. for C18H12N2OS: C, 71.03; H, 3.97; N, 9.20; S, 10.54; Found: C, 71.38; H, 3.58; N, 8.90; S, 10.82.
2-Amino-4-(3,4-dimethoxyphenyl)-4H-benzo[h]chromene-3-carbonitrile (6q)
Yield 73 %; mp: 140–141 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.24 (1H, d, J = 7.8 Hz), 7.88(1H, d, J = 7.54 Hz), 7.54–7.65 (3H, m), 7.11–7.16 (3H, m), 6.72–6.91 (3H, m), 4.84 (1H, s), 3.82 (3H, s), 3.80 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 31.11 (CH), 55.67 (OCH3), 56.66 (OCH3), 59.68 (C), 113.32 (Ar–C), 115.86 (Ar–C), 118.59 (Ar–C), 120.24 (Ar–C), 120.95 (Ar–C), 121.17 (Ar–C), 125.71 (Ar–C), 125.77 (Ar–C), 127.42 (Ar–C), 132.76 (Ar–C), 133.75 (Ar–C), 143.54 (Ar–C), 147.84 (C–OCH3), 150.31 (C–OCH3), 160.34 (C–NH2). Anal. Calcd. for C22H18N2O3: C, 73.73; H, 5.06; N, 7.82; Found: C, 73.44; H, 5.26; N, 8.10.
7-Amino-5-(4-bromophenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7g)
Yield 79 %; mp: 170–171 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.17 (1H, s, D2O exchangeable proton), 7.47 (2H, d, J = 8.1 Hz), 7.18 (2H, d, J = 8.4 Hz), 7.15 (2H, bs, D2O exchangeable proton), 4.23 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.72 (CH), 58.72 (C), 88.40 (C), 119.51 (CN), 120.22 (C–Br), 130.15 (Ar–C), 131.58 (Ar–C), 144.06 (Ar–C), 149.95 (C=O), 152.81 (C–NH2), 158.00 (C), 162.96 (C=O). Anal. Calcd. for C14H9BrN4O3: C, 46.56; H, 2.51; Br, 22.12; N, 15.51; Found: C, 46.26; H, 2.86; Br, 22.31; N, 15.23.
7-Amino-5-(4-methoxyphenyl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7i)
Yield 70 %; mp: 237–238 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.98 (2H, d, J = 7.5 Hz), 7.19 (2H, d, J = 7.5 Hz), 4.56 (1H, s), 3.89 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 35.27 (CH), 55.55 (OCH3), 58.22 (C), 88.44 (C), 114.22 (Ar–C), 120.15 (CN), 130.11 (Ar–C), 134.50 (Ar–C), 149.59 (C=O), 152.18 (C–OCH3), 156.00 (C–NH2), 158.55 (C), 162.69 (C=O). Anal. Calcd. for C15H12N4O4: C, 57.69; H, 3.87; N, 17.94; Found: C, 57.99; H, 3.96; N, 17.52.
7-Amino-5-(thiophen-2-yl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7n)
Yield 74 %; mp: 172–173 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.14 (1H, s, D2O exchangeable proton), 7.40 (1H, d, J = 7.8 Hz), 6.83–6.86 (2H, bs), 6.8 (1H, s, D2O exchangeable proton), 4.52 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 25.80 (CH), 58.10 (C), 80.10 (C), 119.10 (CN), 123.50 (Ar–C), 125.40 (Ar–C), 127.00 (Ar–C), 139.80 (Ar–C), 150.40 (C=O), 158.90 (C–NH2), 160.84 (C), 163.68 (C=O). Anal. Calcd. for C12H8N4O3S: C, 50.00; H, 2.80; N, 19.43; S, 11.12; Found: C, 48.10; H, 3.21; N, 20.21; S, 11.21.
7-Amino-5-(3-methylthiophen-2-yl)-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (7u)
Yield 78 %; mp: 171–173 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 11.21 (1H, s, D2O exchangeable proton), 7.80 (2H, bs, D2O exchangeable proton), 7.28 (1H, d, J = 7.6 Hz), 6.26 (1H, d, 7.6 Hz), 4.62 (1H, s), 2.30 (3H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 13.40 (CH3), 22.30 (CH), 58.10 (C), 80.12 (C), 119.10 (CN), 121.10 (Ar–C), 124.30 (Ar–C), 133.60 (Ar–C), 135.40 (Ar–C), 150.6 (C=O), 159.3 (C–NH2), 160.4 (C), 163.8 (C=O). Anal. Calcd. for C13H10N4O3S: C, 51.65; H, 3.33; N, 18.53; S, 10.61; Found: C, 50.21; H, 3.20; N, 20.10; S, 12.31.
3-Amino-1-(2-methoxyphenyl)-1H-benzo[f]chromene-2-carbonitrile (8h)
Yield 70 %; mp: 200–201 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 7.89–7.92 (2H, m), 7.74 (1H, d, J = 7.8 Hz), 7.42 (2H, m), 7.32 (1H, dd, J = 1.2 and 6.9 Hz), 7.13 (1H, m), 7.03 (1H, d, J = 8.4 Hz), 6.77–6.86 (4H, m), 5.60 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 17.31 (CH), 56.44 (OCH3), 57.41 (C), 112.18 (Ar–C), 117.16 (CN), 121.52 (Ar–C), 123.39 (Ar–C), 125.30 (Ar–C), 127.61 (Ar–C), 128.41 (Ar–C), 128.95 (Ar–C), 129.05 (Ar–C), 129.63 (Ar–C), 131.13 (Ar–C), 134.07 (Ar–C), 151.77 (Ar–C), 156.11 (C–OCH3), 160.69 (C–NH2). Anal. Calcd. for C21H16N2O2: C, 76.81; H, 4.91; N, 8.53; Found: C, 76.52; H, 5.12; N, 8.88.
3-Amino-1-(thiophen-2-yl)-1H-benzo[f]chromene-2-carbonitrile (8n)
Yield 77 %; mp: 228–229 °C, 1H NMR (DMSO-d6, 300 MHz, δ, TMS = 0): 8.05 (1H, d, J = 8.1 Hz), 7.93 (2H, d, J = 8.7 Hz), 7.43–7.54 (2H, m), 7.26–7.32 (2H, m), 7.09 (2H, m), 6.86–7.02 (2H, m), 5.71 (1H, s). 13C NMR (DMSO-d6, 500 MHz, δ, TMS = 0): 17.41 (CH), 57.44 (C), 117.3 (CN), 118.81 (Ar–C), 121.25 (Ar–C), 122.15 (Ar–C), 122.51 (Ar–C), 123.22 (Ar–C), 126.38 (Ar–C), 126.61 (Ar–C), 126.95 (Ar–C), 128.57 (Ar–C), 128.79 (Ar–C), 138.81 (Ar–C), 139.18 (Ar–C), 136.81 (Ar–C), 151.75 (Ar–C), 161.09 (C–NH2). Anal. Calcd. for C18H12N2OS: C, 71.03; H, 3.97; N, 9.20; S, 10.54; Found: C, 71.32; H, 3.66; N, 8.96; S, 10.76.
Xanthine oxidase assay
Bovine milk xanthine oxidase (grade 1, ammonium sulphate suspension, Sigma-Aldrich) activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a Hitachi U-3010 UV–visible spectrophotometer at 25 °C (Escribano et al., 1988; Takano et al., 2005). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.6), 75 µM xanthine and 0.08 U of xanthine oxidase. Inhibition of xanthine oxidase activity by various inhibitors was measured by following the decrease in the uric acid formation at 293 nm at 25 °C. The enzyme was preincubated for 5 min, with test compound, dissolved in DMSO (1 % v/v), and the reaction was started by the addition of xanthine. Final concentration of DMSO (1 % v/v) did not interfere with the enzyme activity. All the experiments were performed in triplicate, and values were expressed as means of three experiments.
Molecular modelling study
The 3D structural coordinates of XO were obtained from protein databank (PDB ID: 1VDV) (Fukunari et al., 2004). The ligand structure was prepared in ChemDraw, and energy was minimized MM2 module of Chem3D ultra (ChemDraw Ultra 6.0 and Chem3D Ultra, 2000). The ligand was docked at the binding site using the GOLD 5.1 (GOLD, Evaluation Version 5.1 2012). Gold performs genetic algorithm-based ligand docking to optimize the conformation of ligand at the receptor binding site. GoldScore scoring function was used to find out the binding pose. GoldScore comprises four components: protein–ligand hydrogen bond energy, protein–ligand van der Waals (vdw) energy, ligand internal vdw energy and ligand torsional strain energy.
References
Abd-El-Aziz AS, El-Agrody AM, Bedair AH, Corkery TC, Ata A (2004) Synthesis of hydroxyquinoline derivatives, aminohydroxychromene, aminocoumarin and their antibacterial activities. Heterocycles 63:1793–1812
Ali MA, El-Remaily EA (2013) Synthesis of pyranopyrazoles using magnetic Fe3O4 nanoparticles as efficient and reusable catalyst. Tetrahedron. doi:10.1016/j.tet.2014.03.024
Andreani LL, Lapi E (1960) Aspects and orientations of modern pharmacognosy. Boll Chim Farm 99:583–586
Aytemir MD, Calis U, Ozalp M (2004) Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4h-pyran-4-one derivatives. Arch Pharm 337:281–288
Banerjee S, Horn A, Khatri H, Sereda G (2011) A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett 52:1878–1881
Biagi G, Giorgi I, Pacchini F, Livi O, Scartoni V (2001) 2-Alkyloxyalkylthiohypoxanthines as new potent inhibitors of xanthine oxidase. Farmaco 56:809–813
Bihani M, Bora PP, Bez G, Askari H (2013) Amberlyst A21: a reusable solid catalyst for green synthesis of pyran annulated heterocycles at room temperature. C R Chim 16:419–426
Bolligarla R, Das SK (2011) Synthesis of new intramolecular charge transfer A–D–A tetrathiafulvalene-fused triads exhibiting large solvent sensitive emission behaviour. Tetrahedron Lett 52:2496–2500
Bonsignore L, Loy G, Secci D, Calignano A (1993) Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem 28:517–520
Bora PB, Bihani M, Bez G (2013) Multicomponent synthesis of dihydropyrano[2,3-c]pyrazoles catalyzed by lipase from Aspergillus niger. J Mol Catal B Enzym 92:24–33
Borges F, Fernandes E, Roleira F (2002) Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 9:195–217
ChemDraw Ultra 6.0 and Chem3D Ultra (2000) Cambridge Soft Corporation, Cambridge, USA
Colombo L, Giacomo MD, Vinci V, Colombo M, Manzoni L, Scolastico C (2003) Synthesis of new bicyclic lactam peptidomimetics by ring-closing metathesis reactions. Tetrahedron 59:4501–4513
Copeland RA (2005) evaluation of enzyme inhibitors in drug discovery. Wiley, Hoboken
Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van-Poel B, Pieters L, Vlietinck AJ, Berghe DV (1998) Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61:71–76
Da-Silva SL, Da-Silva A, Honorio KM, Marangoni S, Toyama MH, Da-Silva ABF (2004) The influence of electronic, steric and hydrophobic properties of flavonoid compounds in the inhibition of the xanthine oxidase. J Mol Struct Theochem 684:1–7
Devi I, Kumar BSD, Bhuyan PJ (2003) A novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines using microwave heating in the solid state. Tetrahedron Lett 44:8307–8310
Dhiman R, Sharma S, Singh G, Nepali K, Bedi PMS (2012) Design and synthesis of aza-flavones as a new class of xanthine oxidase inhibitors. Arch Pharm Chem Life Sci 346:7–16
Elnagdi MH, Elfaham HA, Elgemeie GEH (1983) Utility of α,β-unsaturated nitriles in heterocyclic synthesis. Heterocycles 20:519–550
Escribano J, Gracia-Canovas F, Garcia-Carmona F (1988) A kinetic study of hypoxanthine oxidation by milk xanthine oxidase. Biochem J 254:829–833
GOLD, Evaluation Version 5.1 (2012) Cambridge Crystallographic Data Centre. Cambridge, UK
Fan X, Feng D, Qu Y, Zhang X, Wang J, Loiseau PM, Andrei G, Snoeck R, Clercq ED (2010) Practical and efficient synthesis of pyrano[3,2-c]pyridone, pyrano[4,3-b]pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents. Bioorg Med Chem Lett 20:809–813
Fukunari A, Okamoto K, Nishino T, Eger BT, Pai EF, Kamezawa M, Yamada I, Kato N (2004) Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: a potent xanthine oxidoreductase inhibitor with hepatic excretion. J Pharmacol Exp Ther 311:519–528
Gao S, Tsai CH, Tseng C, Yao CF (2008) Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron 64:9143–9149
Goldmann S, Stoltefus J (1991) 1,4-Dihydropyridines: effects of chirality and conformation on the calcium antagonist and calcium agonist activities. Angew Chem Int Ed Engl 30:1559–1578
Gong K, Wang HL, Luo J, Liu ZL (2009) One-pot synthesis of polyfunctionalized pyrans catalyzed by basic ionic liquid in aqueous media. J Heterocycl Chem 46:1145–1150
Green GR, Evans JM, Vong AK (1995) Pyrans and their benzo derivatives synthesis. In: Katritsky AR, Rees C, Scriven EFV (eds) Comprehensive heterocyclic chemistry II. Pergamon Press, Oxford, p 469
Hasaninejad A, Golzar N, Beyrati M, Zare A, Doroodmand MM (2013) Silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride (SB-DBU)Cl as a highly efficient, heterogeneous and recyclable silica-supported ionic liquid catalyst for the synthesis of benzo[b]pyran, bis(benzo[b]pyran) and spiro-pyran derivatives. J Mol Catal A Chem 372:137–150
Hille R (2006) Structure and function of xanthine oxidoreductase. Eur J Inorg Chem 2006:1913–1936
Hosseini-Monfared H, Meyer H, Janiak C (2013) Dioxygen oxidation of 1-phenylethanol with gold nanoparticles and N-hydroxyphthalimide in ionic liquid. J Mol Catal A Chem 372:72–78
Jain S, Rajguru D, Keshwal BS, Acharya AD (2013) Solvent-free green and efficient one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives. ISRN Org Chem, Article ID 185120, 5
Jiang-Cheng X, Wan-Mei L, Hui Z, Yi-Feng L, Peng-Fei Z (2011) One-pot synthesis of tetrahydrochromene derivatives catalyzed by lipase. Tetrahedron 67:9582–9587
Khaksar S, Rouhollahpour A, Talesh SM (2012) A facile and efficient synthesis of 2-amino-3-cyano-4H-chromenes and tetrahydrobenzo[b]pyrans using 2,2,2-trifluoroethanol as a metal-free and reusable medium. J Fluor Chem 141:11–15
Khurana JM, Nand B, Saluja P (2010) DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron 66:5637–5641
Kidwai M, Sexena S (2006) Convenient preparation of pyrano benzopyranes in aqueous media. Synth Commun 36:2737–2742
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) A facile one-pot green synthesis and antibacterial activity of 2-amino-4H-pyrans and 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes. Eur J Med Chem 44:3805–3809
Lin M, Chen CS, Chen T, Liang YC, Lin JK (2002) Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun 294:167–172
Maalej E, Chabchoub F, Oset-Gasque MJ, Esquivias-Perez M, Gonzalez MP, Monjas L, Perez C, Rios CL, Rodriguez-Franco MI, Iriepa I, Moraleda I, Chioua M, Romero A, Marco-Contelles J, Samadi A (2012) Synthesis, biological assessment, and molecular modeling of racemic 7-aryl-9,10,11,12-tetrahydro-7H-benzo[7,8]chromeno[2,3-b]quinolin-8-amines as potential drugs for the treatment of Alzheimer’s disease. Eur J Med Chem 54:750–763
Mazumder A, Wang S, Neamati N, Nicklaus M, Sunder S, Chen J, Milne GW, Rice WG, Burke TR Jr, Pommier Y (1996) Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J Med Chem 39:2472–2481
Nepali K, Agarwal A, Sapra S, Mittal V, Kumar R, Banerjee UC, Gupta MK, Satti NK, Suri OP, Dhar KL (2011a) N-(1,3-Diaryl-3-oxopropyl)amides as a new template for xanthine oxidase inhibitors. Bioorg Med Chem 19:5569–5576
Nepali K, Singh G, Turan A, Agarwal A, Sapra S, Kumar R, Banerjee UC, Verma PK, Satti NK, Gupta MK, Suri OP, Dhar KL (2011b) A rational approach for the design and synthesis of 1-acetyl-3,5-diaryl-4,5-dihydro(1H)pyrazoles as a new class of potential non-purine xanthine oxidase inhibitors. Bioorg Med Chem 19:1950–1958
Oettl K, Reibneggar G (1999) Pteridines as inhibitors of xanthine oxidase: structural requirements. Biochem Biophys Acta 1430:387–395
Pacher P, Nivorozhkin A, Szabό C (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58:87–114
Paul S, Pradhan K, Ghosh S, De SK, Das AR (2013) Uncapped SnO2 quantum dot catalyzed cascade assembling of four components: a rapid and green approach to the pyrano[2,3-c]pyrazole and spiro-2-oxindole derivatives. Tetrahedron. doi:10.1016/j.tet.2014.02.077
Perez-Perez M, Balzarini J, Rozenski J, Clercq ED, Herdewijn P (1995) Synthesis and antiviral activity of phosphonate derivatives of enantiomeric dihydro-2H-pyranyl nucleosides. Bioorg Med Chem Lett 5:1115–1118
Pochet L, Doucet C, Schynts M, Thierry N, Boggetto N, Pirotte B, Jiang KY, Masereel B, de Tullio P, Delarge J, Reboud-Ravaux M (1996) Esters and amides of 6-(chloromethyl)-2-oxo-2H-1-benzopyran-3-carboxylic acid as inhibitors of alpha-chymotrypsin: significance of the “aromatic” nature of the novel ester-type coumarin for strong inhibitory activity. J Med Chem 39:2579–2585
Robins RK, Revankar GR, O’Brien DE, Springer RH, Albert TNA, Senga K, Miller JP, Streeter DG (1985) Purine analog inhibitors of xanthine oxidase-structure activity relationships and proposed binding of the molybdenum cofactor. J Heterocycl Chem 22:601–634
Rostamnia Z, Morsali A (2014) Size-controlled crystalline basic nanoporous coordination polymers of Zn4O(H2N-TA)3: catalytically study of IRMOF-3 as a suitable and green catalyst for selective synthesis of tetrahydro-chromenes. Inorg Chim Acta 411:113–118
Sadegh R, Ali M (2014) Size-controlled crystalline basic nanoporous coordination polymers of Zn4O(H2N-TA)3: catalytically study of IRMOF-3 as a suitable and green catalyst for selective synthesis of tetrahydro-chromenes. Inorg Chim Acta 411:113–118
Safaei HR, Shekouhy M, Rahmanpur S, Shirinfeshan A (2012) Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans. Green Chem 14:1696–1704
Sanchez A, Hernandez F, Cruz PC (2012) Infrared irradiation-assisted multicomponent synthesis of 2-amino-3-cyano-4H-pyran derivatives. J Med Chem Soc 56:121–127
Sharma S, Sharma K, Ojha R, Kumar K, Singh G, Nepali K, Bedi PMS (2014) Microwave assisted synthesis of naphthopyrans catalysed by silica supported fluoroboric acid as a new class of non purine xanthine oxidase inhibitors. Bioorg Med Chem Lett 24:495–500
Shukla S, Kumar D, Ojha R, Gupta MK, Nepali K, Bedi PMS (2014) 4,6-Diaryl/heteroarylpyrimidin-2(1H)-ones as a new class of xanthine oxidase inhibitors. Arch Pharm Chem Life Sci 347:486–495
Singh H, Sharma S, Ojha R, Gupta MK, Nepali K, Bedi PMS (2014) Synthesis and biological evaluation of naphthoflavones as non purine xanthine oxidase inhbitors. Bioorg Med Chem Lett 24:4192–4197
Star AE, Marby TJ (1971) Flavonoid frond exudates from two Jamaican ferns, Pityrogramma tartarea and P. calomelanos. Phytochemistry 10:2817–2818
Stockert AL, Shinde SS, Anderson RF, Hille R (2002) The reaction mechanism of xanthine oxidase: evidence for two-electron chemistry rather than sequential one-electron steps. J Am Chem Soc 124:14554–14555
Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA (2005) Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci 76:1835–1847
Uher M, Konecny V, Rajniakove O (1994) Synthesis of 5-hydroxy-2-hydroxymethyl-4H-pyran-4-one derivatives with pesticide activity. Chem Pap 48:282–284
Virdi HS, Sharma S, Mehndiratta S, Bedi PMS, Nepali K (2014) Design, synthesis and evaluation of 2,4-diarylpyrano[3,2-c]chromen-5(4H)-one as a new class of non-purine xanthine oxidase inhibitors. J Enzyme Inhibition Med Chem. doi:10.3109/14756366.2014.961446
Wang S, Milne GWA, Yan X, Posey IJ, Nicklaus MC, Graham L, Rice WG (1996) Discovery of novel, non-peptide HIV-1 protease inhibitors by pharmacophore searching. J Med Chem 39:2047–2054
Wang XS, Yang GS, Zhao G (2008) Enantioselective synthesis of naphthopyran derivatives catalyzed by bifunctional thiourea-tertiary amines. Tetrahedron Asymmetry 19:709–714
Wang HJ, Lu J, Zhang ZH (2010) Highly efficient three-component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives. Monatsh Chem 141:1107–1112
Xiang-Shan W, Zhao-Sen Z, Da-Qing S, Xian-Yong W, Zhi-Min Z (2005) One-pot synthesis of 2-amino-4-aryl-4H-pyrano[3,2-c]coumarin derivatives using KF/Al2O3 as catalyst. J Org Chem 25:1138–1141
Xu JC, Li WM, Zheng H, Lai YF, Zhang PF (2011) One-pot synthesis of tetrahydrochromene derivatives catalyzed by lipase. Tetrahedron 67:9582–9587
Yu G, Da-Ming D (2012) Enantioselective synthesis of 2-amino-5,6,7,8-tetrahydro-5-oxo-4H-chromene-3-carbonitriles using squaramide as the catalyst. Tetrahedron Asymmetry 23:1343–1349
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, R., Naaz, F., Sharma, S. et al. Screening of a library of 4-aryl/heteroaryl-4H-fused pyrans for xanthine oxidase inhibition: synthesis, biological evaluation and docking studies. Med Chem Res 24, 3334–3349 (2015). https://doi.org/10.1007/s00044-015-1382-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1382-0