Abstract
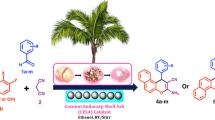
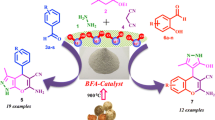
A simple and eco-friendly protocol for the facile synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyridine derivatives was developed using naturally sourced coconut endocarp shell ash (CESA) as a catalyst at room temperature in eco-compatible solvent system. The CESA catalyst was obtained from renewable feedstock, biodegradable waste, by simple thermal treatment on coconut endocarp shell (CESA), and the formation of its catalytic phase was characterized by DSC-TGA, FT-IR, XRD, EDX, BET, and SEM techniques. Derivatives of tetrahydrobenzo[b]pyran and 1, 4-dihydropyridine are obtained with an excellent yield, ranging from 82 to 99% in shorter reaction times. The remarkable advantages of the process presented here are that it is operationally clean, environmentally benign, and the product does not require chromatographic separation. Furthermore, the catalyst can be recovered conveniently and reused five times without significant loss of its catalytic activity.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) have been recognized as an attractive and powerful strategy for the efficient synthesis of a wide variety of highly functionalized organic molecules [1]. Therefore, the academic and industrial research community has increasingly focused on greener synthetic methodologies by the development of MCRs to synthesize many small biologically active heterocyclic compounds [2, 3].
The primary building blocks of natural oxygen-containing heterocyclic compounds are the fused 2H-chromene and 4H-chromene scaffolds, mainly found in natural sources of edible fruits and vegetables [4]. Chromene compounds play an important role in drug research because of their many pharmaceutical and biological activities [5], such as anticancer [6], anti-bacterial [7], antitumor [8], antiallergic [9], antioxidant [10], anti-tuberculosis [11], anti-proliferative [12], and anti-viral activities [13]. Generally, these biological activities of chromene derivatives depend on the nature of attached groups either 4H-pyran or the adjacent rings. Especially, 2-amino-4H-chromene derivatives have a potential application in rheumatoid, psoriasis, and cancer treatment [14]. Moreover, these compounds are used for the treatment of neurodegenerative disorders including Parkinson’s disease, Alzheimer's disease, and Down’s syndrome [15, 16]. Furthermore, 4H-chromene scaffolds have other properties such as laser dyes [17], cosmetics, biodegradable agrochemicals [18], pigments [19], optical brighteners [20], fluorescence makers [21], and pH-sensitive fluorescent materials for visualization of biomolecules [22]. For instance, fused 2-amino-4H-chromene derivatives are important as antibacterial (Fig. 1a), anticancer (Fig. 1b), and inhibitors of insulin-regulated aminopeptidase (IRAP) for enhancing memory and learning functions (Fig. 1c), antimitotic, and antitubercular (Fig. 1d), respectively [23, 24].
Therefore, because of important properties of chromene derivatives in medicinal chemistry, the scientific community has been focused on the development of MCRs strategies for environmentally friendly methodologies to synthesize tetrahydrobenzo[b]pyrans scaffolds based on the cyclization of malononitrile (or ethyl acetoacetate), aldehyde, and diverse enolizable electron-rich C-H activated acidic compounds. Realizing the importance of tetrahydrobenzo[b]pyran derivatives in last two decades, several modified methods have been reported using homogeneous or heterogeneous catalysts, including Cao-ZrO2 [25], DABCO [26], K3PO4 [27], potassium phthalimide-N-oxyl [28], ceria-vanadia/silica [29], MgO [30], 2-aminopyridine [31], LiBr [32], triethanolamine [33], taurine [34], N(Et)3 [35], ionic liquids such as PEG1000-DAIL [36], [Et3NH][HSO4] [37], Eu(III)/IDA/CPTS/CoFe2O4 [38], and Fe3O4@SiO2@NiSB [39].
On the other hand, 1, 4-dihydropyridines (1, 4-DHP) exhibited a wide range of biological and pharmacological activities, particularly in the treatment of cardiovascular diseases as calcium channel antagonists, as well as analgesics [40, 41], vasodilators [42], anti-tumor [43], and anti-inflammatory [44]. Nowadays, several DHPs are commercial products, such as felodipine (Fig. 1e), amlodipine (Fig. 1f), diludine (Fig. 1g), and nimodipine (Fig. 1h), which are manufactured and used worldwide [44, 45]. Hence, several attempts have been reported for the synthesis of 1,4-DHP derivatives using various catalysts, such as ([bmim]BF4) [46], BF3-SiO2 [47], ([BPy][BF4]) [48], Mg3N2 [49], P(C6H5)3 [50], chitosan-based vanadium oxo [51], silica gel/NaHSO4 [52], HClO4-SiO2 [53], TMSCl-NaI [54], AlCl3.6H2O [55], silica SO3H-[BMIM][PF6] [56], modified eggshell [57], and FeCl3 [58]. However, the synthesis of tetrahydrobenzo[b]pyrans and 1,4-DHPs reported methods have their own merits, but they suffer from some drawbacks, such as use of heavy metals or use of toxic, expensive, and hazardous organic solvents, high expensive catalyst loading, tedious workup procedure, harsh reaction conditions, long reaction times, and low yield of products [36, 38, 48]. To avoid these limitations, it is necessary to develop a simple, cost-effective, and green procedures using safe reagents and solvents for the synthesis of tetrahydrobenzo[b]pyran and 1, 4-DHP derivatives.
Nowadays, the efficiency of a chemical reaction could be measured not only by its overall yield and selectivity parameters but also by the use of raw materials, human resources, use of hazardous chemicals, time and energy requirements, and experimental procedures involved in the synthesis. Therefore, the development of an environmentally benign, efficient protocol is still on demand. The introduction of clean procedures by utilizing eco-friendly green catalysts has attracted a great attention from researchers. The increasing interest in natural resources as a catalyst because they are easily available at low cost, biodegradable, non-hazardous and non-toxic agents that can carry organic transformations in an environmentally benign manner [59].
Therefore, the prime aim of the present work is to explore the utilization of naturally sourced catalysts in organic synthesis. So, the exploration of waste biomass for the development of promising catalysts to make synthetic protocols environmentally benign can considerably avoid the problem of solid waste biomass disposal and reduce possible environmental pollution [60]. A plant, Cocos nucifera, belongs to the family Aceraceae (Palm tree). Coconuts are cultivated in more than 90 countries around the world on about 10 million hectares. Almost 75% of the world coconut production is grown in India, Indonesia, and the Philippines [61, 62]. The coconut shell is rich with organic materials, containing 33.61% cellulose, 36.51% lignin, 29.27% pentose, and 0.61% ash and inorganic material [63].
In continuation of our current research interest is the replacement of less efficient and traditional protocols that makes the system more environmentally friendly and cost-effective [64,65,66,67]. The present research revealed that a waste-derived catalyst is an efficacious and promising alternative to hazardous reagents. Herein, we report the first time for the synthesis of tetrahydrobenzo[b]pyran from aldehyde, malononitrile, and dimedone, as well as 1,4-dihydropyridines from aldehyde, ethyl acetoacetate, and ammonium acetate via one-pot multi-component reaction using coconut endocarp shell ash (CESA) as a catalyst at room temperature (RT) in an eco-compatible solvent ethanol (Scheme 1).
Experimental
Materials and methods
All chemicals and laboratory-grade reagents utilized for this study were purchased from Sigma-Aldrich and Merck and used without further purification. Analytical thin-layer chromatography (TLC) technique is used to check the purity of substance and completion of the reaction was performed using Merck silica gel 60 F-254 Al-sheets. The isolated pure form of products was confirmed and characterized by spectroscopic data (IR, 1H NMR, 13C NMR, Mass spectra). Infrared (IR) spectra were recorded in KBr pellets on a Bruker ALPHA FT-IR spectrometer. The 1H and 13C NMR spectral data were recorded on Brucker-AC spectrometers in CDCl3 and DMSO as solvent (for 1H NMR 300 MHz and 13C NMR 75 MHz). The melting point of purified compounds was determined using DBK programmable melting point apparatus as an open capillary method and is uncorrected. For elemental analysis, SHIMADZU AA-7000 atomic absorption spectrophotometer (AAS) was used. Thermal gravimetric analysis (TGA) was obtained using a TA SDT Q600 V20.9 Build instrument in the presence of air at a linear heating rate at 10 °C min−1 from 25 to 1000 °C. For scanning electron microscope (SEM) A JEOL (Tokyo, Japan) JSM-5200 were used. N2 adsorption–desorption isotherm was obtained with a Micrometrics ASAP 2010 surface area and porosity analyzer. Quanta 200 3D FEI scanning electron microscope was used for energy-dispersive X-ray spectroscopy analysis.
Preparation of coconut endocarp shell ash (CESA) catalyst
For the preparation of the CESA catalyst, biowaste endocarp shell of Cocos nucifera were obtained from the local food market. The collected coconut endocarp shell (CES) (Fig. 2a) were cleaned by using deionized water to remove dirt and they were sun-dried for two days. The dried CES (50 g) were broken into small pieces using mortar with help of pestle (Fig. 2b). CES was thermally treated in a silica crucible up to 800 °C in a muffle furnace for two hours. After heating, fine soft ash (Fig. 2c) was obtained and which was denominated as coconut endocarp shell ash (CESA). Further, the CESA was used as catalyst for synthesis of tetrahydrobenzo[b]pyrans and 1, 4-dihydropyridines.
General procedure for the synthesis of tetrahydrobenzo[b]pyran (4a–p)
In a clean and dry 25 mL round-bottom flask, a mixture of aromatic aldehydes 1a–p (1 mmol), dimedone 2 (1 mmol), malononitrile 3 (1 mmol), and ethanol (5 mL) were magnetically stirred at room temperature in the presence of CESA (10 wt. %) as catalyst until desired products 4a–p, were obtained. The reaction progress was checked by TLC (ethyl acetate: hexane 1:9). After completion of the reaction, reaction mixture was subjected for solvent- extraction using ethyl acetate (4 × 10 mL). The combined organic phase was washed with water, dried (Na2SO4) and the solvent removed under reduced pressure to afford a crude product. Further pure product were obtained by recrystallization using 96% ethanol. The identity of the products were characterized by FT-IR, 1H NMR, and 13C NMR spectroscopic methods. Melting point of the products 4a–p were also measured and is in harmony with the literature values previously reported elsewhere.
General procedure for the synthesis of 1, 4-dihydropyridines (7a–n)
A mixture of aromatic aldehydes 1a–n (1 mmol), ethyl acetoacetate 5 (2 mmol), and ammonium acetate 6 (1 mmol) was stirred at room temperature using a CESA catalyst (10 wt. %) in ethanol (5 mL) for a certain time period as indicated in Table 2. Their action was monitored by TLC (ethyl acetate: hexane 1:9). After completion of the reaction, the appropriate workup from the method above was used. All synthesized compounds 7a–n are known and established from their physical and spectral data and is in harmony with the literature values previously reported elsewhere.
Results and discussion
Characterization of coconut endocarp shell ash (CESA) catalyst
To investigate the active sites of catalyst, CESA was characterized by using different analytical techniques such as DSC-TGA, FTIR, XRD, EDX, BET, and SEM.
DSC-TGA analysis
We investigated the suitable calcination temperature with weight change of parent coconut endocarp shell (CES) powder by using DSC-TGA inflow of nitrogen atmosphere. The sample loading was typically 7–8 mg (Fig. 3). The TGA result showed the temperature ranged from 0 to 1000 °C defects the decomposition of parent CES. The three distinct stages of weight loss observed, one at a temperature below 150 °C in the first step was 9.737% due to decomposition of water and organic volatile material. Another major loss has occurred in the second step between 218.42 °C to 372.24 °C which was 53.51% support for the decomposition of organic polymers and chlorides. In a third step, 15.62% weight loss in the range of 400–800 °C was due to metal carbonates. Carbonate minerals are characterized by an exothermic peak caused by the evolution of CO2 in the DSC thermal curves. As the weight remains constant after 900 °C, this temperature was suitable for the complete conversion of parent CES to coconut endocarp shell ash (CESA) catalyst.
FT-IR analysis
Fourier transform infrared (FT-IR) spectra were used to investigate the study of active functional groups in the material, providing strong relevance to the presence of metal oxides, carbonates, and hydroxide bonds. In the FT-IR spectrum of parent CES (Fig. 4a), the observation of strong absorption bands at 1693 cm−1, 1647 cm−1, 1517 cm−1, 1366 cm−1, 1068 cm−1, 991 cm−1, 859 cm−1 and 667 cm−1 due to the presence of metal carbonates and the weak absorption band occurred at 1749 cm−1 corresponds to C = O group of carbonate. After thermal treatment at 900 °C (Fig. 4b), the absorption bands occurred in the range of 1695 cm−1, 1659 cm−1, 1515 cm−1, 1341 cm−1, 1028 cm−1, 628 cm−1 are clearly indicates the presence of metal oxides also the absorption band at 3362 cm−1 due to the presence of a small concentration of OH group in the CESA supports the formation of metal hydroxides due to absorption of moisture from the environment.
XRD analysis
The phase composition of the CESA catalyst is evident from X-ray diffraction analysis (Fig. 5). The X-ray diffraction pattern of the parent CES displayed very broad peaks at 2θ = 20. 50°, 20. 95°, 24. 22°, 29. 86°, 30. 19°, 34. 65°, 39. 80° and 40. 60° suggesting the amorphous nature of this material. After thermal treatment, the crystal structure of CESA shows peaks at 2θ = 28. 08°, 28. 84°, 29. 86°, 30. 19°, 44. 87°, 47. 17°, 49. 89°, 68. 54° and 75. 66° hint the presence of characteristic patterns of metal carbonates. The peaks appearing at 2θ = 31. 01°, 33. 59°, 34. 65°, 40. 60°, 41. 05° and 43. 12° were characteristic of metal oxides. At 2θ = 20. 50°, 21. 03° and 22. 01° metal hydroxides were detected. These active phases of the catalyst were utilized for the conversion of the reactant into the product.
EDX analysis
The EDX spectrum of CESA catalyst is presented in Fig. 6. The report reveals a very high distribution of oxides of K, Na, Si, Ca, Mg, P, and moderate to less distribution of elements including B, Mn, Fe, and Cu present. The presence of O and Cl suggests that K, Na, Si, Ca, Mg, and P may exist in the form of corresponding oxides, carbonates and chlorides which imparts basicity to the CESA catalyst and play an extreme role in the catalyst.
BET analysis
N2 adsorption–desorption isotherms as well as the corresponding pore size distribution curve for the prepared CESA catalyst were shown in Fig. 7. The surface area of the CESA catalyst was found to be 168.92 m2/g, while the pore volume and average pore radius were 0.093536 cm3/g−1 and 2.215 nm, respectively. The pore size distribution curve indicates mesopores of very uniform sizes. The isotherms of the prepared CESA catalyst were type III isotherms with an H4-type hysteresis loop.
SEM analysis
The surface morphology and shape of the CESA catalyst were studied by scanning electron microscopy (SEM). A SEM image of the prepared CESA catalyst is depicted in Fig. 8. It illustrates the porous nature, which provides a smooth and soft active surface area for catalyzing the reaction effectively.
Catalytic performance
At the outset of the protocol, the efficiency of naturally sourced CESA catalyst was launched by choosing 4-chlorobenzaldehyde 1b (1 mmol), dimedone 2 (1 mmol), and malononitrile 3 (1 mmol) as model substrates for the synthesis of 2-amino-4-(4-chlorophenyl) -5, 6, 7, 8-tetrahydro-7, 7-dimethyl-5-oxo-4H-chromene-3-carbonitrile 4b. Initially, we focused on identifying the optimal reaction conditions for our proposed synthetic conversion, and those are shown in Table 1.
Preliminary optimization conditions indicated that the trace amount of the desired product 4b was obtained in the absence of catalyst and solvent-free conditions, even under room temperature or at reflux conditions (Table 1, entries 1, 2). However, the model reaction was performed in the ethanol and water as a solvent, in absence of catalyst, only a small amount of the desired product 4b was obtained at room temperature (Table 1, entries 3, 4). These results indicated that a catalyst must be necessary for this reaction. We observe that, due to the addition of a catalytic amount of CESA, the yield of desired product 4b was improved in the absence of solvents and there was no further improvement in the desired product yield even at reflux conditions (Table 1, entry 5, 6). The result shows that there is a need for the solvent to improve the product yields. To optimize a suitable reaction medium, the model reaction was tested in various organic solvents as well as in water with a catalytic amount of CESA catalyst (wt. %) at room temperature. Notably, when this model reaction was carried out in ethanol as a solvent with CESA catalyst by varying the catalytic amounts (2, 5, 10, 12 wt. %) gave 90% to 98% corresponding product yield after 05 min at room temperature (Table 1, entries 7–10).
It was found that when increasing the catalytic amount from 2 to 10 wt. % in ethanol, the yield also increased (Table 1, entries 7–9) which among them 10 wt. % of catalyst had the best performance (Table 1, entry 9). No improvements in product yields were observed after increasing the CESA catalytic amount (Table 1, entry 10). It was found that there is no improved effect on product yield at refluxed conditions (Table 1, entry 11). On the other hand, optimization of model reaction was also examined in other solvents like MeOH, toluene, THF, H2O, and acetonitrile afforded the product yield from 38 to 78% (Table 1, entries 12–16). In general, the best yield of 4b was achieved by employing 10 wt. % of CESA catalyst in EtOH solvent (5 mL) at room temperature, which is considered as the optimized reaction conditions for the model reaction (Table 1, entry 9).
With the optimized reaction conditions in hand, the scope of this method to prepare tetrahydrobenzo[b]pyran with respect to various substituted aromatic aldehydes 1a–p was evaluated with dimedone 2 and malononitrile 3 in the presence of CESA catalyst. The results are summarized in Table 2. As shown in Table 2, the introduction of both electron-acceptor and electron-donor groups on nucleus of aryl aldehydes, reacted successfully in reaction conditions and gave high product yields 4a–p. It was found that aromatic aldehydes with electron acceptor groups accelerate the reaction rate as compared to the electron-donor groups. It was also noticed that the ortho-substituted aromatic aldehydes have lower yields compared to the meta- and para-substituted aromatic aldehydes. All the desired products 4a–p of this reaction within short reaction times give well to excellent yields under the optimized reaction conditions. The structural assignments of the isolated pure products 4a–p were characterized by FT-IR, 1H NMR, 13C NMR spectral data and is in harmony with literature.
The excellent performance of the prepared CESA catalyst in the synthesis of tetrahydrobenzo[b]pyran derivatives encouraged us to study its effect on the synthesis of biologically active 1,4-dihydropyridine derivatives 7a–n via one-pot four-component reaction of aryl aldehydes 1a–n (1 mmol), ethyl acetoacetate 5 (2 mmol), ammonium acetate 6 (1 mmol) under the optimized reaction conditions. Several derivatives of the 1, 4-dihydropyridine scaffolds have been prepared and the results are presented in Table 3. It was found that the CESA catalyst affords good to excellent yield of desired product in shorter reaction time.
The plausible mechanisms for the synthesis of tetrahydrobenzo[b]pyran derivatives catalyzed by CESA catalyst in a one-pot multi-component reaction of aromatic aldehyde, dimedone, and malononitrile in ethanol is illustrated in Scheme 2. As it is demonstrated, the CESA catalyst may activate both carbonyls of aldehyde and dimedone synergistically. Initially, the formation of benzylidinecyclohexanedione (1) intermediate by Knoevenagel condensation between aldehyde and dimedone was followed by the nucleophilic Michael addition of malononitrile to give the intermediate (2). Further cyclization yields an intermediate (3), which is then tautomerized to yield tetrahydrobenzo[b]pyran derivatives.
The most important point of these catalysts was their recyclability. The recyclability of the CESA catalyst was checked for the synthesis of tetrahydrobenzo[b]pyran (4b) using 4-chlorobenzaldehyde 1b under the optimized reaction conditions. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (4 X 20 mL) to isolate product 4b without column purification. The remaining aqueous phase of the CESA catalyst was oven-dried for three hours at temperature 120 °C and utilized for the next run under the same reaction conditions on model reaction. The results indicate that the CESA catalyst could be reused in a series of five consecutive runs without significant loss of the catalytic activity (Fig. 9). The heterogeneity of recovered CESA catalyst was determined by using flame AAS through a hot filtration test. Results reveals similar composition of detectable metals with EDS (Fig. 6) having K (38%), Ca (27%), Si (15%), Na (6%), Fe (5%), Mg (3%), Cl (2.9%), Mn (0.2%), Cu (0.012%), and Zn (0.0.5%).
Finally, to demonstrate the catalytic efficiency and capability of the present CESA catalyst to prepare the product of 4b and 7b, it has been compared with several previously reported catalysts in Table 4. Each of these reported methods has its own advantages, but some of them suffer from disadvantages such as the use of the expensive catalysts, toxic chemical, harsh reaction condition, long reaction time and poor yield of product. So the present method demonstrated that the use of a naturally sourced biodegradable catalyst, a green reaction medium, with a shorter reaction time, and reusability of catalyst, while a small quantity of this inexpensive and readily available catalyst is sufficient to obtain excellent yields of the desired product. Therefore the present CESA catalyst provides better catalytic activity and is superior in accelerating the reactions for the synthesis of the biologically active tetrahydrobenzo[b]pyrans and 1, 4-dihydropyridines and could be an alternative to other reported catalysts.
Conclusion
In conclusion, we have reported waste biomass coconut endocarp shell ash (CESA) catalyst as a readily available, green, environmentally benign, and energy-efficient natural catalyst for the synthesis of tetrahydrobenzo[b]pyran and 1, 4-dihydropyridine derivatives in ethanol at room temperature, which are often encountered in pharmacologically and biologically active compounds. The most important advantages of the present protocol are that it not only offers improvements in the reaction rates and obtains excellent desired yields, but also recyclability of the catalyst, avoids the use of corrosive catalysts and toxic reagents, and heavy metals. Further promising points of this present protocol include a clean, safe reaction profile, biodegradable, and highly efficient, low-cost obtained from natural renewable resources.
References
A. Yaghoubi, M.G. Dekamin, ChemistrySelect 2, 9236 (2017)
C. Altug, A.K. Burnett, E. Caner, Y. Durust, M.C. Elliott, R.P.J. Glanville, C. Guy, A.D. Westwell, Tetrahedron 67, 9522 (2011)
M. Nasr-Esfahani, T. Abdizadeh, J. Nanosci. Nanotechnol. 13, 5004 (2013)
S. Hatakeyama, N. Ochi, H. Numata, S. Takano, J. Chem. Soc. Chem. Commun. 17, 1202 (1988)
L.F. Tietze, Angew. Chem. Int. Ed. Engl. 22, 828 (1983)
A. Adili, Z.L. Tao, D.F. Chen, Z.Y. Han, Org. Biomol. Chem. 13(8), 2247 (2015)
S. Limsuwan, E.N. Trip, T.R.H.M. Kouwen, S. Piersma, A. Hiranrat, W. Mahabusarakam, S.P. Voravuthikunchai, J.M.V. Dijl, O. Kayser, Phytomedicine 16, 645 (2009)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
K. Görlitzer, A. Dehne, E. Engler, Arch. Pharm. 316, 264 (1983)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Chem. Lett. 19, 1139 (2009)
Z.Q. Xu, K. Pupek, W.J. Suling, L. Enache, M.T. Flavin, Bioorg. Med. Chem. 14, 4610 (2006)
M. Brunavs, C.P. Dell, P.T. Gallagher, W.M. Owton, C.W. Smith, Eur. Pat. Appl. EP 557075 A1 19930825 (1993)
M. Rueping, E. Sugiono, E. Merino, Chem. Eur. J. 14, 6329 (2008)
Y. Gao, W. Yang, D.M. Du, Tetrahedron Asymmetry 23(5), 339 (2012)
S.K. Kundu, J. Mondal, A. Bhaumik, Dalton Trans. 42, 10515 (2013)
Y. Dgachi, L. Ismaili, D. Knez, M. Benchekroun, H. Martin, N. Szałaj, S. Wehle, O.M. Bautista-Aguilera, V. Luzet, A. Bonnet, B. Malawska, S. Gobec, M. Chioua, M. Decker, F. Chabchoub, J. Marco-Contelles, ChemMedChem 11, 1318 (2016)
G.A. Reynolds, K.H. Drexhage, Opt. Commun. 13, 222 (1975)
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A. EI-Taweel, Heterocycles 26 903 (1987)
G. P. Ellis, in The Chemistry of Heterocyclic of Compounds. Chromenes, Harmones and Chromones; ed. By A. Weissberger, E.C. Taylor, (John Wiley: New York, 1977) 11
H. Langhals, Angew. Chem. Int. Ed. 43, 5290 (2004)
E.R. Bissell, A.R. Mitchell, R.E. Smith, J. Org. Chem. 45, 2283 (1980)
C.G. Knight, T. Stephens, Biochem. J. 258, 683 (1989)
M.G. Dekamin, M. Eslami, Green Chem. 16(12), 4914 (2014)
B. Karmakar, R. Nandi, Res. Chem. Intermed. 47, 2161 (2021)
S. Pradhan, V. Sahu, B.G. Mishra, J. Mol. Catal. A Chem. 425, 297 (2016)
D. Tahmassebi, J.A. Bryson, S.I. Binz, Synth. Commun. 41, 2701 (2011)
D.M. Pore, K.A. Undale, B.B. Dongare, U.V. Desai, Catal. Lett. 132, 104 (2009)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
S.N. Maddila, S. Maddila, W.E. Van-Zyl, S.B. Jonnalagadda, ChemistryOpen 5, 38 (2016)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
R. Ramesh, P. Vadivel, S. Maheswari, A. Lalitha, Res. Chem. Intermed. 42, 7625 (2016)
W.B. Sun, P. Zhang, J. Fan, S.H. Chen, Z.H. Zhang, Synth. Commun. 40, 587 (2010)
R. Rahnamafa, L. Moradi, M. Khoobi, Res. Chem. Intermed. 46(4), 2109 (2020)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
D. Azarifar, R. Nejat-Yami, F. Sameri, Z. Akrami, Lett. Org. Chem. 9, 435 (2012)
H. Zhi, C. Lu, Q. Zhang, J. Luo, Chem. Commun. 20, 2878 (2009)
V.U. Mane, S.M. Chavan, B.R. Choudhari, D.V. Mane, J. Pharm. Chem. Biol. Sci. 6(4), 311 (2019)
T. Tamoradi, B. Karmakar, M. Kamalzare, M. Bayat, A.T. Kal-Koshvndi, A. Maleki, J. Mol. Struct. 1219, 128598 (2020)
H. Malekim, J. Rakhtshah, B. Shaabani, Appl. Organomet. Chem. 34(8), e5683 (2020)
B. Loev, M.M. Goodman, K.M. Snader, R. Tedeschi, E. Macko, J. Med. Chem. 17, 956 (1974)
D.J. Triggle, Eur. J. Pharmacol. 375, 311 (1999)
R. Mannhold, B. Jablonka, W. Voigdt, K. Schonafinger, K. Schraven, Eur. J. Med. Chem. 27, 229 (1992)
S. Ghosh, F. Saikh, J. Das, A.K. Pramanik, Tetrahedron Lett. 54, 58 (2013)
R.S. Thombal, V.H. Jadhav, J. Chem. Applied Biochem. 2(1), 111 (2015)
A. Sakamoto, S.T. Ohnishi, R. Ogawa, J. Anaesth. 7(2), 193 (1993)
J.S. Yadav, B.V.S. Reddy, A.K. Basak, A.V. Narsaiah, Green Chem. 5, 60 (2003)
B. Sadeghi, A. Namakkoubi, A. Hassanabadi, J. Chem. Res. 37(1), 11 (2013)
X.Y. Wu, Synth. Commun. 42, 454 (2012)
K.L. Brigwood, E.G. Veitch, S.V. Ley, Org. Lett. 10(16), 3627 (2008)
A. Debache, W. Ghalem, R. Boulcina, A. Belfaitah, S. Rhouatiand, B. Carboni, Tetrahedron Lett. 50, 5248 (2009)
M. Safaiee, B. Ebrahimghasri, M.A. Zolfigol, S. Baghery, A. Khoshnood, D.A. Alonso, New J. Chem. 42, 12539 (2018)
M.A. Chari, K. Syamasundar, Catal. Commun. 6, 624 (2005)
M. Maheswara, V. Siddaiah, Y.K. Rao, Y.-M. Tzeng, C. Sridhar, J. Mol. Catal. A Chem. 260, 179 (2006)
G. Sabitha, G. Reddy, C.S. Reddy, J.S. Yadav, Tetrahedron Lett. 44, 4129 (2003)
S.D. Sharma, P. Hazarika, D. Konwar, Catal. Commun. 9, 709 (2008)
P. Sharma, M. Gupta, Green Chem. 2015(17), 1100 (2015)
S.T. Morbale, S.S. Shinde, S.D. Jadhav, M.B. Deshmukh, S.S. Patil, Der. Pharm. Lett. 7(12), 169 (2015)
A. Keyhani, F. Hatamjafari, Orient. J. Chem. 29(2), 783 (2013)
K. Venkateswarlu, Env. Chem. Lett. 19, 3887 (2021)
J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu, B. Han, Angew. Chem. Int. Ed. 2015(54), 9399 (2015)
M. Satheesh, M. Pugazhvadivu, B. Prabu, V. Gunasegaran, A. Manikandan, J. Nanosci. Nanotechnol. 19, 4123 (2019)
T.L. Ting, R.P. Jaya, N.A. Hassan, H. Yaacob, D.S. Jayanti, M.A.M. Ariffine, J. Teknologi. 78, 85 (2016)
A. Apasi, P.B. Madakson, D.S. Yawas, V.S. Aigbodion, Tribo in Industry 34, 36 (2012)
M.U. Patil, S.K. Shinde, S.P. Patil, S.S. Patil, Res. Chem. Intermed. 46, 4923 (2020)
S.K. Shinde, S.A. Damate, S.T. Morbale, M.U. Patil, S.S. Patil, RSC Adv. 7, 7315 (2017)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. sIntermed. 44(3), 1775 (2018)
A. Pawar, S. Gajare, A. Jagdale, S. Patil, W. Chandane, G. Rashinkar, S. Patil, Catal. Lett. 152, 1854 (2021)
Acknowledgements
The authors are grateful to the Indian Institute of Chemical Technology (IICT), Hyderabad and Common Facility Center (CFC), Shivaji University Kolhapur for spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.P., Shinde, S.K., Patil, M.U. et al. Coconut endocarp shell ash (CESA): a versatile and waste-originated catalyst for the synthesis of tetrahydrobenzo[b]pyrans and 1, 4-dihydropyridines. Res Chem Intermed 48, 3589–3612 (2022). https://doi.org/10.1007/s11164-022-04770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04770-1