Abstract
We have explored a number of protic ionic liquids (PILs) as a catalyst for the synthesis of biscoumarins by condensation of 4-hydroxycoumarin with an aromatic aldehyde. Methylimidazolium- and triethylammonium-based PILs were synthesized by simple neutralization reaction with protic acids. Triethylammonium hydrogen sulfate [Et3NH][HSO4] was found to be the best among the studied PILs concerning the yield of products and reaction time period. Different biscoumarin derivatives were synthesized based on 4-hydroxycoumarin and various substituted aromatic aldehydes at optimum reaction conditions. Obtained products were separated just by simple filtration. The facile method does not require additional purification for formed products. The catalyst has shown better yields along with outstanding recyclability, providing an environmental benign protocol for the synthesis of biscoumarin derivatives.
Graphical Abstract
Screening of simple protic ionic liquids as a catalyst in the synthesis of biscoumarins, out of which [Et3NH][HSO4] was found to be best among the studied PILs.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) have been successfully employed in a variety of reactions as environmentally benign catalysts and solvents; indeed, they provide the possibility for green manufacturing in the chemical industry [1]. They have attracted increasing interest in the context of green organic syntheses, and in fact were initially introduced as alternative green reaction media because of their unique chemical and physical properties, such as non-volatility, non-flammability, thermal stability and controlled miscibility, as well as excellent recyclability, showing their significant role in monitoring reactions as solvent or catalyst in both homogeneous and heterogeneous systems [1–5]. Brønsted or Lewis acidic ionic liquids have been used extensively for organic transformations as environmentally friendly catalysts [6]. Brønsted acidic or protic ionic liquids (PILs), a class of ionic liquids, are easily produced through the combination of a Brønsted acid and Brønsted base. PILs encompass a proton available for hydrogen bonding which differentiate them from aprotic ILs [7]. PILs having the useful characteristics of solid acids and mineral liquid acids, and are designed to replace traditional mineral liquid acids such as sulfuric acid and hydrochloric acid in chemical procedures; in fact, they have potential as dual solvent–catalysts in organic reactions [8–10].
The acid strength of PILs is an important factor for their use as catalysts in organic transformations. The structure of the cations and anions as well as their combination decides the Brønsted acidity of a PIL. Such acidic character makes them especially different from other ILs and enhances their applicability as selective catalysts in organic synthesis. PILs are of special importance because they simultaneously possess proton acidity and the characteristic properties of ionic liquids [11]. Various methods have been used to measure the acidity of PILs including the determination of basicity of associated anions, the proton NMR technique [12, 13], the Hammett acidity indicator with UV–visible spectroscopy [14] or titration against alkalis [15]. As the acid strength of a PIL is strongly correlated to its catalytic activity, it greatly affects the product formation. For instance, in the case of Friedel–Crafts alkylation of phenol with tert-butyl alcohol, a weak acid catalyst gave 2-tert-butylphenol, a medium acid catalyst providing 4-tert-butylphenol, while in the presence of a strong acid catalyst, 2,4-di-tert-butylphenol is formed. Accordingly, Duan et al. have tested a series of pyridinium-based PILs as catalysts for this alkylation. On the basis of acidity, the selectivity in the reaction varies, as an IL with bis(trifluoromethylsulfonyl)imide anion showed a strong acidic nature, whereas an IL with methanesulfonate demonstrated weak acidity [16]. Similarly, for the same reaction, a series of SO3H functionalized imidazolium PILs along with [HSO4] anions and having moderate acidity exhibited almost similar selectivities [17]. In another example, in the Saucy–Marbet reaction, the PIL [Et3NH][HSO4] furnished the best results among the five studied PILs incorporated with [HSO4] anions. Also, due to their lipohilic nature, the conversion efficiency and the selectivity of the PILs was seen to be decreased as the alkyl chain length in the IL moiety increased [18].
Due to their specific, selective and excellent catalytic performances, PILs have been employed in numerous organic transformations, a few of them to be listed as, Knoevenagel [19, 20] and Pechmann [21] and in other condensation reactions [22, 23], the Diels–Alder reaction [24], the multicomponent Hantzsch reaction [25], the Mannich reaction [26], esterification [27], the Biginelli reaction [28], the Beckmann rearrangement [29], etc.
Biscoumarins are vital organic compounds which have received considerable attention in synthetic and medicinal chemistry because of their broad spectrum of biological activities as well as interesting potential in therapeutic applications. They often possess interesting pharmacological properties such as antitumor and antibacterial [30–32], anticancer [33], urease and α-glucosidase inhibitors [34, 35], antifungal [36], antiproliferative activities [37], etc. 3,3′-Arylmethylene-bis-4-hydroxycoumarins, commonly known as biscoumarins, are usually synthesized by condensation of 4-hydroxycoumarin with various substituted aldehydes [38]. Several attempts have been made in the synthesis of biscoumarins with different catalysts and media such as molecular iodine [39], metal salts as homogeneous catalysts [40, 41], heterogeneous solid acid catalysts [42–44], nanocomposites and nanoparticles [45, 46], and bio-supported acidic catalysts [47], while in a few cases mineral acids like H2SO4 have been engaged [48]. However, many reported methods are associated with inherent disadvantages like harsh reaction conditions, prolonged reaction times, and expensive and less reusable catalysts, as well as time-consuming work-up procedures. To overcome such circumstances and to obtain a more advantageous method, a new environmentally benign protocol needs to be developed. Concerning green methodology, ionic liquids are one of the best choices as catalysts. Many researchers have explored a variety of ILs in the synthesis of biscoumarin derivatives, for example, 1-butyl-3-methylimidazolium bromide, [bmim][Br] [49], polymeric IL, poly(4-vinylpyridine-co-1-sulfonic acid butyl-4-vinylpyridinium) hydrogen sulfate [50], benzimidazolium-based ILs [51], N-hydroxyethylammonium formate/acetate [52], [pyridine–SO3H][Cl] [53], etc.
Ionic liquids have emerged as strong alternatives for conventional catalytic systems in synthetic chemistry. In the synthesis of biscoumarins, the environmentally benign properties of ILs, such as non-toxic, easy to handle, negligible vapor pressure, high thermal stability, etc., have made them able to replace the traditional catalysts like mineral acids, heterogeneous or supported acid catalysts, metal salts, etc. Reusability of catalysts is the most vital part in any synthesis. After a reaction, ILs can be easily separated and reused for five to six more reaction cycles without significant loss in catalytic activity, thus emphasizing their cost-effectiveness in biscoumarin synthesis [51]. ILs can also reduce costs by avoiding the use of expensive metal salts like ruthenium chloride [41]. ILs are more advantageous materials for modifying inconvenient operating conditions; for instance, [pyridine–SO3H][Cl] [53] and [MIM(CH2)4SO3H][HSO4] [54] have reduced the reaction time up to 10–12 min, and the reaction temperature has been possibly minimized almost to room temperature instead of high refluxing temperatures [52]; also, the yield of biscoumarins can be increased above 90% by using minimum amounts of catalyst (10–20 mol%) along with easy work-up procedures in the synthesis of biscoumarins [49–51]. However, comparatively few reports are found that describe ILs as effective catalysts for the synthesis of biscoumarins.
Taking into account the requirements in the synthesis of biscoumarins, we have developed a new straightforward protocol by using simple protic ionic liquids (PILs) as cost-effective catalysts. Ten numbers of PILs based on 1-methylimidazolium and triethylammonium cations were synthesized and tested in the synthesis of biscoumarin derivatives. The reaction conditions including the reflux temperature, catalyst loading, and time span for reaction were optimized; also, the recyclability of catalyst was investigated.
Our aim was to develop a simple and cost-effective protocol for the synthesis of biscoumarins. The imidazolium- and triethylammonium-based PIL catalysts studied in this work are easily prepared by one-step neat neutralization reactions with easily available mineral acids like H2SO4, HCl, HNO3, H3PO4 and CH3COOH. We are proposing a facile synthetic method for biscoumarins by using these PILs as catalysts under mild reaction conditions.
Experimental
Materials and equipments
All chemicals were purchased from commercial sources and used without any further purification. Melting points were recorded on a Buchi B-545 apparatus in open capillary tubes and are uncorrected. IR spectra were recorded on a Perkin-Elmer FT-IR-1600 spectrophotometer. 1HNMR and 13C NMR spectra were recorded on a Bruker Avance (300 and 75 MHz, respectively) spectrometer. Mass spectra were recorded on a Shimadzu QP2010 GCMS.
Syntheses of PILs
PILs were synthesized by simple neutralization reactions (Scheme 1). To 1-methylimidazole was slowly added an equimolar amount of an acid, i.e. H2SO4, HCl, HNO3, H3PO4 or CH3COOH under ice-cold conditions. The reaction mixture was stirred for an appropriate time to form the corresponding ionic liquids (Table 1, entries 2–6). The formed ILs were washed with ethyl acetate (3 × 10 mL) followed by n-hexane, and dried in vacuum under reduced pressure. A similar methodology was used for the synthesis of the respective triethylammonium-based PILs (Table 1, entries 7–11).
General procedure for synthesis of biscoumarins
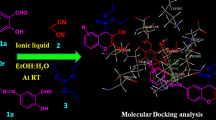
A typical procedure involves, in a round-bottom flask equipped with a reflux condenser, in which a mixture of 4-hydroxycoumarin (2 mmol), arylaldehyde (1 mmol), PIL catalyst (10 mol%) and 5 mL of ethanol was stirred at 80 °C. The reaction was monitored by TLC. After completion of the reaction, a solid product is obtained which is filtered and recrystallized with ethanol (Scheme 2).
Spectral data of ionic liquids
1. Triethylammonium hydrogen sulfate [Et 3 NH][HSO 4 ] (Entry 7, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.15–1.19 (t, 9H), 3.04–3.12 (m, 6H), 8.98 (s, 1H);
13CNMR (75 MHz, DMSO d6): δ (ppm) 8.88, 46.40.
2. 3-Methylimidazolium hydrogen sulfate [MIM][HSO 4 ] (Entry 2, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 3.77 (s, 3H), 4.35 (s, 1H), 7.32 (s, 1H), 7.42 (s, 1H), 8.43 (s, 1H).
3. 3-Methylimidazolium chloride [MIM][Cl] (Entry 3, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 3.88 (s, 3H), 5.45 (s, 1H), 7.55–7.56 (t, 1H), 7.63 (d, 1H), 9.02 (s, 1H).
4. 3-Methylimidazolium nitrate [MIM][NO 3 ] (Entry 4, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 3.88 (s, 3H), 5.45 (s, 1H), 7.55 (d, 1H), 7.63 (s, 1H), 9.02 (s, 1H).
5. 3-Methylimidazolium dihydrogen phosphate [MIM][H 2 PO 4 ] (Entry 5, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 3.72 (s, 3H), 4.54 (s, 1H), 7.07 (s, 1H), 7.20 (s, 1H), 8.00 (s, 1H).
6. 3-Methylimidazolium acetate [MIM][CH 3 COO] (Entry 6, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.89 (s, 3H), 3.64 (s, 3H), 5.16 (s, 1H), 6.88 (s, 1H), 7.01 (s, 1H), 7.54 (s, 1H).
7. Triethylammonium chloride [Et 3 NH][Cl] (Entry 8, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.21–1.26 (t, 9H), 3.03–3.10 (m, 6H), 10.38 (s, 1H).
8. Triethylammonium nitrate [Et 3 NH][NO 3 ] (Entry 9, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.21–1.26 (t, 9H), 3.03–3.10 (m, 6H), 10.38 (s, 1H).
9. Triethylammonium dihydrogen phosphate [Et 3 NH][H 2 PO 4 ] (Entry 10, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.20 (s, 9H), 3.00 (s, 6H), 9.85 (s, 1H).
10. Triethylammonium acetate [Et 3 NH][CH 3 COO] (Entry 11, Table 1 )
1HNMR (300 MHz, DMSO d6): δ (ppm) 1.12 (s, 3H), 1.87 (s, 9H), 2.91–2.93 (d, 6H), 9.98 (s, 1H).
Spectral data of selected biscoumarins
1. 3,3′-(phenylmethylene)bis(4-hydroxy-2H-1-benzopyran-2-one) (Entry 1, Table 3 )
White solid, IR (KBr, cm−1) data: 3417, 3069, 2924, 2739, 1659, 1615, 1568, 1496, 1346, 1308, 756; 1HNMR (300 MHz, DMSO d6): δ (ppm) 6.21 (s, 1H), 7.02–7.07 (t, 2H, J = 15 Hz), 7.09–7.12 (d, 1H, J = 9 Hz), 7.15–7.20 (t, 2H, J = 15 Hz), 7.25–7.30 (t, 4H, J = 15 Hz), 7.51–7.57 (m, 2H), 7.80–7.82 (t, 2H, J = 6 Hz), 13CNMR (75 MHz, DMSO d6): δ (ppm) 36.47, 104.34, 118.99, 123.98, 124.43, 125.82, 127.12, 128.43, 132.10, 141.10, 152.74, 165.26, 166.57; Anal. Calcd. C, 72.81, H 3.91; Found C, 72.67, H 3.98; GC–MS, m/z = 412.
2. 3,3′-(4-methoxyphenylmethylene)bis(4-hydroxy-2H-1-benzopyran-2-one) (Entry 2, Table 3 )
White solid, IR (KBr, cm−1) data: 3372, 2938, 2728, 1667, 1605, 1569, 1510, 1356, 1306, 1258, 1219, 1093, 769; 1HNMR (300 MHz, DMSO d6): δ (ppm) 3.68 (s, 3H), 6.24 (s, 1H), 6.75–6.78 (d, 2H, J = 9 Hz), 7.00–7.03 (d, 2H, J = 9 Hz), 7.26–7.33 (m, 4H), 7.53–7.58 (q, 2H), 7.84–7.87 (t, 2H, J = 9 Hz); 13CNMR (75 MHz, DMSO d6): δ (ppm) 35.72, 55.40, 104.64, 113.68, 116.29, 118.84, 124.04, 124.38, 128.16, 132.14, 132.54, 152.68, 157.66, 165.26, 166.27; Anal. Calcd. C, 70.58, H 4.10; Found C, 71.57, H 3.93; GC–MS, m/z = 442.
3. 3,3′-[(1H-imidazol-2-yl)methylene]bis(4-hydroxy-2H-1-benzopyran-2-one) (Entry 6, Table 3 )
White solid, IR (KBr, cm−1) data: 3358, 3110, 2918, 2684, 1671, 1610, 1556, 1498, 1405, 1276, 1186, 1049, 760; 1HNMR (300 MHz, DMSO d6): δ (ppm) 6.53–6.54 (d, 1H, J = 3 Hz), 7.15 (s, 1H), 7.18 (s, 2H), 7.20 (s, 2H), 7.43–7.46 (d, 2H, J = 9 Hz), 7.84–7.87 (t, 2H, J = 9 Hz), 13.58 (s, 1H); 13CNMR (75 MHz, DMSO d6): δ (ppm) 30.93, 57.00, 99.65, 115.90, 118.47, 120.12, 123.24, 124.79, 131.67, 149.38, 153.18, 164.71, 169.69; Anal. Calcd. C, 65.67, H 3.51, N 6.96; Found C, 65.85, H 3.69, N 6.58; GC–MS, m/z = 402.
4. 3,3′-[(2H-1,3-benzodioxol-5-yl)methylene]bis(4-hydroxy-2H-1-benzopyran-2-one) (Entry 7, Table 3 )
White solid, IR (KBr, cm−1) data: 3428, 3075, 2898, 2725, 2605, 1662, 1615, 1568, 1504, 1488, 1436, 1345, 1309, 1234, 1098, 1040, 763; 1HNMR (300 MHz, DMSO d6): δ (ppm) 5.90 (s, 2H), 6.29 (s, 1H), 6.59–6.70 (m, 3H), 7.30–7.32 (d, 4H, J = 6 Hz), 7.53–7.58 (t, 2H, J = 15 Hz), 7.92–7.94 (d, 2H, J = 6 Hz); 13CNMR (75 MHz, DMSO d6): δ (ppm) 35.87, 101.13, 104.93, 107.64, 108.13, 116.42, 117.14, 119.73, 124.23, 124.44, 131.91, 132.59, 146.06, 147.98, 152.44, 164.56, 165.89; Anal. Calcd. C, 68.42, H 3.53; Found C, 68.88, H 3.26; GC–MS, m/z = 456.
5. 3,3′-[(4-nitrophenyl)methylene]bis(4-hydroxy-2H-1-benzopyran-2-one) (Entry 9, Table 3 )
Yellow solid, IR (KBr, cm−1) data: 3417, 3058, 2933, 2717, 2608, 1655, 1608, 1564, 1522, 1491, 1350, 1311, 1215, 1181, 1098, 773; 1HNMR (300 MHz, DMSO d6): δ (ppm) 6.50 (s, 1H), 7.27–7.32 (m, 4H, J = 9 Hz, J = 3 Hz), 7.36–7.39 (d, 2H, J = 9 Hz), 7.54–7.59 (t, 2H, J = 15 Hz), 7.94–7.97 (dd, 2H), 8.05–8.08 (d, 2H, J = 9 Hz); 13CNMR (75 MHz, DMSO d6): δ (ppm) 36.35, 104.04, 116.49, 116.77, 123.61, 124.34, 124.64, 127.92, 132.91, 146.18, 146.46, 152.45, 165.13, 166.36; Anal. Calcd. C 65.65, H 3.31, N 3.06; Found C 65.37, H 3.35, N 2.82; GC–MS, m/z = 457.
Results and discussion
A series of methylimidazolium- and triethylammonium-based simple protic ionic liquids have been synthesized and their potential as a catalyst in the synthesis of biscoumarin derivatives was studied. A condensation reaction between 4-hydroxycoumarin and an aromatic aldehyde leads to the formation of biscoumarin in the presence of the PIL as catalyst and ethanol as solvent (Scheme 2).
Synthesized PILs were screened as catalysts in a model reaction to select the best one among them. In a model reaction, benzaldehyde was used to synthesize the biscoumarin derivative with 4-hydroxycoumarin. On the basis of the results summarized in Table 1, [Et3NH][HSO4] and [MIM][HSO4] have been found to be superior catalysts concerning the yield of the product and the time required for completion of the reaction. Also, this suggests that HSO4 − is a better anion for PILs due to its higher acidity among the other anions pertaining to the catalytic activities. It was found that PILs based on [Et3NH] cation had a comparatively better catalytic performance. Thus, [Et3NH][HSO4] was chosen as the catalyst for further optimization of the reaction conditions and syntheses of biscoumarins. Additionally, [Et3NH][HSO4] is more beneficial from the economical and accessibility point of view, as well as being stable both in air and water [55].
The reaction temperature is the crucial factor in the succession of the reaction as it significantly affects the rate of the reaction. To find out the optimum reflux temperature, the reaction was carried out at different temperatures (Table 2). It was found that, at room temperature, for a prolonged time it generated a negligible yield, while increases in temperature improved the product yield. The maximum product formation was found at 80 and 100 °C, and therefore the reflux temperature was fixed at 80 °C in the synthesis of biscoumarins. The optimum loading of the catalyst for completion of the reaction was demonstrated with different amounts of PIL, i.e. 5, 10, 20, 30, 40 and 50 mol% (Table 2). However, higher amounts of PIL did not show any improvement in the yield of the product, and 10 mol% of catalyst was sufficient to promote the reaction. Therefore, further syntheses of biscoumarins from various aromatic aldehydes were carried at 80 °C in the presence of 10 mol% of [Et3NH][HSO4] and ethanol as solvent.
Under optimized conditions, [Et3NH][HSO4] was explored as the catalyst in the preparation of different biscoumarin derivatives from various aromatic aldehydes. A series of arylaldehydes containing both electron-donating and -withdrawing substituents rendered good to excellent yields (Table 3). It can be seen that aldehydes comprising electron-donating substituents provide relatively better yields within shorter reaction times. The formation of the products was confirmed by comparing their melting points with literature values, while the structures of the selected biscoumarin derivatives were established by IR, 1HNMR, 13CNMR and GC–MS spectra. All the characterizations and interpretations are in good agreement with the expected structures of the biscoumarins.
In the view of green chemistry, the reusability of the catalyst is a vital part in its application. After completion of the reaction, the product can be easily separated just by filtration, and the filtrate containing the PIL was evaporated to regain the catalyst. The obtained PIL was again washed with ethyl acetate followed by n-hexane and dried in vacuum. The rejuvenated catalyst was again employed in the model reaction to test its consistency in performance. Astoundingly, [Et3NH][HSO4] showed efficient catalytic activity for up to six cycles without a significant decrease in product yield (Fig. 1).
The plausible reaction mechanism in the synthesis of biscoumarin involves the Knoevenagel condensation followed by a Michael addition. An aldehyde is initially activated by the catalyst through hydrogen bonding, which facilitates the nucleophilic attack of 4-hydroxycoumarin followed by the loss of H2O to form an intermediate. Further, this intermediate is activated again by the PIL catalyst and, due to the nucleophilic attack of the second molecule of 4-hydroxycoumarin, a Michael addition product that is biscoumarin is formed [47, 56] (Scheme 3).
Conclusion
In conclusion, we have developed an efficient and environmentally benign synthesis route for biscoumarin derivatives in the presence of a simple protic ionic liquid, [Et3NH][HSO4]. The synthesized inexpensive PIL catalyst provides a green protocol through good to excellent yields, moderate reaction time, and easy work-up procedure as well as exceptional recyclability. Hence, our proposed method offers a better alternative to existing methods. However, based on this study, we are proceeding to develop a more facile biscoumarin synthesis protocol by using ionic liquids, which can optimistically alter the reaction operating conditions such as reaction time, reflux temperature or reusability of the catalyst.
Supporting information
Electronic supplementary material contents 1HNMR, 13CNMR spectra of [Et3NH][HSO4]; 1HNMR, 13CNMR and GC–MS spectra of selected biscoumarin derivatives.
References
N.V. Plechkova, K.R. Seddon, Chem. Soc. Rev. 37, 123 (2008)
T. Welton, Chem. Rev. 99, 2071 (1999)
S. Zhu, R. Chen, Y. Wu, Q. Chen, X. Zhang, Z. Yu, Chem. Biochem. Eng. Q. 23, 207 (2009)
R. Sheldon, Chem. Commun. 2399 (2001)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, N. Zanatta, H.G. Bonacorso, Chem. Rev. 108, 2015 (2008)
A.R. Hajipour, F. Rafiee, Org. Prep. Proced. Int. 42, 285 (2010)
T.L. Greaves, C.J. Drummond, Chem. Rev. 108, 206 (2008)
P.P. Salvi, A.M. Mandhare, A.S. Sartape, D.K. Pawar, S.H. Han, S.S. Kolekar, C. R. Chim. 14, 883 (2011)
M. Picquet, I. Tkatchenko, I. Tommasi, P. Wasserscheid, J. Zimmermann, Adv. Synth. Catal. 345, 959 (2003)
K.E. Johnson, R.M. Pagni, J. Bartmess, Monatsh. Chem. 138, 1077 (2007)
B. Tamami, A. Sardarian, E. Ataollahi, Turk. J. Chem. 40, 422 (2016)
S.A. Siddiqui, T.M. Potewar, R.J. Lahoti, K.V. Srinivasan, Synthesis, 2849 (2006)
S.S. Palimkar, S.A. Siddiqui, T. Daniel, R.J. Lahoti, K.V. Srinivasan, J. Org. Chem. 68, 9371 (2003)
Z. Du, Z. Li, S. Guo, J. Zhang, L. Zhu, Y. Deng, J. Phys. Chem. B 109, 19542 (2005)
Z. Fei, D. Zhao, T.J. Geldbach, R. Scopelliti, P.J. Dyson, Chem. Eur. J. 10, 4886 (2004)
Z. Duan, Y. Gu, J. Zhang, L. Zhu, Y. Deng, J. Mol. Catal. A: Chem. 250, 163 (2006)
J. Gui, H. Ban, X. Cong, X. Zhang, Z. Hu, Z. Sun, J. Mol. Catal. A: Chem. 225, 27 (2005)
C. Wang, W. Zhao, H. Li, L. Guo, Green Chem. 11, 843 (2009)
A.G. Ying, H.D. Liang, R.H. Zheng, C.H. Ge, H.J. Jiang, C.L. Wu, Res. Chem. Intermed. 37, 579 (2011)
R.V. Hangarge, D.V. Jarikote, M.S. Shingare, Green Chem. 4, 266 (2002)
S. Rezayati, F. Sheikholeslami-Farahani, F. Rostami-Charati, S.A.S. Abad, Res. Chem. Intermed. 42, 4097 (2016)
G. Karthikeyan, P.T. Perumal, Can. J. Chem. 83, 1746 (2005)
Z.Y. Yu, Q.S. Fang, J. Zhou, Z.B. Song, Res. Chem. Intermed. 42, 2035 (2016)
E. Janus, I. Goc-Maciejewska, M. Łożyński, J. Pernak, Tetrahedron Lett. 47, 4079 (2006)
D. Patil, D. Chandam, A. Mulik, P. Patil, S. Jagadale, R. Kant, V. Gupta, M. Deshmukh, Catal. Lett. 144, 949 (2014)
G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang, D. Sun, Green Chem. 6, 75 (2004)
H.-P. Zhu, F. Yang, J. Tang, M.-Y. He, Green Chem. 5, 38 (2003)
R. Zheng, X. Wang, H. Xu, J. Du, Synth. Commun. 36, 1503 (2006)
S. Guo, Z. Du, S. Zhang, D. Li, Z. Li, Y. Deng, Green Chem. 8, 296 (2006)
J. Li, Y.-P. Sui, J.-J. Xin, X.-L. Du, J.-T. Li, H.-R. Huo, H. Ma, W.-H. Wang, H.-Y. Zhou, H.-D. Zhan, Z.-J. Wang, C. Li, F. Sui, X. Li, Bioorg. Med. Chem. Lett. 25, 5520 (2015)
J. Li, Z. Hou, F. Li, Z.-D. Zhang, Y. Zhou, X.-X. Luo, M.-K. Li, J. Mol. Struct. 1075, 509 (2014)
Y.-P. Sui, H.-R. Huo, J.-J. Xin, J. Li, X.-J. Li, X.-L. Du, H. Ma, H.-Y. Zhou, H.-D. Zhan, Z.-J. Wang, C. Li, F. Sui, M.-K. Li, Molecules 20, 17614 (2015)
J.-J. Xin, J. Li, Z.-D. Zhang, X.-B. Hu, M.-K. Li, J. Mol. Struct. 1084, 200 (2015)
K.M. Khan, S. Iqbal, M.A. Lodhi, G.M. Maharvi, Z. Ullah, M.I. Choudhary, A. Rahman, S. Perveen, Bioorg. Med. Chem. 12, 1963 (2004)
K.M. Khan, F. Rahim, A. Wadood, N. Kosar, M. Taha, S. Lalani, A. Khan, M.I. Fakhri, M. Junaid, W. Rehman, M. Khan, S. Perveen, M. Sajid, M.I. Choudhary, Eur. J. Med. Chem. 81, 245 (2014)
Z.N. Siddiqui, M.T.N. Musthafa, A. Ahmad, A.U. Khan, Arch. Pharm. 344, 394 (2011)
I. Kostova, G. Momekov, Eur. J. Med. Chem. 41, 717 (2006)
A. Das Gupta, S. Samanta, R. Mondal, A.K. Mallik, Bull. Korean Chem. Soc. 33, 4239 (2012)
M. Kidwi, V. Bansal, P. Mothsra, S. Saxena, R.K. Somvanshi, S. Dey, T.P. Singh, J. Mol. Catal. A: Chem. 268, 76 (2007)
J.N. Sangshetti, N.D. Kokare, D.B. Shinde, Green Chem. Lett. Rev. 2, 233 (2009)
K. Tabatabaeian, H. Heidari, A. Khorshidi, M. Mamaghani, N.O. Mahmoodi, J. Serb. Chem. Soc. 77, 407 (2012)
F. Shirini, M. Abedini, S.A. Kiaroudi, Phosphorus, Sulfur Silicon Relat. Elem. 189, 1279 (2014)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 4343 (2012)
A.R. Kiasat, L. Hemat-Alian, Res. Chem. Intermed. 41, 873 (2015)
J. Albadi, A. Mansournezhad, S. Salehnasab, Res. Chem. Intermed. 41, 5713 (2015)
M. Nikpassand, L.Z. Fekri, L. Karimian, M. Rassa, Curr. Org. Synth. 12, 358 (2015)
R. Rezaei, M.R. Sheikhi, Res. Chem. Intermed. 41, 1283 (2015)
J.M. Khurana, A. Lumb, A. Chaudhary, B. Nand, J. Heterocycl. Chem. 51, 1747 (2014)
J.M. Khurana, S. Kumar, Monatsh. Chem. 141, 561 (2010)
K.P. Boroujeni, P. Ghasemi, Z. Rafienia, Monatsh. Chem. 145, 1023 (2014)
W. Li, Y. Wang, Z. Wang, L. Dai, Y. Wang, Catal. Lett. 141, 1651 (2011)
A. Tzani, A. Douka, A. Papadopoulos, E.A. Pavlatou, E. Voutsas, A. Detsi, ACS Sustain. Chem. Eng. 1, 1180 (2013)
M.A. Zolfigol, A.R. Moosavi-Zare, M. Zarei, C. R. Chim. 17, 1264 (2014)
N. Tavakoli-Hoseini, M.M. Heravi, F.F. Bamoharram, A. Davoodnia, M. Ghassemzadeh, J. Mol. Liq. 163, 122 (2011)
Z.N. Siddiqui, K. Khan, ACS Sustain. Chem. Eng. 2, 1187 (2014)
R. Rezaei, F. Moezzi, M.M. Doroodmand, Chin. Chem. Lett. 25, 183 (2014)
Acknowledgements
Authors SKP and MMV under UGC-BSR Meritorious Students fellowship and SCB under RGNF are grateful to the University Grants Commission (UGC), India, for financial support and DST-FIST, New Delhi, India, for instrument facilities at the Department of Chemistry, Shivaji University, Kolhapur.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.K., Awale, D.V., Vadiyar, M.M. et al. Simple protic ionic liquid [Et3NH][HSO4] as a proficient catalyst for facile synthesis of biscoumarins. Res Chem Intermed 43, 5365–5376 (2017). https://doi.org/10.1007/s11164-017-2932-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2932-5