Abstract
Starch–sulfuric acid-catalyzed, simple, one pot, solvent-free, environmentally benign synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives has been achieved by reaction of aromatic aldehydes with 4-hydroxycoumarin. The catalyst is reusable and has remarkable activity. This procedure has several advantages including high yields, short reaction times, easy work-up, and use of an inexpensive, moderately acidic, safe catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coumarin derivatives have important pharmacological properties, for example anticoagulant, insecticidal, antihelminthic, hypnotic, antifungal, phytoalexin, and HIV protease inhibition [1–3]. The minimum active pharmacophore is a coumarin dimer containing an aryl substituent on the central linker methylene [4]. Addition of 4-hydroxy and 7-hydroxy substituents to the coumarin rings improves the potency of the compounds [3]. In addition, the coordination ability of the biscoumarins as ligands has been proved in complexation with lanthanum(III) ions, so this class of complexes has cytotoxic activity [5]. The most direct procedure for preparation of bis-coumarins is condensation of an aldehyde with 4-hydroxycoumarin in the presence of a variety of catalysts, for example molecular iodine [6], 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) [7], MnCl2 [8], POCl3 in dry dimethylformamide (DMF) [9], Et2AlCl3 [10], SO3H-functionalized ionic liquids [11], [bmim][BF4] [12], tetrabutylammonium bromide (TBAB) [13], Zn(Proline)2 [14], sodium dodecyl sulfate (SDS) [15], triethylbenzylammonium chloride (TEBA) [16], phosphotungstic acid [17], H14[NaP5W30O110])–SiO2 [18], tris(hydrogensulfato)boron [19], sulfated titania [20], and ethanol or acetic acid under reflux [21]. The reaction has also been conducted under thermal solvent-free microwave conditions [22].
Green chemistry emphasizes the development of environmentally benign chemical processes and technology [23, 24]. Recently, the direction of science and technology has been shifting toward more eco-friendly, natural product resources and reusable catalysts. Natural biopolymers are attractive candidates in the search for solid support catalysts [25, 26]. It was therefore thought worthwhile to develop a new and mild method using an inexpensive biopolymer-based catalyst that can be easily separated, reused, and is not contaminated by the products.
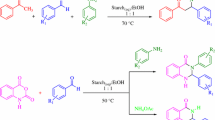
Herein, we report an efficient one-pot synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives by reaction of a variety of aldehydes with 4-hydroxycoumarin under solvent-free conditions in the presence of starch–sulfuric acid (Scheme 1).
Experimental
General
All chemicals and analytical-grade solvents were purchased from Merck or Fluka. Melting points of all products were determined in open glass capillaries on a Mettler 9100 melting point apparatus. Infrared (IR) spectra were recorded by use of a 4300 Shimadzu FT-IR spectrometer. 1H NMR spectra were recorded on a Bruker 400-MHz spectrometer. Elemental analysis was performed on a Heraeus CHN Rapid analyzer. Most of the products were characterized by comparison of their melting points and IR and 1H NMR spectra with those of authentic samples.
Preparation of starch–sulfuric acid
Chlorosulfonic acid (1.0 g, 9 mmol) was added dropwise, at 0 °C, over a period of 2 h, to a magnetically stirred mixture of 5.0 g starch in 20 ml n-hexane. HCl gas was removed from the reaction vessel immediately. When addition was complete, the mixture was stirred for 2 h. The mixture was then filtered and washed with 30 ml acetonitrile and dried at room temperature to afford 5.25 g of starch–sulfuric acid as a white powder. The sulfur content of starch–sulfuric acid samples by conventional elemental analysis was 0.55 mmol/g. The number of H+ sites on the starch–SO3H, determined by acid–base titration, was 0.50 meq/g. This value corresponds to approximately 90 % of the sulfur content, indicating that most of the sulfur species on the sample are in the form of the sulfonic acid groups.
Typical experimental procedure for synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives
A mixture of 4-hydroxycoumarin (2 mmol), aldehyde (1 mmol), and starch–sulfuric acid (0.1 g) was ground and heated to 80 °C for the appropriate time (Table 2). After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature and extracted with EtOAc (2 × 5 mL). The solution was concentrated and the crude product was recrystallized from ethanol yielding the pure biscoumarin. For reaction of formaldehyde, which needed water as solvent, the components mentioned above were mixed in 5 mL of water and stirred magnetically at 80 °C for 15 min. After completion of the reaction, the reaction mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. The pure product was obtained by crystallization from ethanol. Most of the biscoumarin derivatives are well known in the literature and were identified by comparison of their physical and spectral data.
Spectral analysis of compounds (3a–m, 4)
Compound 3a
Yellow crystals, IR (KBr): 3,425, 3,030, 1,660, 1,610, 761 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.50 (1H, s, CH), 7.22–8.25 (13H, m, 13 × CH); 11.33 (1H, s, OH), 11.57 (1H, s, OH); Anal. Calcd for C25H16O6 (411.78): C, 72.81; H, 3.91. Found: C, 72.69; H, 3.83.
Compound 3b
White crystals, lR (KBr): 3,427, 3,030, 1,670, 1,605, 1,094, 765 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.40 (1H, s, CH), 7.32–8.40 (12H, m, 12 × CH); 11.33 (1H, s, OH), 11.57 (1H, s, OH); Anal. Calcd for C25H15ClO6 (445.68): C, 67.20; H, 3.38. Found: C, 67.0; H, 3.30.
Compound 3c
Yellow crystals, lR (KBr): 3,435, 2,925, 1,651, 1,618, 1,060, 762 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.14 (1H, s, CH), 7.24–8.03 (12H, m, 12 × CH); 10.92 (H, s, OH), 11.63 (H, s, OH), Anal. Calcd for C25H15ClO6 (445.68): C, 67.20; H, 3.38. Found: C, 67.15; H, 3.35.
Compound 3d
Yellow crystals, IR (KBr): 3,450, 3,035, 1,657, 1,615, 1,348, 760 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.63 (1H, s, CH), 7.42–8.35 (12H, m, 12 × CH); 11.44 (1H, s, OH), 11.57 (1H, s, OH); Anal. Calcd for C25H15NO8 (458.43): C, 65.65; H, 3.31; N, 3.06. Found: C, 65.78; H, 3.34; N, 2.83.
Compound 3e
Yellow crystals, lR (KBr): 3,420, 2,926, 1,656, 1,616, 1,335, 765 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.13 (1H, s, CH), 7.26–8.17 (12H, m, 12 × CH); 11.58 (2H, s, 2 × OH), Anal. Calcd for C25H15NO8 (458.43): C, 65.65; H, 3.31; N, 3.06. Found: C, 65.69; H, 3.30, N, 2.90.
Compound 3f
White crystals, IR (KBr): 3,386, 3,035, 1,668, 1,607, 1,258, 769 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.72 (3H, s, OCH3), 6.45 (1H, s, CH), 7.12–8.18 (12H, m, 12 × CH), 11.34 (1H, s, OH), 11.52 (1H, s, OH); Anal. Calcd for C26H18O7 (443.56): C, 70.58; H, 4.10. Found: C, 70.75; H, 3.96.
Compound 3g
White crystals, IR (KBr): 3,410, 3,075, 1,670, 1,614, 1,350, 764 cm−1; 1H NMR (400 MHz, CDCl3): δ 2.36 (3H, s, CH3), 6.12 (1H, s, CH), 7.11–8.07 (12H, m, 12 × CH), 11.32 (1H, s, OH), 11.57 (1H, s, OH); Anal. Calcd for C26H18O6 (427.50): C, 73.23; H, 4.25. Found: C, 73.15; H, 4.43.
Compound 3h
White crystals, IR (KBr): 3,335, 3,040, 1,668, 1,607, 1,325, 766 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.44 (1H, s, CH), 7.15–8.13 (12H, m, 12 × CH), 9.82 (1H, s, OH), 11.33 (1H, s, OH), 11.68 (1H, s, OH); Anal. Calcd for C25H16O7 (429.82): C, 70.09; H, 3.76. Found: C, 70.69; H, 4.08.
Compound 3i
Yellow crystals, IR (KBr): 3,435, 2,915, 1,645, 1,610, 1,450, 925 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.33 (1H, s, CH), 7.15–7.88 (12H, m, 12 × CH), 11.44 (1H, s, OH), 11.58 (1H, s, OH); Anal. Calcd for C25H15O6Br: (491.29): C, 61.12; H, 3.08. Found: C, 61.25; H, 3.11.
Compound 3j
Yellow crystals, IR (KBr): 3,325, 3,025, 1,725, 1,670, 1,614, 760 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.56 (1H, d, CH), 6.68 (1H, d, CH =), 6.75 (1H, d, CH=), 7.14–8.05 (12H, m, 12 × CH); 11.42 (1H, s, OH), 11.58 (1H, s, OH); Anal. Calcd for C27H18O6 (437.55): C, 73.97; H, 4.14. Found: C, 73.78; H, 4.08.
Compound 3k
White crystals, IR (KBr): 3,330, 3,073, 1,670, 1,614, 1,330, 765 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.76 (3H, s, CH3O), 3.89 (3H, s, CH3O), 6.10 (1H, s, CH), 6.73–8.09 (11H, m, 11 × CH), 11.32 (1H, s, OH), 11.55 (1H, s, OH); Anal. Calcd for C27H20O8: (473.75): C, 68.43; H, 4.25. Found: C, 68.21; H, 4.12.
Compound 3l
Yellow crystals, IR (KBr): 3,338, 3,083, 1,662, 1,610, 1,337, 765 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 3.20 (6H, s, 2 × CH3), 6.31 (1H, s, CH), 7.23–7.84 (12H, m, 12 × CH); Anal. Calcd for C27H21NO6 (456.47): C, 66.53; H, 4.34; N, 2.87. Found: C, 65.29; H, 3.24; N, 2.98.
Compound 3m
Black amorphous solid, IR (KBr): 3,030, 1,657, 1,604, 1,325, 765 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.0 (1H, s, CH), 6.32–6.58 (3H, m, 3 × CH), 7.33–8.35 (8H, m, 8 × CH); Anal. Calcd for C23H14O7 (402.42): C, 68.66; H, 3.51. Found: C, 68.46; H, 3.48.
Compound 3n
Cream crystals, IR (KBr): 3,443, 2,926, 1,650, 1,600, 762 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 3.85 (2H, s, CH2), 7.26–8.01 (8H, m, 8 × CH), 11.32 (2H, s, 2 × OH); Anal. Calcd for C19H12O6 (336.29): C, 67.86; H, 3.60. Found: C, 67.82; H, 3.63.
Compound 4
Yellow crystals, mp: 300–302 °C; IR (KBr): 3,436, 3,074, 1,662, 1,603, 1,315, 762 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 6.35 (2H, s, 2 × CH), 7.05–7.92 (20H, m, 20 × CH); Anal. Calcd for C44H26O12 (745.24): C, 70.60; H, 3.75. Found: C, 70.35; H, 3.66
Results and discussion
Starch–sulfuric acid (SSA) is readily prepared by dropwise addition of chlorosulfonic acid to a mixture of starch in n-hexane at 0 °C. It is important to note that this reaction is easy and clean without any work-up procedure because HCl gas is evolved from the reaction vessel immediately. This white homogeneous, nonhygroscopic solid acid is stable under the reaction conditions (Scheme 2).
To evaluate the feasibility of SSA as a catalyst for synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives (Scheme 1), reaction of a 1:2 molar ratio of benzaldehyde and 4-hydroxycoumarin, respectively, to give α,α′-(benzylidene)-bis-(4-hydroxycoumarin), was conducted under different conditions both in the absence and presence of SSA; the results are listed in Table 1. In the absence of SSA only 25 % yield of the product was obtained, with recovery of starting material, even after heating at 80 °C for 2 h (entry 1, Table 1) whereas in the presence of SSA (0.05 g), under the same conditions the yield increased to 60 % (entry 2, Table 1). On the basis of this result, further studies were conducted and it was found that 0.1 g SSA was optimum for this reaction, and gave the product in 90 % yield in just 10 min (entry 3, Table 1). The reaction was also examined in EtOH, H2O, CHCl3, and toluene as solvents. In the presence of solvents the reaction was sluggish; solvent selection was critical and had a significant effect on product yields. When ethanol or water was replaced by chloroform and toluene, there was a significant increase in the product yield (entries 4–7, Table 1). Reaction temperature was also optimized. Below 80 °C the reaction proceeded slowly giving a relatively low yield and no improvement was observed above 80 °C. All further studies were carried out under solvent-free conditions with 0.1 g catalyst at 80 °C.
The scope and generality of this procedure were demonstrated by applying it to a broad range of aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents. All the aromatic aldehydes reacted very well under the optimized reaction conditions, giving good-to-excellent yields of the desired products without formation of any side products, and all the reactions were complete within 5–12 min (entries 1–13, Table 2). Variation of the structure the aromatic aldehydes had no significant effect on the yield, and with aldehydes bearing sensitive functional groups, for example Cl, NO2, OCH3, and CH=CH, the reaction proceeded smoothly to afford the corresponding products in excellent yields (entries 2–6 and 10, Table 2). The reaction proceeded more quickly with aldehydes containing para and meta nitro groups and required only 5 min to give the corresponding α,α′-benzylidene bis(4-hydroxycoumarins) in excellent yields (entries 4 and 5, Table 2). A longer reaction time (12 min) was needed for reaction of p-hydroxybenzaldehyde (entry 8, Table 2). SSA also worked well even with the acid-sensitive aldehyde furfural, without leading to formation of any side products (entry 13, Table 2). The reaction worked very well for aromatic aldehydes but poorly for aliphatic aldehydes, because of their low boiling points under solvent-free conditions. For aliphatic aldehydes the reaction was therefore conducted in water as solvent (entry 14, Table 2).
The reaction was also found to be applicable to dialdehydes, as exemplified by use of 1,4-benzenedialdehyde. Two carbonyl groups from the dialdehyde were reacted with four molecules of 4-hydroxycoumarin to afford the product 4 (Scheme 3) in 80 % yield.
With regard to the mechanism, the transformation involves a Knoevenagel condensation of 4-hydroxycoumarin with the aldehydes followed by a Michael addition with another molecule of 4-hydroxycoumarin (Scheme 4).
Starch–sulfuric acid was recovered by filtration after addition of ethanol to the stirred reaction mixture. Conventional elemental analysis showed the presence of sulfur, indicating sulfur had not leached out. For reusability experiments, recovered catalyst was dried in an oven at 80 °C for 1 h prior before use. The results, given in Table 3, indicate the catalyst was reusable five times without any significant loss of activity.
To show the merit of this work, we compared our results with those from synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives by use of previously reported catalysts. The yield of products in the presence of starch–sulfuric acid is comparable with that reported for other catalysts. However, reaction in the presence of most catalysts required longer reaction times than in this work (Table 4).
To compare the efficiency of SSA with that of simple silica gel and hydrated silica gel, the reaction was conducted in the presence these materials under the same reaction conditions. In the presence of silica gel 75 % yield of the product was obtained after heating for 13 min. Reaction with hydrated silica gel required less time (10 min) and 80 % of the product was obtained.
Conclusions
We have described a simple and highly efficient procedure for preparation of biscoumarins using starch–sulfuric acid as catalyst. The attractive features of the procedure are that it is environmentally benign because the reaction is solvent-free and the biodegradable acid catalyst can be recycled. Mild reaction conditions and short reaction times lead to high yields.
References
J.H. Lee, H.B. Bang, S.Y. Han, J.G. Jun, Tetrahedron Lett. 48, 2889 (2007)
R.D.R.S. Manian, J. Jayashankaran, R. Raghunathan, Tetrahedron Lett. 48, 1385 (2007)
H. Zhao, N. Neamati, H. Hong, A. Mazumder, S. Wang, S. Sunder, G.W.A. Milne, Y. Pommier, T.R. Burke, J. Med. Chem. 40, 242 (1997)
G.K. Maria, E. Marian, J. Med. Pharm. Chem. 3, 583 (1961)
I. Kostava, I. Manolov, I. Nicolova, S. Konstantonov, M. Karaivanova, Eur. J. Med. Chem. 36, 339I (2001)
M. Kidwai, V. Bansal, P. Mothsra, S. Saxena, R.K. Somvanshi, S. Dey, T.P. Singh, J. Mol. Catal. A Chem. 268, 76 (2007)
H. Hagiwara, N. Fujimoto, T. Suzuki, M. Ando, Heterocycles 53, 549 (2000)
J.N. Sangshetti, N.D. Kokare, D.B. Shinde, Green Chem. Lett. Rev 2, 233 (2009)
M.H.A. Elgamal, N.M.M. Shalaby, M.A. Shaban, H. Duddeck, B. Mikhova, A. Simon, G. Toth, Monatsh. Chem. 128, 701 (1997)
H. Hagiwara, S. Miya, T. Suzuki, M. Ando, I. Yamamoto, M. Kato, Heterocycles 51, 497 (1999)
W. Li, Y. Wang, Z. Wang, L. Dai, Y. Wang, Catal. Lett. 141, 1651 (2011)
J.M. Khurana, S. Kumar, Monatsh. Chem. 141, 561 (2010)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
Z. Siddiqui, F. Farooq, Catal. Sci. Technol. 1, 810 (2011)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7, 890 (2010)
J. Wang, D.Q. Shi, Q.Y. Zhuang, X.S. Wang, S.J. Tu, Chin. J. Org. Chem. 25, 926 (2005)
P. Singh, P. Kumar, A. Katyal, Catal. Lett. 134, 303 (2010)
M.M. Heravi, F. Nahavandi, S. Sadjadi, Synth. Commun. 40, 498 (2010)
Z. Karimi-Jaberi, M.R. Nazarifar, B. Pooladian, Chin. Chem. Lett. 23, 781 (2012)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 4343 (2012)
N. Hamdi, M.C. Purta, P. Valerga, Eur. J. Med. Chem. 43, 2541 (2008)
G.X. Gong, J.F. Zhou, L.T. An, X.L. Duan, S.J. Ji, Synth. Commun. 39, 497 (2009)
P.T. Anastas, J.C. Warner, Green chemistry: theory and practice (Oxford University Press, Oxford, 1998)
P.T. Anastas, T. Williamson, Green chemistry, frontiers in benign chemical synthesis and process (Oxford University Press, Oxford, 1998)
J.P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, Eur. J. Med. Chem. 13, 67 (1978)
A. Buleon, P. Colonna, V. Planchot, S. Ball, Int. J. Biol. Macromol. 23, 85 (1998)
Acknowledgments
The authors are grateful to Firoozabad University Research Council for the partial financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, R., Sheikhi, M.R. Starch–sulfuric acid as a bio-supported and recyclable solid acid catalyst for rapid synthesis of α,α′-benzylidene bis(4-hydroxycoumarin) derivatives. Res Chem Intermed 41, 1283–1292 (2015). https://doi.org/10.1007/s11164-013-1272-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1272-3