Abstract
Biscoumarin derivatives are synthesized via a simple, one-pot, pseudo three-component reaction between various arylaldehydes and 4-hydroxycoumarin using phospho sulfonic acid as a reaction mediator under solvent-free neat conditions in good to excellent yields. High yields, short reaction time, easy work-up, elimination of solvents and environmentally benign milder reaction conditions are the advantages of this procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multi-component reactions (MCRs), are a promising and vital field of chemistry because by the synthesis of complicated molecules can be achieved in a very fast, efficient and time saving manner without the isolation of any intermediate [1–3]. On the other hands, the use of heterogeneous catalytic methods for synthesis of fine chemicals is a promising field of research with potential application in pharmaceutical and related fine chemical industries [4]. Therefore, the possibility of performing multicomponent reactions under solvent-free conditions with a heterogeneous catalyst could enhance their efficiency from an economic as well as ecological point of view.
Biscoumarins have received considerable attention of synthetic and medicinal chemists because of their broad spectrum of biological and pharmaceutical activities [5–7]. A number of biscoumarins have also been found to be urease inhibitors [8]. Although some types of these compounds could be isolated from plants [9], attempts have been made to use alternative catalysts for biscoumarin synthesis. A literature search revealed that a number of catalytic methods have been developed for the synthesis of biologically important biscoumarin derivatives, specially the bridge substituted dimers of 4-hydroxycoumarin, by the reaction of 4- hydroxycoumarin and various aldehydes [10–15]. However, each of the methods has its own disadvantages, such as long reaction time, harsh reaction conditions, the use of large excess of reagents, low yield and the use of toxic, corrosive, expensive, or non-reusable catalysts.
This finding prompted us towards further investigation in search for a new catalyst, which will carry out the synthesis of biscoumarins under simpler experimental set up and eco-friendly conditions. Therefore, we now wish to explore a straightforward convergent one-pot synthesis of bis-(4-hydroxycoumarin-3-yl) methanes using phospho sulfonic acid as an efficient solid acid catalyst under solvent-free conditions through domino Knoevenagel condensation/Michael addition sequence (Scheme 1).
Experimental section
General
All commercially available chemicals were purchased from Fluka and Merck companies and used without further purification. IR spectra were recorded on a BOMEM MB-Series 1998 FT-IR spectrophotometer. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker Advanced DPX 400 MHz FT-NMR spectrometer using TMS as internal standard. Reaction monitoring was accomplished by TLC on silica gel polygram SILG/UV 254 plates.
Preparation of phospho sulfonic acid
A 50 mL suction flask was equipped with a constant pressure dropping funnel. The gas outlet was connected to a vacuum system through an adsorbing solution of alkali trap. Diammonium hydrogen phosphate (2 g, 15 mmol) was charged in the flask and chlorosulfonic acid (5.24 g, ca. 3 mL, 45 mmol) in CH2Cl2 (10 mL) was added dropwise over a period of 30 min at room temperature. After completion of the addition, the mixture was shaken for 2 h, while the residual HCl was eliminated by suction. Then the mixture was washed with excess dried CH2Cl2. Finally, a white solid powder (4.2 g) was obtained.
General procedure
A mixture of benzaldehyde (1 mmol), 4-hydroxycoumarin (2 mmol) and PSA (0.05 g) was heated at 100 °C. Completion of the reaction was indicated by TLC [ethyl acetate/n-hexane (1:2)]. After completion of the reaction the insoluble crude product was dissolved in hot ethyl acetate and phospho sulfunic acid was filtered. The crude product was purified by recrystallization in ethanol to afford the pure product.
Selected spectral data
Product (2a): 3,3-(Benzylidene)-bis-(4-hydroxycoumarin): white crystal, melting point (228–230), IR (KBr, cm−1): 1098, 1347, 1493, 1605, 1657, 3084.57; 1H NMR: (CDCl3): 6.12 (s, 1H), 7.25–8.21 (m, 13H), 11.33 (s, 1H), 11.56 (s, 1H); 13C NMR: (CDCl3): 36.17, 103.89–152.54 (Caromat), 164.62 (C–OH),165.84 (C–OH),166.90 (CO),169.34 (CO).
Product (2b): 3,3-(4-Ethoxybenzylidene)-bis-(4-hydroxycoumarin): red crystal, melting point (229–231), IR (KBr, cm−1): 1087, 1345.53, 1448.4, 1609.39, 1667, 3072.64; 1H NMR: (CDCl3): 2.8 (t, 3H), 5.2 (q, 2H), 5.56 (s, 1H), 6.48–7.8 (m, 12H), 10.49 (s, 1H), 10.62 (s, 1H); 13C NMR: (CDCl3): 31.1, 98.1–152.2 (Caromat), 152.5, 162.3, (C–OH), 163.9 (C–OH), 189.9 (CO), 191.1(CO).
Product (2c): 3,3-(4-Nitrobenzylidene)-bis-(4-hydroxycoumarin): yellow crystal, melting point (227–229), IR (KBr, cm−1): 1100.18, 1357.81, 1493.17, 1608.91, 1660.48, 3087.35; 1H NMR: (CDCl3): 6.13 (s, 1H), 7.41–8.21 (m, 12H), 11.40 (s, 1H), 11.59 (s, 1H); 13C NMR: (CDCl3): 36.52, 103.27–152.57 (Caromat), 146.86, 164.86 (C–OH),166.46 (C–OH), 167.02 (CO), 169.14 (CO).
Product (2e): 3,3-(4-Triflurobenzylidene)-bis-(4-hydroxycoumarin): white crystal, melting point(266–268), IR(KBr, cm−1): 1112.8, 1324.79, 1483.52, 1609.17, 1664.88, 3074.72; 1H NMR: (CDCl3): 5.6 (s, 1H), 6.8–7.9 (m, 12H), 9.68 (s, 1H), 10.6 (s, 1H); 13C NMR: (CDCl3): 32.3,73.1, 97.7–148.2 (Caromat), 162.3 (C–OH), 164 (C–OH), 190.1 (CO), 191.3(CO).
Results and discussion
Phospho sulfonic acid, PSA, was easily prepared by simple mixing of diammonium hydrogen phosphate and chlorosulfonic acid in CH2Cl2 at room temperature (Scheme 2).
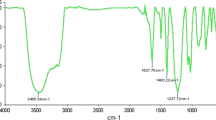
The presence of sulfonyl group in PSA is confirmed by FT-IR analysis. The FT-IR spectrum of the catalyst (KBr disk), showed O=S=O asymmetric and symmetric stretching peaks in 1124 and 1075 cm−1, respectively, and the S–O stretching peak in 678 cm−1, which strictly confirms the sulfonic group linkage. The sulfonic acid loading of PSA was calculated based on titration of the proton-exchanged brine solution and shown the loading of 11 mmol SO3H g−1 of acidic catalyst.
In order to carry out the synthesis of biscoumarin under environmentally benign conditions, Initially, the synthesis of 3,3’-(benzylidene)-bis(4-hydroxycoumarin) was selected as a model reaction to optimize the reaction conditions. The reaction was carried out by heating a mixture of benzaldehyde (1 mmol) and 4-hydroxycoumarin (2 mmol) in the presence of various amount of PSA at different temperatures under solvent free conditions. As can be seen from Table 1, the shortest time and best yield was achieved in the presence of 0.05 g of catalyst at 100 °C (Entry 4).
Encouraged by this result, a wide variety of aromatic aldehydes, containing both electron withdrawing and donating substitutes were treated under the optimized conditions and afforded the corresponding biscoumarin (Table 2) in good to excellent yields and short reaction times. With regard to the substituents, both aldehydes with electron withdrawing and electron donating groups participated in the reaction, but the former were better. The structure of the products was established from their IR spectral data and comparison of their melting points with those of authentic samples. Also, the structure of some products was confirmed by 1H and 13C NMR spectral data.
To compare the advantage of the use of PSA over the reported catalysts, the model reaction of, benzaldehyde (1 mmol) and 4-hydroxycoumarin (2 mmol) was considered as a representative example (Table 3). These results clearly demonstrate that PSA is an equally or more efficient catalyst for this three-component reaction.
Conclusion
In conclusion, we have reported an easy, efficient and green protocol for the synthesis of biscoumarins from the one-pot pseudo three-component condensation reaction of various arylaldehydes and 4-hydroxycoumarin using phospho sulfonic acid as a novel environmentally safe heterogeneous solid acid catalyst under solvent-free conditions. The method offers marked improvement with its operational simplicity, low reaction time and high yields of pure products.
References
L. Rong, X. Wei, S. Tao, Y. Lu, R. Xie, J. Zhou, Z. Zong, An efficient and convenient one-pot multicomponent synthesis of novel pyrimidine derivatives: N-(4-aryl-6-(pyridin-2-yl) pyrimidin-2-yl)cyanamides. Res. Chem. Intermed. (2012). doi:10.1007/s11164-012-0724-5
J.M. Khurana, S. Kumar, Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 50, 4125–4127 (2009)
A.R. Kiasat, A. Mouradezadegun, S.J. Saghanezhad, Phospho sulfonic acid: a novel and efficient solid acid catalyst for the one-pot preparation of 2h-indazolo[2,1-b]phthalazine-triones. J. Serb. Chem. Soc. (2012). doi:10.2298/JSC120508088K
S.N. Ghattali, K. Saidi, H. Khabazzadeh, (NH4)2.5H0.5PW12O40 catalyzed rapid and efficient one-pot synthesis of dihydropyridines via the Hantzsch reaction under solvent-free conditions. Res. Chem. Intermed. (2012). doi:10.1007/s11164-012-0962-6
H. Mehrabi, H. Abusaidi, Synthesis of biscoumarin and 3,4–dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (sds) in neat water. J. Iran. Chem. Soc. 7, 890–894 (2010)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Synthesis and pharmacological activity of 2-oxo(2H)1-benzopyran-3-carboxamide derivatives. Eur. J. Med. Chem. 28, 517–520 (1993)
K.M. Khan, S. Iqbal, M.A. Lodhi, G.M. Maharvi, Z. Ullah, M.I. Choudhary, A. Rahman, S. Perveen, Biscoumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg. Med. Chem. 12, 1963–1968 (2004)
N. Tavakoli-Hoseini, M.M. Heravi, F.F. Bamoharram, A. Davoodnia, M. Ghassemzadeh, An unexpected tetracyclic product isolated during the synthesis of biscoumarins catalyzed by [MIM(CH2)4SO3H][HSO4]: Characterization and X-ray crystal structure of 7-(2-hydroxy-4-oxo-4H-chromen-3-yl)-6H,7H-chromeno[4,3-b]chromen-6-one. J. Mol. Liq. 163, 122–127 (2011)
V. Padalkar, K. Phatangare, S. Takale, R. Pisal, V. Chaskar, Silica supported sodium hydrogen sulfate and Indion190 resin: an efficient and heterogeneous catalysts for facile synthesis of bis-(4-hydroxycoumarin-3-yl) methanes. J. Saudi Chem. Soc. (2011). doi:10.1016/j.jscs.2011.12.015
J.M. Khurana, S. Kumar, Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 50, 4125–4127 (2009)
Z. Karimi-Jaberi, M.R. Nazarifar, B. Pooladian, Synthesis of functionalized furo[3,2-c]coumarins via a one-pot oxidative pseudo three-component reaction in poly(ethylene glycol). Chin. Chem. Lett. 23, 781–784 (2012)
H. Mehrabi, H. Abusaidi, Synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat water. J. Iran. Chem. Soc. 7, 890–894 (2010)
G. Palmisano, F. Tibiletti, A. Penoni, F. Colombo, S. Tollari, D. Garella, S. Tagliapietra, G. Cravotto, Ultrasound-enhanced one-pot synthesis of 3-(Het)arylmethyl-4- hydroxycoumarins in water. Ultrason. Sonochem. 18, 652–660 (2011)
Z. Zareai, M. Khoobi, A. Ramazani, A. Foroumadi, A. Souldozi, K. Slepokura, T. Lis, A. Shafiee, Synthesis of functionalized furo[3,2-c]coumarins via a one-pot oxidative pseudo three-component reaction in poly(ethylene glycol). Tetrahedron 68, 6721–6726 (2012)
R. Kenchappa, Y.D. Bodke, A. Chandrashekar, S. Telkar, K.S. Manjunatha, M.A. Sindhe, Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-subtituted phenyl) pyridine derivatives as potent biological agents. Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2013.03.020
K. Tabatabeian, H. Heidary, A.R. Khorshidi, M. Mamaghani, N.O. Mahmoodi, Synthesis of biscoumarin derivatives by the reaction of aldehydes and 4-hydroxycoumarin using ruthenium(III) chloride hydrate as a versatile homogeneous catalyst. J. Serb. Chem. Soc. 77, 407–413 (2012)
M.M. Heravi, S. Sadjadi, N.M. Haj, H.A. Oskooie, F.F. Bamoharram, Role of various heteropolyacids in the reaction of 4-hydroxycoumarin, aldehydes and ethylcyanoacetate. Catal. Commun. 10, 1643–1646 (2009)
B. Karmakar, A. Nayak, J. B. Sulfated titania catalyzed water mediated efficient synthesis of dicoumarols—a green approach J. Tetrahedron Lett. 4343–4346 (2012)
Acknowledgments
We gratefully acknowledge the support of this work by Shahid Chamran University Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiasat, A.R., Hemat-Alian, L. Phospho sulfonic acid: a versatile and efficient solid acid catalyst for facile synthesis of bis-(4-hydroxycoumarin-3-yl) methanes under solvent-free conditions. Res Chem Intermed 41, 873–880 (2015). https://doi.org/10.1007/s11164-013-1239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1239-4