Abstract
The wide utility and catalytic efficiency of microbial pectinase in various industries has greatly increased its global demand. Among the natural sources of pectinases, microbial pectinases are used frequently for its ease of production and unique physicochemical properties. Yet similar to other industrial enzymes, pectinases also face the constraint of thermo-tolerance and low yield in its economised production. The current review addresses the various strategies adopted to meet the high yield and thermo-tolerance of pectinases as well as the various attempts made in the field of pectinases to its improved production and better catalytic efficiency. The utilisation of natural as well as recombinant microbial sources, metagenomic approaches, metabolic engineering, site directed mutagenesis and media engineering techniques adopted in the field of pectinases have been discussed. The significance of pectinases in various industries is depicted by enlisting its applications. To the best our knowledge the current review is unique being the first attempt to compile the recent advancements in the field of pectinases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pectinases traced back as the first enzyme used in homes to its commercialized production since 1930, accounts for a major proportion of the industrial enzymes. They have attained more value with the multitude applications such as production of functional foods (Khan et al. 2013; Prathyusha and Suneetha 2011), retting and degumming of fibres in textile industry (Cao et al. 1992), production of good quality paper (Ahlawat et al. 2008), fermentation of coffee and tea, oil extractions and treatment of pectic waste water, bioethanol production etc. (Kashyap et al. 2001). The term pectinase refers to heterogeneous enzymes including homogalacturonan-degrading polygalacturonases (PG) or pectin depolymerase; polymethylgalacturonases (PMG); lyases or transeliminases and pectin esterases (PE), which is also known as pectin methyl esterases (PME).These enzymes are capable of either lysing the glycosidic bonds, debranching or modifying pectin, the most abundant component of fruits (Cuesta 2016). Pectin functions as a cross linking polysaccharide in the primary cell wall and middle lamella of fruits and vegetables cross linking cellulose and hemicellulose fibres and the use of pectinases improve access of cellulases to their substrates (Giacobbe et al. 2014). Pectin hydrolases are produced mainly by fungi, being more active on acid or neutral medium at temperatures between 40 and 60 °C, whereas bacterial pectinases are more active in acidic conditions (Pedrolli et al. 2009). As per reports available approximately 75% of the industrial enzymes are hydrolases, with carbohydralases being the second largest group (Priya and Sashi 2014).
Pectinases are classified based on their mode of action into polygalacturonase (EC 3.2.1.15), Pectin esterase (EC 3.1.1.11), Pectin lyase (EC 4.2.2.10) and Pectate lyase (EC 4.2.2.2). These enzymes act on O-α-(1,4) poly galacturonopyranose structures with activities and specificities that depend partly upon the degree of methylation (Saadoun et al. 2013). Based on their cleavage specificity pectinases can be grouped into ones cleave pectin smooth regions or pectin hairy regions (Pedrolli et al. 2009). Protopectinases solubilizes protopectin and forms soluble pectin, pectin methyl esterases and pectin acetyl esterases eliminates methoxyl and acetyl residues from pectin which give rise to polygalacturonic acid, polygalacturonase breaks the glycosidic α-(1–4) bonds between galacturonic residues by hydrolysis and trans-elimination reactions. Pectinases are divided as acidic or alkaline based on pH; whereas they are termed as endo or exo when enzyme action is random or at terminal end respectively.
A critical study on the various reviews on pectinases indicate different aspects of this group of enzymes such as its structural–functional characteristics (Gummadi et al. 2007), purification (Gummadi and Panda 2003), applications (Khan et al. 2013; Pedrolli et al. 2009; Sharma et al. 2013) etc. The current review lays down the various attempts made in the field of pectinases to its increased production, improved thermo-tolerance and better catalytic efficiency of this enzyme. The increasing global demand of pectinases could be addressed by the adoption of either natural or recombinant enzymes by strain improvement, genetic engineering, metagenomic studies, site-directed mutagenesis, directed evolution and media engineering.
2 Significance of pectinase thermo-tolerance and enzyme yield
Most of the commercially available pectinases are combinations of pectate lyases, polygalacturonases and pectin methyl esterases mainly derived from Aspergillus sp (Kashyap et al. 2001). Of these acidic pectinases are widely used in food industry, while alkaline pectinases find applications in a variety of industrial processes. A close study of these enzymes reveal that these enzymes are exposed to extreme processing conditions with temperatures ranging from 30 to 70 °C in the food industry, for instance in sugar extraction and temperatures higher in biotech industries (Singh et al. 1999). A comparative analysis on the thermal inactivation of commercial pectinases revealed that some of the brands lost half of its activity on exposure to 50 °C for even 2 min of exposure (Ortega et al. 2004). Thus the choice of pectinases with better thermo-tolerance becomes essential for its effective utilization. Apart from tolerating high temperatures, thermo-stable enzymes would be advantageous in having a little activity at lower temperatures, longer shelf life, resistant to organic solvents, high and low pH solubility, less viscous and high reaction rates (Kusuma and Sri 2014). Moreover, by the use of thermo- tolerant enzymes the chance of enzyme inactivation during attempts to kill contaminating pathogens becomes quite rare.
The high yield and thermo-tolerance of pectinases can be addressed by use of natural or recombinant high yielding isolates in combination with efforts using metagenomic, metabolic engineering, site-directed mutagenesis and various production optimization strategies. Deep insights on the molecular characterisation, functional analysis, genetic studies and mutation strategies have been some of the key factors contributing to pectinase enzyme yield, its thermo-tolerance and alkaline stability.
2.1 High yielding natural strains
Pectinase is naturally present in plants and in fruits for natural ripening of fruits, yet microbial sources are commonly used for large scale production for application level studies and for industrial use due to ease of maintenance and production. Pectinolytic organisms can be isolated from spoiled fruits walls, soil, decaying agro-waste, animals etc. The predominantly pectinase producing microorganisms include Pseudomonas, Xanthomonas, Erwinia, soil isolates such as Actinomycetes and Streptomycetes and various fungi. Aspergillus niger, Aspergillus versicolor, Aspergillus flavus, Fusarium oxysporum, Rhizopus stolonifer, Mucor racemous, Mucor hiemalis, Penicillium jenseni, Penicillium citrinum and Trichoderma viride are the main fungal source of pectinases (Priya and Sashi 2014). Fungal organisms A. niger is a good source of pectinase. A. niger IM 6 gives maximum enzyme activity at 40 °C with 60% moisture on seventh-day solid state fermentation (Akhter et al. 2011). Almost 82% of pectinase activity was retained by A. niger strain MCAS2 pectinase even at 100 °C and the enzyme was found to be stable even at alkaline conditions (Khatri et al. 2015). Table 1 enlists some of the natural thermo-stable pectinases.
Thermostable pectinases have widely reported in various studies and these natural strains are selected when higher temperatures of processing are required. A comparative analysis shows that the optimal temperature for a Polygalacturonase from Streptomyces sp. QG-11-3 is 60 °C and the hyper-thermophilic bacterium Thermotoga maritima is at 80 °C (Kluskens et al. 2005). Most predominantly Bacillus sp. are good sources of thermo-stable enzymes (Rebello et al. 2017). The thermal stability of polymethyl galacturonase from thermo-stable Bacillus sp. BR1390 indicated 100% activity at 60 °C after 60 min and a residual activity of 50% at 90 °C was observed after 30 min of incubation (Banafshe and Hamid 2014).Various studies indicate that the thermo-stability is attributed to the presence of cysteine residue present in the amino acid sequences of pectinases enabling the formation of disulphide bonds as well as conferring a strong hydrophobicity to the pectinase (Singh et al. 2012a, b; You et al. 2010).
2.2 Recombinant pectinase sources
Recombinant pectinases also find various applications in foods and quite often the natural non-GMO food products also may be finally treated with recombinant pectinases to improve food quality. The most common source of recombinant pectinase used in the food industry includes mainly fungal derived pectinases such as Aspergillus, Penicillium and Trichoderma varieties as per GMO compass reports (http://www.gmo-compass.org/eng/database/ enzymes/92.pectinase.html). Bacillus derived recombinant pectinases are used predominantly in industrial purposes.
The use of highly efficient pectinases with peculiar properties under the promoter control of various expression hosts has been done in Escherichia coli, Pichia pastoris, Saccharomyces cerevisiae, P. griseoroseum have been reported as shown in Table 2. A comparative analysis of the yield, thermo-stability and pH tolerance of these enzymes helps greatly to validate their use in different fields. Recombinant expression of thermo-alkaline Pel (BacPelA) gene from Bacillus clausii in E. coli resulted in recombinant mature BacPelA with an enzyme activity of 8378.2 U ml−1 (A235) by high-cell-density cultivation in fed-batch fermentation with productivity of 239.4 U ml−1 h−1 and this represents the highest Pel yield reported to date (Zhou et al. 2017). In another study recombinant Aspergillus expressed in P. pastoris achieved a maximal activity of 2408.70 U ml−1 in the culture supernatant of by high cell density batch fermentation, equivalent to a 4.8 times greater yield than that from shake-flask culture (Abdulrachman et al. 2017). P. pastoris based expression of an acid stable endo-polygalacturonase gene from Penicillium oxalicum produced a yield of 1828.7 U ml−1 (Cheng et al. 2017).The long lag time in basal salts medium (BSM) and an occurrence of proteolysis associated with recombinant pectinase production in P. pastoris KM71 has been found to be overcome by using synthetic FM22 medium for inoculum and proteolysis control by growth at lower pH (Charoenrat et al. 2013).
Recombinant P. griseoroseum T20 produced by transformation of P. griseoroseum with the plasmid pAN52pgg2, containing the gene encoding PG of P. griseoroseum, under control of the gpd promoter gene from Aspergillus nidulans yielded 266- and 27-fold greater levels of pectin lyase (PL) and polygalacturonase (PG) respectively than the wild-type strain (Teixeira et al. 2011). The use of a constitutive promoter such as the promoter of gpd gene instead of the indigenous promoter of Penicillium greatly enhanced the pectinase production. Attempts in the generation of catabolite repression resistant mutant strains of Penicillium griseoroseum mutants by UV-induced spontaneous mutations resulted in a 7.8-fold increase of pectinase production than the wild strain (Lima et al. 2017).
2.3 Metagenomic approach
Metatranscriptomics studies of the rumen of a dairy cow revealed the presence of pectinase producing microbes such as Bacteroides; Prevotella sp., Bacteroides sp. and the Ruminococcus (Comtet-Marre et al. 2017); while studies in sheep previously indicated the pectinase genes of Butyrivibrio, Prevotella, Bacteroides and Fibrobacter (Yuan et al. 2012). Studies on a soil metagenome yielded an ORF of a pectate lyase similar to Bacillus licheniformis and it was expressed in E. coli (Singh et al. 2012a). As noted this enzyme worked at a broad range pH and temperature not requiring Ca2+ for its activity.
Recombinant expression of pelB gene a soil metagenome product in E. coli turned out to be good bioscouring agent in the textile pretreatment process (Wang et al. 2014). The further optimization of this recombinant in a 7L bioreactor resulted in the production of pectinase with activity of 1816.2 Uml−1. New insights on the presence and role of multiple pectin degrading enzymes such as pectin lyase, polygalacturonase, galactosidase, arabinofuranosidase and rhamnosidase was reported from a thermophilic compost metagenome (Wang et al. 2016). The above paper was also unique making a first report on the role of actinomycetes in pectin degradation.
2.4 Metabolic engineering strategies
Protoplast fusion between complementary auxotrophic and morphological mutant strains of P. griseoroseum and P. expansum was induced by polyethylene glycol and calcium ions (Ca2+) to obtain recombinant RGE27 with a threefold increase in polygalacturonase and 1.2-fold pectin lyase production than the parental strain (Varavallo et al. 2007). A semi rational approach based screening and comparative analysis on poly galacturonidase (PGL) production using first, 6 signal peptides (amyX, bpr, vpr, yvgO, wapA and nprE) in Bacillus subtilis, yielded a bpr directed efficient PGL secretory expression with a PGL titre to 313.7 U ml−1 (Zhang et al. 2013). Further optimization and use of strong promoter P43 and Shine–Dalgarno sequence increased PGL titre to 446.3 U ml−1; whereas fed-batch studies in a fermenter yielded a titre of 632.6 Uml−1 with a productivity of 17.6 U ml−1 h−1, which was the highest secretory production of PGL by the B. subtilis system.
2.5 Site-directed mutagenesis
Site-directed mutagenesis (SDM) research on pectate lyase was initially done to elucidate its mechanism of action and active site analysis. SDM studies on pelC and pelE pectate lyases of Erwinia chrysanthemi revealed that the active site included also the Ca2+ binding site (Jurnak et al. 1996). Mutagenesis studies revealed that the amino acids around the Ca2+ binding site are involved in the catalysis reaction of pectate lyase. Another study on the pectate lyase of A. niger revealed that the substrate binds to the enzyme as a Ca2+-substrate complex, thus explaining the absolute requirement of pectate lyases for Ca2+-ions (Benen et al. 2003).
Improvements in the thermo-stability of pectate lyase of Xanthomonas campestris origin (PL Xc ) was achieved by a single beneficial mutation (R236F) based on melting temperature guided sequence alignment; resulted in a 6 °C increase in T m and a 23-fold increase in the half-life at 45 °C without compromising the enzymes catalytic efficiency (Xiao et al. 2008). Combination of R236F with another beneficial mutation (A31G) caused a hydrophobic desolvation of the enzyme with a two-fold increase in specific activity of the enzyme maintaining the improved T m value.
Site-directed mutagenesis of polygalactauronidase of S. cerevisiae expressed in P. pastoris showed that aspartic acid residues at positions 179, 200, and 201 and histidine 222 were critical for enzyme activity (Blanco et al. 2002). Mutation of the two potential glycosylation sites at residues 318 and 330 revealed that double mutations at these two sites by converting asparagine to aspartate caused a 50% reduction in enzyme activity when compared to the wild-type PGU1 transformant.
Directed evolution studies conducted on pectate lyase generated 12 mutants (A118H, A182 V, T190L, A197G, S208 K, T219 M, T223E, S255R, S263 K, N275Y, Y309 W, and S312 V) with thermo-tolerance greater than the parent strain (Solbak et al. 2005). However the best performing isolate had eight point mutations which contributed to a melting temperature 16 °C greater than the wild strain. Directed evolution of pectin methylesterase of E. chrysanthemi involving a four amino acid substitution led to the formation a mutant enzyme with T m value of 11 °C from the wild type, maintaining its wild type kinetic properties.
2.6 Efforts to optimised industrial production
Pectinases are produced commercially by both submerged (SmF) and solid state fermentation (SSF), with the latter giving better enzyme yields. Various factors such as substrate selection, process conditions, water content, incubation time, inoculums size, pH, temperature, presence of inhibitors/activators and addition of carbon and nitrogen sources are crucially influencing the pectinase biosynthesis. A comparison on pectinase yield by 6 different fungus on different substrates viz, wheat bran, rice bran, orange peels, peanut shells, canola oilseed cake and sugarcane bagasse found wheat bran a promising substrate (Amin et al. 2017a). The role of ammonium sulphate in the induction of pectinase production was also evident with fungi such as Aspergillus fumigatus and A. alliaceus BIM-83 (Phutela et al. 2005; Sapunova et al. 1997). The former isolate A. fumigatus attained a maximum activity of 1116 Ug−1 for pectinase and 1270 Ug−1 for polygalacturonase at pH 4.0 and 5.0, respectively on growth on wheat bran (Phutela et al. 2005). Intermittent agitation exhibited a positive effect in the SSF of pectinase production in pilot scale bed reactor (Finkler et al. 2017).
Production of thermostable alkaline pectinases by Bacillus pumilus dcsr1 was increased 1.7-fold at optimized conditions with a 14.2-fold high enzyme production obtained in solid state fermentation than in submerged fermentation (Sharma and Satyanarayana 2012). An enzyme yield of 348 ± 11.8 Ug−1 was obtained on agro residue used as substrate moistened with mineral salt solution and optimum water activity was 0.92, optimum pH 9.0 and optimum temperature obtained was 40 °C.
An evaluation on the various bioreactors used in the production of pectinases indicate that better results were obtained by solid state fermentation as shown in Table 3. Pectinase production by A. niger LB-02-SF in a bench-scale rotating drum bioreactor indicated that enzyme production was favoured in conditions limiting the fungal growth, without any temperature control but with an intermediate air flow. Thus the best conditions for biomass growth were not the best for pectinase production (Poletto et al. 2017).
A novel strategy extraction protocol aiming to reduce the cost and increase the enzyme yield was adopted in the downstream process of pectinase extraction (Wolf-Marquez et al. 2017). The above extraction strategy utilised the salting-out potential of two biocompatible cholinium-based ionic liquids (N1112OHCl and N1112OHH2PO4) in aqueous solutions of Tergitol, enabling 90% extraction of pectinase. Further on the exposure of pectinase to pulsed electric field was found to increase its thermal stability and activity without altering its structure (Zhang et al. 2017). Mild ultrasound treatment of pectinase increased its immobilization yield as well as its catalytic activity, but reduced its thermo-stability, reaction stability and reusability due to structural changes (Ma et al. 2017).
2.7 Structural and genetic factors contributing to enzyme properties
The presence of various conserved aminoacid residues in pectinases, the protein conformation and interacting bonding forces play a great role conferring to the properties such as thermotolerance, alkaline stability and catalytic efficiency to these enzymes. Polygalacturonases generally possess a conserved right-handed parallel β-helical structure with ten complete turns (Bonivento et al. 2008), with the active site open on both sides in endo-PGs or occluded on one side in the exo-PG (Abbott and Boraston, 2007). A unique tetrameric β-helical structure was also reported in exopolygalacturonase from Thermotoga maritime, the most thermotolerant pectinase reported so far (Pijning et al. 2009). The tetrameric nature of this protein accounts to its thermotolerance, substrate specificity (exo-activity and acceptance of non-methylated, saturated polygalacturonate only), as well as product specificity (release of mono-galacturonate).
Three conserved aspartate residues of pectinase interact with the substrate, with one residue (Asp173) acting as an acid, while the other two (Asp153 and Asp174) act as a base in the hydrolytic cleavage of the substrates (Pijning et al. 2009). Recent studies indicate that cation-π interactions of endo-polygalacturonases critically affect the thermotolerance and catalytic efficiency of these enzymes. Three single mutants of polygalacturonases viz, H58Y, T71Y and T304Y promoted the cation-π interactions of the enzyme, thereby increasing its thermostability (T m increased by 0.6–3.9 °C) and catalytic efficiency to a 32-fold (Tu et al. 2016).
The genetic analysis of various pectinase coding genes in different microbes indicate that this enzyme could be coded by post-translational modification a polypeptide encoded by a single gene as in Fusarium moniliforme (Caprari et al. 1993) or encoded by family of diverged genes as in A. niger (Bussink et al. 1992). Microarray analysis of A. niger grown on main sugar components of pectin revealed the expression of 46 pectolytic genes in the isolate (Martens-Uzunova and Schaap 2009) and most of these multigenes have been generated by gene duplication (Carroll et al. 2005).
Presence of substrates such as pectin, galacturonic acid and polygalacturonic acid induce the secretion of pectate lyase, polygalacturonase and pectin methyl esterase encoding genes, but glucose represses these genes. A clear involvement of carbon catabolite repressor protein (Cre A) is also found in the process of repression (Maldonado and Saad, 1998). The presence of various conserved sequence 5′-TYATTGGTGGAA-3′ and 5′-CCCTGA-3′ aiding in gene expression were identified in A. niger (Visser et al. 2004).
3 Applications of pectinases
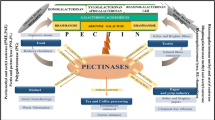
Pectinases basically find applications both in acidic and alkaline conditions particularly in the food and textile industry respectively. Applications studies with pectinases are ongoing in global research fields to obtain maximum fastened activity with enzymes. The wide applications of this enzyme has attributed to its increasing global demand and some of these applications are reviewed here (Fig. 1).
3.1 Protoplast isolation
Protoplasts can be isolated by mechanical or enzymatic methods. Pectinases finds application for protoplast isolation in combination with other enzymes like cellulases and chitinases. Protoplasts have a wide range of applications in genetic transformations, membrane studies etc. An enzyme cocktail consisting of commercial cellulases, crude pectinases and crude chitinases was used to release maximum number of protoplast from Pleurotus eous and P. flabellatus mycelia (Parani and Eyini 2011). Mycelia extracted and incubated with enzyme cocktail for 3 h, KCl (0.6 M) as osmotic stabilizer, phosphate buffer of pH 6.0 and 3 days old culture gave maximum protoplast yield.
3.2 Fruit juice clarification
Fungal pectinolytic preparations are widely used for the clarification of fruit juices. Acid pectinases are mainly used to remove pectin in fruit juice. Pectinases produced by A. niger (fungal pectinases) are commonly used to clarify fruit juices. Fruits are rich in pectin, crushing of fruit gives highly viscous fruit juice. Pectin is responsible for haze and precipitate formation in juice. The gelatinous nature of juice will result in clear juice extraction difficulties. Pectinase usages in extraction process improve juice quality. Pectinases increases filtration efficiency of fruit juice by decreasing turbidity of fruit juices (Saadoun et al. 2013). Pectinases degrades gel structure and decreases viscosity of juice. More than 90% improvement than mechanical extraction of clear juice and enzyme treatment also improves colour, flavour and nutritional characteristics of juice. Mainly used in apple juice preparation, clearness will be high when compared to pectinase untreated juice and on enzymatic treatment total pectin content of juice decreases (Pedrolli et al. 2009).
The benefits of using pectinases in juices include also the release of various phenolic compounds from fruits which serve as good antioxidants aiding in prevention of various ailments such as cancers and coronary heart diseases (Aliaa et al. 2010). Strawberry and raspberry juices need enzymatic de-pectinization to remove the colloidal nature (Versari et al. 1998). The pectin, phenolic substances and protein of juices result in formation of irreversible complexes that enzyme cannot break so it is essential to remove pectin. Pectinases depolymerize highly esterified pectin in apple juice. A combination of pectinase, cellulase and amylase gives juice yield up to 100% (Pasha et al. 2013). The enzyme treated banana can be used to produce banana wine, which gives cleared banana wine without affecting any other characteristics of banana wine (Tapre and Jain 2014).
3.3 Retting and degumming of fibre crops
Fibres containing gum should be degummed for its usage in textile industry. Pectin cements the fibres together and this pectin should be degraded. As chemical degumming causes pollution, an alternative use of pectinases and xylanases mixture serves as an eco-friendly and economical solution for pollution with non biodegradable pollutants (Sharma and Satyanarayana 2012). Alkaline pectinases are commonly used for retting and degumming process of fibres like ramie, flax, sunn hemp and jute. Pectinolytic enzymes secreted by soft rot bacteria cause maceration of woody fabrics that are long, strong and stiff, which softens fibres (Liao 1989).
Pectinase based enzyme retting is an eco-friendly process and has several advantages over water retting providing a high yield, quality and consistent quality to fibres. Moreover, it is a faster process and produces fewer odours. Green stem of ankra plant wet retting takes 10–12 days but 6% NaOH treatment with enzyme ankra twigs fibre extraction takes only 1 day. The use of medium twigs for enzyme treatment resulted in 81.5% pulp yield (Kuhad and Singh 2013).
In another study banana fibre separation was attained by using pectinase enzyme produced by A. niger (Azzaz et al. 2013). In higher plant tissues cells are united together through middle lamella are rich in pectin, on enzyme treatment middle lamella will be destroyed and separation of fibres can be observed. Scanning electron microscopy reveals that pectin degraded and fibres are separated. Apart from use in treatment of natural fibres, pectinase treatment was also found to increase the mechanical properties of reinforced thermoplastic composites (Saleem et al. 2008).
3.4 Animal Feeds
Reports on the supplementation of pectinases in animal feed suggest that it helps to increase the absorption of nutrients by animals aided by degradation of fibres entrapping them (Hoondal et al. 2002).
3.5 Liquefaction and saccharification of biomass
Bioethanol obtained from enzyme treated biomass is a suitable alternative to fossil fuels and it helps to remove green house gas emissions. As the complex pectinaceous structures in feed stock degraded and hydrolysed by pectinases, the rate of ethanol production increases (Chen et al. 2012). Enzymatic hydrolysis of biomass is efficient treatment without generation of toxic waste and economically feasible process. Liquid hot water treatment enlarges the accessible and susceptible surface area of sugar and makes it accessible to hydrolytic enzymes. Substrate with enzyme cocktail of 2.5U gives high level of galacturonic acid released shows high percentage of saccharification.0.25–2.0 g lemon peel in 15 ml buffer with enzyme gives 94.59% saccharification and 1 g yield of sugar. Increase in lemon peel concentration decreases saccharification yield and reducing sugars and it may be due to feed back inhibition i.e. high end product (reducing sugar) concentration which results in enzyme inactivation (Mostafa et al. 2013).
3.6 Coffee and tea fermentation
Alkaline pectinases are generally produced by bacteria Bacillus species. Some fungi and yeasts also produces alkaline pectinases which are used for coffee and tea fermentation (Pedrolli et al. 2009). Soft ripe coffee fruits sorted and passed into mechanical pulper to remove coffee skin. Only coffee seed is released through pulper screen, thin viscous inner mesocarp which is a highly hydrated layer called mucilage can be removed by natural fermentation then washed dried to 35% moisture content.
Pectinase treatment increase tea fermentation rate and destroy foam forming property of instant tea powders by destroying pectin (Pasha et al. 2013). Pectinase from A. niger, Byssochlamys fulva and Mucor circinelloids used for fermentation of tea leaves from Camellia sinensis plant, increased production of phenolic compounds increases tea quality. Polygalacturonase are mainly used to increase tea quality (Thakur and Gupta 2012).
3.7 Wine industry
Pectinases are also widely used in wine making industry to increase the quality of wine (Rehman et al. 2015). The use of pectinases in combination of other enzymes such as hemicellulases, glucanases and glycosidases in the wine industry is considered to be the oldest application of this enzyme. The use of pectinase in wine industry in the grape must is done mainly to support the extraction process to maximize juice yield, facilitate filtration and intensify the flavour and colour (Sieiro et al. 2012). However, the use of pectinases is often low in commercial preparations to avoid the production of excess amount of methanol by the action of pectin methyl esterases.
3.8 Essential oil extraction
The utility of pectinase in the extraction of essential oils from various sources such as olives (Ortiz et al. 2017), flaxseed oil (Kulkarni et al. 2017), dates (Mehanni et al. 2017) etc. have been widely studied. Pectinase treatment yields oil of superior quality than organically extracted oil with lower fatty acids, peroxide value and colour intensity (Mehanni et al. 2017). Moreover the retaining of phospholipids in solid phase reduces the cost for refining process.
3.9 Paper bleaching, deinking and recycling
Pectinases in combination with xylanases are primarily used in the paper industry as a bio-bleaching agent. Unlike the conventional chemical bleaching agents, the use of enzymes is found to be eco-friendly, less harsh and good in improving the quality of the paper. Apart from reducing the kappa number and permanganate number of pulp, biological bleaching using pectinases in combination with xylanases brighten the paper and improve its physical properties (Kaur et al. 2010; Nathan et al. 2017). The replacement of chemical pectinases also contribute to reduce the chlorine disposal into the environment compared to those chemical alternative strategies. Recent studies indicate that ultra filtered concoction of pectinase and xylanase give better result than crude enzyme (Sharma et al. 2017). Biological deinking and bleaching would aid in lowering the BOD and COD of waste water before disposal (Singh et al. 2012a).
3.10 Others
The use of pectinase in the production of non-digestible oligosaccharides especially bioactive compounds has also been reviewed (Bezerra et al. 2017). The use of enzyme decoctions of pectinases and other enzymes are used to obtain good preparation of viruses from plant tissues (Butot et al. 2007). The role of pectinases in treatment of waste water is also evident in the remediation of water from pectin waste exuding industrial units (Singh et al. 2012a).This makes wastewater devoid of pectinaceous matter and ready for treatment by activated sludge treatment (Hoondal et al. 2002).
4 Conclusion and future perspectives
Application of pectinases in various fields shows the potential of green process. The various applications of pectinases as noted in the above section, greatly demands its economised production. Yet the properties of thermo-tolerance and high yield would surely be an advantage to these enzymes to its effective utilisation. Thus the use of various proteomic, genomic and production optimization methodologies either singly or combinatorial should be attempted to meet the increasing global demands of pectinases. Fine tuning will lead to the development of an economically viable strategy.
References
Abbott DW, Boraston AB (2007) The structural basis for exopolygalacturonase activity in a family 28 glycoside hydrolase. J Mol Biol 368(5):1215–1222
Abdulrachman D et al (2017) Heterologous expression of Aspergillus aculeatus endo-polygalacturonase in Pichia pastoris by high cell density fermentation and its application in textile scouring. BMC Biotechnol 17:15. doi:10.1186/s12896-017-0334-9
Ahlawat S, Mandhan RP, Dhiman SS, Kumar R, Sharma J (2008) Potential application of alkaline pectinase from Bacillus subtilis SS in pulp and paper industry. Appl Biochem Biotechnol 149:287–293
Akhter N, Morshed MA, Uddin A, Begum F, Sultan T, Azad AK (2011) Production of pectinase by Aspergillus niger cultured in solid state media. Int J Biosci 1:33–42
Aliaa ARN, Mazlina MKS, Taip FS (2010) Impact of commercial pectolytic enzymes on selected properties of white dragon fruit juice. J Inst Eng Malays 71:25–31
Almeida C, Brainyik TA, Moradas-Ferreira P, Teixeira J (2003) Continuous production of pectinase by immobilized yeast cells on spent grains. J Biosci Bioeng 96(6):513–518
Amin F, Bhatti HN, Bhatti IA, Asgher M (2013) Utilization of wheat bran for enhanced production of exopolygalacturonase by Penicillium notatum using response surface methodology. Pak J Agric Sci 50:469–477
Amin F, Bhatti HN, Bilal M, Asgher M (2017a) Multiple parameter optimizations for enhanced biosynthesis of exo-polygalacturonase enzyme and its application in fruit juice clarification. Int J Food Eng 13(2). doi:10.1515/ijfe-2016-0256
Amin F, Bhatti HN, Bilal M, Asgher M (2017b) Purification, kinetic, and thermodynamic characteristics of an exo-polygalacturonase from Penicillium notatum with industrial perspective. Appl Biochem Biotechnol. doi:10.1007/s12010-017-2455-y
Azzaz HH, Murad HA, Khalif AM, Morsy TA, Mansour AM (2013) Pectinase production optimization and its application in banana fibre degradation Egypt. J Nutr Foods 16:117–125
Banafshe R, Hamid RKH (2014) Isolation and partial characterization of a bacterial thermostable polymethyl galacturonase from a newly isolated Bacillus sp. strain BR1390. Iran. J Biotechnol 12(4):e1133
Benen JAE, van Alebeek GJWM, Voragen AGJ, Visser J (2003) Mode of action analysis and structure-function relationships of Aspergillus niger pectinolytic enzymes. In: Voragen F, Schols H, Visser R (eds) Advances in pectin and pectinase research. Springer, Netherlands, pp 235–256. doi:10.1007/978-94-017-0331-4_18
Bennamoun L et al (2016) Production and properties of a thermostable, pH-stable exo-polygalacturonase using Aureobasidium pullulans isolated from Saharan soil of Algeria grown on tomato pomace. Foods 5:72
Bezerra T, Montibra R, Hansen EB, Contiero J (2017) Microbial glycosidases for nondigestible oligosaccharides production. In: Senturk M (ed) Enzyme inhibitors and activators. InTech Open Access Publishers, pp 181–206. doi:10.5772/65935
Blanco P, Thow G, Simpson CG, Villa TG, Williamson B (2002) Mutagenesis of key amino acids alters activity of a Saccharomyces cerevisiae endo-polygalacturonase expressed in Pichia pastoris. FEMS Microbiol Lett 210:187–191
Bonivento D et al (2008) Crystal structure of the endopolygalacturonase from the phytopathogenic fungus Colletotrichum lupini and its interaction with polygalacturonase inhibiting proteins. Proteins Struct Funct Bioinf 70(1):294–299
Bussink HJD, Buxton FP, Fraaye BA, Graaff LHd, Visser J (1992) The polygalacturonases of Aspergillus niger are encoded by a family of diverged genes. Eur J Biochem 208:83–90
Butot S, Putallaz T, Sanchez G (2007) Procedure for rapid concentration and detection of enteric viruses from berries and vegetables. Appl Environ Microbiol 73(1):186–192
Byrne CE, Cavalitto SF, Voget CE (2017) Purification and characterization of two inducible exopolygalacturonases from Aspergillus kawachii. Biocatal Agric Biotechnol 10:38–45. doi:10.1016/j.bcab.2017.02.005
Cao J, Zheng L, Chen S (1992) Screening of pectinase producer from alkalophilic bacteria and study on its potential application in degumming of ramie. Enzyme Microb Technol 14:1013–1016
Caprari C et al (1993) Fusarium moniliforme secretes four endopolygalacturonases derived from a single gene product. Physiol Mol Plant Path 43:453–462
Carroll SB, Grenier JK, Weatherbee SD (2005) From DNA to diversity. Molecular genetics and the evolution of animal design, 2nd edn. Blackwell Scientific, Malden
Celestino SMC et al (2006) Purification and characterization of a novel pectinase from Acrophialophora nainiana with emphasis on its physicochemical properties. J Biotechnol 123:33–42. doi:10.1016/j.jbiotec.2005.10.024
Charoenrat T et al (2013) Improvement of recombinant endoglucanase produced in Pichia pastoris KM71 through the use of synthetic medium for inoculum and pH control of proteolysis. J Biosci Bioeng 116:193–198. doi:10.1016/j.jbiosc.2013.02.020
Chen Q, Jin Y, Zhang G, Fang Y, Xiao Y, Zhao H (2012) Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 5:3019–3032
Cheng Z et al (2017) Identification of an acidic endo-polygalacturonase from Penicillium oxalicum CZ1028 and its broad use in major tropical and subtropical fruit juices production. J Biosci Bioeng 23(6):665–672. doi:10.1016/j.jbiosc.2017.01.013
Colla E, Santos LO, Deamici K, Magagnin GN, Vendruscolo M, Costa JAV (2016) Simultaneous production of amyloglucosidase and exo-polygalacturonase by Aspergillus niger in a rotating drum reactor. Appl Biochem Biotechnol 181(2):627–637
Comtet-Marre S et al (2017) Metatranscriptomics reveals the active bacterial and eukaryotic fibrolytic communities in the rumen of dairy cow fed a mixed diet. Front Microbiol 8:67. doi:10.3389/fmicb.2017.00067
Cuesta UI (2016) A Review of natural and engineered enzymes involved in bioethanol production. University of Montana, Montana
Damak N, Hadj-Taieb N, Bonnin E, Ben Bacha A, Gargouri A (2011) Purification and biochemical characterization of a novel thermoactive fungal pectate lyase from Penicillium occitanis. Process Biochem 46:888–893. doi:10.1016/j.procbio.2010.12.014
Demir H, Tari C (2017) Bioconversion of wheat bran for polygalacturonase production by Aspergillus sojae in tray type solid-state fermentation. Int Biodeterior Biodegrad 106:60–66
Finkler ATJ et al (2017) Intermittent agitation contributes to uniformity across the bed during pectinase production by Aspergillus niger grown in solid-state fermentation in a pilot-scale packed-bed bioreactor. Biochem Eng J 121:1–12. doi:10.1016/j.bej.2017.01.011
Giacobbe S, Pepe O, Ventorino V, Birolo L, Vinciguerra R, Faraco V (2014) Identification and characterisation of a pectinolytic enzyme from Paenibacillus xylanolyticus. BioResources 9:4873–4887
Gummadi SN, Panda T (2003) Purification and biochemical properties of microbial pectinases—a review. Process Biochem 38:987–996
Gummadi SN, Manoj N, Kumar DS (2007) Structural and biochemical properties of pectinases. In: Polaina J, MacCabe AP (eds) Industrial enzymes structure, function and applications. Springer, Netherlands, pp 99–115. doi:10.1007/1-4020-5377-0_7
Hoondal G, Tiwari R, Tewari R, Dahiya NB, Beg Q (2002) Microbial alkaline pectinases and their industrial applications: a review. Appl Microbiol Biotechnol 59(4):409–418
Jurnak F et al (1996) Functional implications of the three-dimensional structures of pectate lyases. In: Visser J, Voragen AGJ (eds) Progress in biotechnology, vol 14. Elsevier, Netherlands, pp 295–308. doi:10.1016/S0921-0423(96)80263-2
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77:215–227. doi:10.1016/s0960-8524(00)00118-8
Kaur A, Mahajan R, Singh A, Garg G, Sharma J (2010) Application of cellulase-free xylano-pectinolytic enzymes from the same bacterial isolate in biobleaching of kraft pulp. Bioresour Technol 101:9150–9155. doi:10.1016/j.biortech.2010.07.020
Khan M, Nakkeeran E, Umesh-Kumar S (2013) Potential application of pectinase in developing functional foods. Annu Rev Food Sci Technol 4:21–34
Khatri BP, Bhattarai T, Shrestha S, Maharjan J (2015) Alkaline thermo-stable pectinase enzyme from Aspergillus niger strain MCAS2 isolated from Manaslu Conservation Area, Gorkha Nepal. SpringerPlus 4:1–8. doi:10.1186/s40064-015-1286-y
Kluskens LD et al (2005) Characterization and mode of action of an exopolygalacturonase from the hyperthermophilic bacterium Thermotoga maritime. FEBS J 272:5464–5473
Ko CH, Tsai CH, Tu J, Tang SH, Liu CC (2011) Expression and thermostability of Paenibacillus campinasensis BL11 pectate lyase and its applications in bast fibre processing. Ann Appl Biol 158:218–225. doi:10.1111/j.1744-7348.2010.00456.x
Kuhad RC, Singh A (2013) Biotechnology for environmental management and resource recovery. Springer, Berlin
Kulkarni NG, Kar JR, Singhal RS (2017) Extraction of flaxseed oil: a comparative study of three-phase partitioning and supercritical carbon dioxide using response surface methodology. Food Bioprocess Technol 10(5):940–948
Kusuma MP, Sri RRD (2014) Thermoalkaline polygalacturonases—a review. Int J Pharm Sci Rev Res 28:162–165
Li X, Wang H, Zhou C, Ma Y, Li J, Song J (2014) Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnol 14:18. doi:10.1186/1472-6750-14-18
Liao CH (1989) Analysis of pectate lyases produced by soft rot bacteria associated with spoilage of vegetables. Appl Environ Microbiol 55(7):1677–1683
Lima JO, Pereira JF, Araujo EFd, Queiroz MVd (2017) Pectin lyase overproduction by Penicillium griseoroseum mutants resistant to catabolite repression. Braz J Microbiol . doi:10.1016/j.bjm.2016.12.009
Ma X et al (2017) Characteristics of pectinase treated with ultrasound both during and after the immobilization process. Ultrason Sonochem 36:1–10. doi:10.1016/j.ultsonch.2016.10.026
Maisuria VB, Nerurkar AS (2012) Biochemical properties and thermal behaviour of pectate lyase produced by Pectobacterium carotovorum subsp. carotovorum BR1 with industrial potentials. Biochem Eng J 63:22–30. doi:10.1016/j.bej.2012.01.007
Maldonado MC, Saad AM (1998) Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Ind Microbiol Biotechnol 20:34–38
Martens-Uzunova ES, Schaap PJ (2009) Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics. Fungal Genet Biol 46:S170–S179
Martin N, Guez MAU, Sette LD, Da Silva R, Gomes E (2010) Pectinase production by a Brazilian thermophilic fungus Thermomucor indicae-seudaticae N31 in solid-state and submerged fermentation. Microbiology 79:306–313. doi:10.1134/s0026261710030057
Martins ES, Silva D, Da Silva R, Gomes E (2002) Solid state production of thermostable pectinases from thermophilic Thermoascus aurantiacus. Process Biochem 37:949–954. doi:10.1016/S0032-9592(01)00300-4
Mehanni AE, El-Reffaei WH, Melo A, Casal S, Ferreira IM (2017) Enzymatic extraction of oil from Balanites Aegyptiaca (desert date) kernel and comparison with solvent extracted oil. J Food Biochem 41(2):e12270. doi:10.1111/jfbc.12270
Mei Y, Chen Y, Zhai R, Liu Y (2013) Cloning, purification and biochemical properties of a thermostable pectinase from Bacillus halodurans M29. J Mol Catal B: Enzym 94:77–81. doi:10.1016/j.molcatb.2013.05.00
Mostafa FA, Ahmed SA, Helmy WA (2013) Enzymatic saccharification of pretreated lemon peels for fermentable sugar production. J Appl Sci Res 9:2301–2310
Nathan VK, Rani ME, Rathinasamy G, Dhiraviam KN (2017) Low molecular weight xylanase from Trichoderma viride VKF3 for bio-bleaching of newspaper pulp. BioResource 12(3):5264–5278
Ortega N et al (2004) Kinetic behaviour and thermal inactivation of pectinlyase used in food processing. Int J Food Sci Technol 39:631–639. doi:10.1111/j.1365-2621.2004.00822.x
Ortiz GE et al (2017) Pectinase production by Aspergillus giganteus in solid-state fermentation: optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J Indus Microbiol Biotechnol 44(2):197–211
Parani K, Eyini M (2011) Mycelial protoplasts release from Pleurotus eous and P.flabellatus. J Biosci Res 2:130–135
Pasha KM, Anuradha P, Subbarao D (2013) Applications of pectinases in industrial sector. Int J Pure Appl Sci Technol 16:89–95
Pedrolli DB, Carmona EC (2010) Purification and characterization of the exopolygalacturonase produced by Aspergillus giganteus in submerged cultures. J Ind Microbiol Biotechnol 37:567–573. doi:10.1007/s10295-010-0702-0
Pedrolli DB, Monteiro AC, Gomes E, Carmona EC (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol J 3:9–18
Phutela U, Dhuna V, Sandhu S, Chadha BS (2005) Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz J Microbiol 36:63–69
Pijning T et al (2009) The crystal structure of a hyperthermoactive exopolygalacturonase from Thermotoga maritima reveals a unique tetramer. FEBS Lett 583(22):3665–3670
Pitol LO, Biz A, Mallmann E, Krieger N, Mitchell DA (2016) Production of pectinases by solid-state fermentation in a pilot-scale packed-bed bioreactor. Chem Eng J 283:1009–1018
Poletto P, TsA Polidoro, Mr Zeni, da Silveira MM (2017) Evaluation of the operating conditions for the solid-state production of pectinases by Aspergillus niger in a bench-scale, intermittently agitated rotating drum bioreactor.LWT-Food. Sci Technol 79:92–101. doi:10.1016/j.lwt.2017.01.018
Prathyusha K, Suneetha V (2011) Bacterial pectinases and their potent biotechnological application in fruit processing/juice production industry: a review. J Phytol 3:16–19
Priya V, Sashi V (2014) Pectinase producing microorganisms. Int J Sci Res Pub 4:1–4
Rebello S, Jose L, Sindhu R, Aneesh EM (2017) Molecular advancements in the development of thermostable phytases. Appl Microbiol Biotechnol 101:2677–2689
Rehman HU et al (2015) Morphological and molecular based identification of pectinase producing Bacillus licheniformis from rotten vegetable. J Genet Eng Biotechnol 13:139–144
Ruiz HA, Rodriguez-Jasso RM, Rodriguez R, Contreras-Esquivel JC, Aguilar CN (2012) Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem Eng J 65:90–95
Saadoun I, Dawagreh A, Jaradat Z, Ababneh Q (2013) Influence of culture conditions on pectinase production by Streptomyces sp (strain J9). Int J Life Sci Med Res 3:148
Saleem Z, Rennebaum H, Pudel F, Grimm E (2008) Treating bast fibres with pectinase improves mechanical characteristics of reinforced thermoplastic composites. Compos Sci Technol 68(2):471–476
Sapunova LI, Lobanok AG, Mikhailova RV (1997) Conditions of synthesis of pectinases and proteases by Aspergillus alliaceus and production of a complex macerating preparation. Appl Biochem Microbiol 33:257–260
Schnitzhofer W et al (2007) Purification and mechanistic characterisation of two polygalacturonases from Sclerotium rolfsii. Enzyme Microb Technol 40:1739–1747. doi:10.1016/j.enzmictec.2006.11.005
Sharma DC, Satyanarayana T (2012) Biotechnological potential of agro residues for economical production of thermoalkali-stable pectinase by Bacillus pumilus dcsr1 by solid-state fermentation and its efficacy in the treatment of ramie fibres. Enzyme Res 2012:1–7
Sharma N, Rathore M, Sharma M (2013) Microbial pectinase: sources, characterization and applications. Rev Environ Sci Biotechnol 12:45–60
Sharma D, Agrawal S, Yadav RD, Mahajan R (2017) Improved efficacy of ultrafiltered xylanase -pectinase concoction in biobleaching of plywood waste soda pulp. 3. Biotech 7(1):2
Sieiro C, da Silva AF, Garcia-Fraga B, Lopez-Seijas J, Villa TG (2012) Microbial pectic enzymes in the food and wine industry. INTECH Open Access Publisher, Rijeka
Singh SA, Ramakrishna M, Rao AGA (1999) Optimization of downstream processing parameters for the recovery of pectinase from the fermented broth of Aspergillus carbonarious. Process Biochem 35:411–417
Singh A, Yadav RD, Kaur A, Mahajan R (2012a) An ecofriendly cost effective enzymatic methodology for deinking of school waste paper. Bioresour Technol 120:322–327. doi:10.1016/j.biortech.2012.06.050
Singh R, Dhawan S, Singh K, Kaur J (2012b) Cloning, expression and characterization of a metagenome derived thermoactive/thermostable pectinase. Mol Biol Rep 39:8353–8361
Solbak AI et al (2005) Discovery of pectin-degrading enzymes and directed evolution of a novel pectate lyase for processing cotton fabric. J Biol Chem 280:9431–9438
Tapre AR, Jain RK (2014) Pectinases: enzymes for fruit processing industry Int Food Res J 21:447–453
Teixeira JA, Gonçalves DB, de Queiroz MV, de Araujo EF (2011) Improved pectinase production in Penicillium griseoroseum recombinant strains. J Appl Microbiol 111:818–825. doi:10.1111/j.1365-2672.2011.05099.x
Thakur J, Gupta R (2012) Improvement of tea leaves fermentation through pectinases. Acta Microbiol Immunol Hung 59:321–334
Tu T et al (2016) Probing the role of cation-π interaction in the thermotolerance and catalytic performance of endo-polygalacturonases. Sci Rep 6:38413
Varavallo MA et al (2007) Isolation of recombinant strains with enhanced pectinase production by protoplast fusion between Penicillium expansum and Penicillium griseoroseum. Braz J Microbiol 38:52–57
Versari A, Biesenbruch S, Barbanti D, Farnell PJ, Galassi S (1998) Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Res Int 30:811–817. doi:10.1016/S0963-9969(98)00050-7
Visser J, Bussink HJ, Witteveen C (2004) Gene expression in filamentous fungi. In: Smith A (ed) Gene expression in recombinant microorganisms. Marcel Dekker Inc., New York, pp 241–308
Wang H, Li X, Ma Y, Song J (2014) Characterization and high-level expression of a metagenome-derived alkaline pectate lyase in recombinant Escherichia coli. Process Biochem 49:69–76. doi:10.1016/j.procbio.2013.10.001
Wang C et al (2016) Metagenomic analysis of microbial consortia enriched from compost: new insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol Biofuels 9:22. doi:10.1186/s13068-016-0440-2
Wolf-Marquez VE et al (2015) Batch and pulsed fed-batch cultures of Aspergillus flavipes FP-500 growing on lemon peel at stirred tank reactor. Appl Biochem Biotechnol 177(6):1201–1215
Wolf-Marquez VE et al (2017) Scaling-up and ionic liquid-based extraction of pectinases from Aspergillus flavipes cultures. Bioresour Technol 225:326–335. doi:10.1016/j.biortech.2016.11.067
Wong LY, Saad WZ, Mohamad R, Tahir PM (2017) Optimization of cultural conditions for polygalacturonase production by a newly isolated Aspergillus fumigatus R6 capable of retting kenaf. Ind Crops Prod 97:175–183. doi:10.1016/j.indcrop.2016.12.019
Xiao Z et al (2008) Improvement of the Thermostability and activity of a pectate lyase by single amino acid substitutions, using a strategy based on melting-temperature-guided sequence alignment. Appl Environ Microbiol 74:1183–1189. doi:10.1128/aem.02220-07
Yang J, Luo H, Li J, Wang K, Cheng H, Bai Y (2011) Cloning, expression and characterization of an acidic endo-polygalacturonase from Bispora sp. MEY-1 and its potential application in juice clarification. Process Biochem 46:272–277. doi:10.1016/j.procbio.2010.08.022
You C, Huang Q, Xue H, Xu Y, Lu H (2010) Potential hydrophobic interaction between two cysteines in interior hydrophobic region improve thermostability of a family 11 xylanase from Neocallimastix patriciarum. Biotechnol Bioeng 105:861–870
Yu P, Zhang Y, Gu D (2017) Production optimization of a heat-tolerant alkaline pectinase from Bacillus subtilis ZGL14 and its purification and characterization. Bioengineered 16:1–11. doi:10.1080/21655979.2017.1292188
Yuan P, Meng K, Huang H, Shi P, Luo H, Yang P (2011) A novel acidic and low-temperature-active endo-polygalacturonase from Penicillium sp. CGMCC 1669 with potential for application in apple juice clarification. Food Chem 129:1369–1375. doi:10.1016/j.foodchem.2011.05.065
Yuan P et al (2012) Abundance and genetic diversity of microbial polygalacturonase and pectate lyase in the sheep rumen ecosystem. PLoS ONE 7:e40940
Zhang J et al (2013) High-level extracellular production of alkaline polygalacturonate lyase in Bacillus subtilis with optimized regulatory elements. Bioresour Technol 146:543–548. doi:10.1016/j.biortech.2013.07.129
Zhang F, Tian M, Du M, Fang T (2017) Enhancing the activity of pectinase using pulsed electric field (PEF) treatment. J Food Eng 205:56–63. doi:10.1016/j.jfoodeng.2017.02.023
Zhou C, Xue Y, Ma Y (2017) Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming. Appl Microbiol Biotechnol 9:1–14
Acknowledgements
Sharrel Rebello acknowledges SERB for National Post Doctoral Fellowship (File no PDF/2015/000472). One of the authors Raveendran Sindhu acknowledges Department of Biotechnology for sanctioning a project under DBT Bio-CARe scheme (File no BT/Bio-CARe/06/890/2011-2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rebello, S., Anju, M., Aneesh, E.M. et al. Recent advancements in the production and application of microbial pectinases: an overview. Rev Environ Sci Biotechnol 16, 381–394 (2017). https://doi.org/10.1007/s11157-017-9437-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-017-9437-y