Abstract
Pectinase, a group of pectin degrading enzymes, is one of the most influential industrial enzymes, helpful in producing a wide variety of products with good qualities. These enzymes are biocatalysts and are highly specific, non-toxic, sustainable, and eco-friendly. Consequently, both pectin and pectinase are crucially essential biomolecules with extensive applicatory perception in the biotechnological sector. The market demand and application of pectinases in new sectors are continuously increasing. However, due to the high cost of the substrate used for the growth of microbes, the production of pectinase using microorganisms is limited. Therefore, low-cost or no-cost substrates, such as various agricultural biomasses, are emphasized in producing pectinases. The importance and implications of pectinases are rising in diverse areas, including bioethanol production, extraction of DNA, and protoplast isolation from a plant. Therefore, this review briefly describes the structure of pectin, types and source of pectinases, substrates and strategies used for pectinases production, and emphasizes diverse potential applications of pectinases. The review also has included a list of pectinases producing microbes and alternative substrates for commercial production of pectinase applicable in pectinase-based industrial technology.
Key points
• Pectinase applications are continuously expanding.
• Organic wastes can be used as low-cost sources of pectin.
• Utilization of wastes helps to reduce pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectinase is a complex heterogeneous group of enzymes that acts on pectic substances. The pectic content is the generic name for a natural heteropolymer compound, acidic in nature, present commonly in plants and fruits (Picot-Allain et al. 2020; Satapathy et al. 2020). The homemade winemaker used pectinases in producing wine two centuries ago. However, the first commercial use of pectinases started in 1930 to clarify fruit juice, and it was later used in making wine and fruit juice (Tapre and Jain 2014; Bhardwaj et al. 2017). Only after 1960, other enzymes were recorded, the chemical nature of plant tissues becomes apparent, and researchers started to use more enzymes proficiently. Of all these enzymes, pectinases are essential catalysts of the commercial sector for the maximum yield of stable and clarified fruit juices (Tapre and Jain 2014). Applications of pectinases are increasing continuously, and the worldwide enzyme market accounts for 25% of pectinases (Amin et al. 2017). The different natural sources for pectinase production are yeast, bacteria, fungi, and plants. Microbial enzymes are replacing the chemical catalysts because enzymatic catalysts are more specific, less aggressive, ecofriendly, and saves energy (Garg et al. 2016; Amin et al. 2019).
As the demand for pectin and pectinase is upgoing, this review aims to briefly explain the different avenues of pectinases applications so that the review encourages the researcher to develop different strategies for maximum pectinases production and to study its applications in different areas. In addition, the review will help to know the ideas regarding its sources, production strategies, and alternative sources of substrate for pectinases production and are described in following paragraphs.
Pectin
Pectin is a naturally occurring biopolymer, one of the main constituents of all higher plants cell wall. It is only a sole polysaccharide in the middle lamella responsible for cell cohesion. It is non-toxic, anionic heteropolysaccharide in nature, accounting for 0.5 to 4.0% of the fresh weight of the plant (Jayani et al. 2005; Picot-Allain et al. 2020). In plants and fruits, pectin provides the rigidity and structure of the cell. The type of pectin plays an essential role in the texture of vegetables and fruits during growth, ripening, and storage. Also, pectin plays an important role on the plant growth and development, as well as morphogenesis, defense, cell–cell adhesion, wall structure, signaling, cell expansion, wall porosity, binding of ions, growth factors and enzymes, pollen tube growth, seed hydration, leaf abscission, and fruit development (Khan et al. 2013; Chen et al. 2015; Yamada et al. 2015). The enzymatic and chemical modifications affect the pectin that is present in fruits. During the ripening of fruits, solubilization of pectin and softening of fruits take place due to the pectinase (Paniagua et al. 2014). The molecular weight of pectin ranges from 60,000 to 318,000 g/mol and varies with the plant source, stage of fruit/plant, extraction condition, and methods (Sayah et al. 2016; Yang et al. 2018).
Structure of pectin
The structure of pectin is complex, not known entirely, and is still in debate. However, two pectic models are prevalent; the “smooth and hairy region” model and the “RG I backbone” model.
The main constituent of pectin polysaccharides is D-galacturonic acid, joined by α (1 → 4) glycosidic bonds. Other sugar units, including ribose, galactose, arabinose, and sucrose, are also found inserted into the polymers, and about 70% galacturonic acid is present in the cell wall (Harholt et al. 2010; Picot-Allain et al. 2020; Shrestha et al. 2020). The structural components and molecular mass of the pectin differ with their origin, cell types, different stages of cellular development, extraction condition, extraction methods, and the thickness of a given cell wall (Harholt et al. 2010; Pancerz et al. 2021). However, the pectin molecule of the same molar mass may have different hydrodynamic properties due to the difference in the degree of methylation (DM), branching, and neutral sugar content. The most common polysaccharide structural components of pectin are homogalacturonan (HG), rhamnogalacturonan I (RG I), and rhamnogalacturonan II (RG II) (Lara-Espinoza et al. 2018; Picot-Allain et al. 2020; Ropartz and Ralet 2020).
Homogalacturonan
This is the simplest and most abundantly found in pectin, accounting for about 75–100% pectin. HG represents the backbone chain of the pectin molecule and contains linear polymers of α-D galacturonic acid (GalA) residue linked with α (1 → 4) glycosidic bonds. The minimum length of HG is estimated to be 100 GalA residues. GalA residues can be acetylated or/and methyl esterified. The number of acetyl esterified GalA and methyl esterified GalA in 100 GalA residues is known as the degree of acetylation (DA) and the degree of methylation (DM), respectively. Both DA and DM have a massive effect on the functional properties of pectin. This HG region is also considered as a smooth zone of pectin (Lara-Espinoza et al. 2018; Ropartz and Ralet 2020).
Rhamnogalacturonan I
RG I is present in the highly branched area containing many neutral sugars such as arabinose, galactose, and mannose as the side chains of a-1, two-linked resides of L-rhamnopyranose. Moreover, the backbone of RG I is formed by the repeated disaccharide structure of rhamnose and galacturonic acid. The galacturonic residue can undergo acetylation and bind with other neutral sugars (galactose, arabinose, and xylose). Therefore, RG I accounts for about 10–25% of pectin (Lara-Espinoza et al. 2018).
Rhamnogalacturonan II
RG II is the most complex branch of a pectic domain, containing an HG backbone, and is the most conserved and widespread domain of the plant. RG II accounts for 0–10% of pectin. A short HM of RG II is substituted by four side chains of several unusual sugar residues. RG I and RG II regions of pectin are known as hairy regions of pectin (Lara-Espinoza et al. 2018; Mellinas et al. 2020).
Application of pectin
Pectin has remarkable properties; therefore, pectin is utilized as a gelling, stabilizing, and emulsifying agent in the food and cosmetic industries. Also, pectin has multiple positive effects on human health, including lowering blood cholesterol and serum glucose levels, inhibiting the growth of cancer cells, and stimulating the immune response (Jackson et al. 2007; Mohnen 2008; Lara-Espinoza et al. 2018). Moreover, pectin is also used to produce various exclusive products, including edible and biodegradable films, adhesives, paper substitutes, foams and plasticizers, surface modifiers for medical devices, and materials for biomedical implantation. Besides, pectin is used as a carrier material in colon-specific drug delivery systems (Chambin et al. 2006; Vityazev et al. 2017; Lara-Espinoza et al. 2018), effectively removing toxic chemicals like lead, cadmium, arsenic, and mercury from the gastrointestinal tract and respiratory organs (Kohn 1982; Lara-Espinoza et al. 2018). Moreover, pectin has been used as a pectin hydrogel in controlled-release matrix tablet formulations and water purification (Aydìn and Akbuǧa 1996; Thakur et al. 2019).
Pectinases
Pectinases are biocatalyst comprising complex enzymes that degrade pectic substances (Khan et al. 2013; Kavuthodi et al. 2015; Oumer 2017). In the past, pectinases were only known as the virulence factor in the decomposition of a plant cell wall. But, today, pectinases are becoming necessary enzymes due to its multiple applications in a wide range of industrial sectors such as food, textile, and biofuel industries and accounts for about 25% of total enzyme market sale (Oumer 2017; Shrestha et al. 2020). Nevertheless, pectinases are the upcoming important biocatalyst because of its versatility, broad-substrate specificity, inducibility, stability, and ability to act on a large variety of pectic substances (Pedrolli et al. 2009; Martín et al. 2019).
Sources of pectinases
There are many sources for pectinases that are widely distributed in nature. The different sources of pectinases are plants, bacteria, fungi, yeasts, insects, nematodes, and protozoa. However, the microbial source is significant due to the fast growth, diversified widespread, shorter fermentation, and more accessible genetic modifications. Microbial pectinases play a vital role in plant pathogenesis, symbiosis, and decomposition of plant deposits (Polizeli et al. 2016; Amin et al. 2019). Also, the microbial pectinases production process has no political or social issues as in plant and animal sources. It is reported that 35% of pectinases are from bacterial source, 55% from fungi and yeast, while only 15% from plant or animal sources (Kavuthodi and Sebastian 2018). Many studies are undergoing related to a high quality of pectinases like the high stability to different physicochemical conditions from microbes of different sources. Some of the microbes capable of producing pectinases at their optimum conditions are listed in Table 1.
Bacterial source
Different bacteria are the ideal source for pectinases production. Bacteria are easy to grow in different laboratory environmental conditions like temperatures and pH, have a shorter lifetime, easy to manipulate genetically, and are environmentally friendly (Amin et al. 2019; John et al. 2020). Pectinases from bacterial sources are usually neutral or slightly alkaline in nature (Hoondal et al. 2002; Bhardwaj et al. 2017; Kavuthodi and Sebastian 2018). For example, pectinases produced from Bacillus tequilensis were stable showing higher pectinase activity at pH 10 (Zhang et al. 2019), and pectin lyase from Paenibacillus xylanolyticus showed stability at pH 9 (Giacobbe et al. 2014) and so on. Alkaline pectinase is predominantly produced from Bacillus sp., Pseudomonas sp., and actinomycetes (Hoondal et al. 2002; Kavuthodi and Sebastian 2018). Bacillus sp. is an essential and highly efficient bacterial source for industrial applications (Kavuthodi and Sebastian 2018) which can be perceived from Table 1 as well.
Fungal source
The fungi are the common source of acidic pectinases (Bhardwaj et al. 2017; Kavuthodi and Sebastian 2018), and the most crucial fungal strain for pectinase production is Aspergillus sp. This strain has outstanding importance because the strain produces non-toxic and economically important metabolites. Also, Aspergillus sp. is assigned as generally regarded as safe microbe (Bhardwaj et al. 2017; John et al. 2020). In addition, Table 1 illustrates that the fungi such as Aspergillus, Candida, Geotricum, and Rhizopus have a longer optimum time on pectinases production than bacteria. In the study, Penicillium notatum, Coriolus versicolor, Ganoderma lucidum, and Trametes hirsuta produced 83.46 U/gds, 73.21 U/gds, 62.29 U/gds, and 47.15 U/gds pectinases respectively when incubated at 30 °C (Amin et al. 2017).
Yeast pectinases
Yeast also acts as the source for pectinases. For example, Wickerhamomyces anomalus, Saccharomycopsis fibuligera, Papiliotrema flavescens, Pichia kudriavzevii, and Saccharomyces cerevisiae have the potential to be used as the starter for coffee fermentation (Haile and Kang 2019). Moreover, Rhodotorula glutinis isolated from fruit has pectinase and tannase activity. It was stated that this yeast could be higher (503.1 U/ml) polygalacturonase producer using immobilized cells (Taskin 2013). The polygalacturonase activity was also observed in Aureobasidium pullulans, Metschnikowia pulcherrima, and Metschnikowia fructicola. However, Belda et al. selected M. pilcherrima as a potential candidate to use on a semi-industrial scale for red wine upgrading (Belda et al. 2016).
Insect pectinases
Commercial pectinases are generally produced from a microbial source. However, insects can also be the source of pectinases. The sugarcane weevil, Sphenophorus levis, has pectin methylesterase and endo-polygalacturonase. The pectinase from sugarcane weevil has been expressed in Pichia pastoris, and P. pastoris could be used as an alternative resource for industrial pectinases (Habrylo et al. 2018). Polygalacturonase producing Bacillus sp. was isolated from the gut of Apis mellifera (honey bee) which was alkaline and slightly thermostable in nature (Paudel et al. 2015). Some of the forest pests and wood-boring cerambycid beetle, Apriona japonica, have cell wall–degrading enzymes, including pectinases (Pauchet et al. 2014).
Plant and animal pectinases
The plants and animal sources are less popular because many social and political issues need to be considered, less yield, time-consuming, and not appropriate to produce pectinase over a wide range of environmental conditions. In addition, plant and animal tissues may contain some harmful materials. Furthermore, sometimes pectinase extracted from diseased plants and animals may have a substantial risk of contamination from diseases and difficulties in isolation and purification, thereby discourage the production of pectinase from diseased plant and animal sources (Bhardwaj et al. 2017). Besides, plant tissue contains phenolic compounds and animal tissue, some inhibitors, and proteases (Bhardwaj et al. 2017; Kavuthodi and Sebastian 2018). Thus, the microbial source is the most valuable source for the pectinase enzyme. However, there should be more exploration for plant and animal pectinases.
Alternative substrates (low-cost pectin sources) for pectinases production
The extracted pectin from different plant sources has humongous applications. Thus, it is wise to use agricultural wastes as alternative sources for pectinase production to benefit economically and reduce agricultural waste disposal. The agro-wastes are renewable, inexpensive, and natural resource, acting as a cost-effective and eco-friendly source for pectinases production. Natural resource utilization will help solve the energy shortage problem, pollution concerns, waste disposal issues and will not compete with the food supply chain (Govindaraji and Vuppu 2020; Shrestha et al. 2020). Various agricultural wastes are incredibly nourishing, which enable and promote the growth of different microbes. Apple pomace, sunflower heads, orange peel, barley grain, and grape pomace have extensively been used as the carbon source for pectinase production (Osofero et al. 2018; John et al. 2020). However, there are lots of agro-waste that have the potential to be used as the source of pectin for pectinases production.
Crop waste
Wheat bran is one of the common agro-industrial waste accounts 15 to 20% and gets discarded during the wheat flour production process. Wheat bran has the potential to be used for industrially important enzymes. It is reported that wheat bran and tea extract together produce a 15.28-fold increase in polygalacturonase with a specific activity of 33.47 U/ml under solid-state fermentation (SSF), at 50 °C and pH 4 (Anand et al. 2017). In another study, when Aspergillus ojae was cultured on media with wheat bran at 37 °C for 4 days under SSF, polygalacturonase of 535.4 U/g substrates was produced (Demir et al. 2014). Similarly, Oumer and Abate observed maximum pectinase production (1272.4 ± 25.5 U/g) by Bacillus subtilis using wheat bran under SSF having initial pH 6.5 and at 37 °C (Oumer and Abate 2018). Kaur and Gupta screened 25 different agro waste such as orange peel, coconut fiber, paddy straw, mustard straw, mustard oil cake, rice bran, and lemon peel for pectinase and pectin lyase production. The maximum pectinase (450.50 ± 12.8 U/gds) was observed in orange peel-containing media at pH 7 and 35 °C. However, the mixture of orange peel and coconut fiber at a ratio of 4:1 revealed as the prominent substrate producing 3315 U/gds of pectinase with moisture content 60% at 35 °C and pH 4 after 4 days of incubation (Kaur and Gupta 2017).
Tobacco stalks are also considered waste, have a high amount of pectin, and Bacillus tequilensis showed pectinase activity of 1370 U/ml in optimal fermentation conditions; 40 h, pH 7, and inoculum amount 3% (Zhang et al. 2019). Aspergillus awamori was exploited to use leaves of Ficus religiosa in solid-state fermentation for producing pectinase. The maximum pectinase was observed in 72 h, at 30 °C, 60% v/w moisture content, and pH 5 (Dasari 2020).
Onion waste has the potential to be used as a substrate in pectinase production. Pereira et al. produced 4.82 U/ml of thermostable pectinase under SSF by using onion waste (Pereira et al. 2017).
Fruit and vegetable waste
When peels from fruits like orange, mango, pomegranate, and mosambi were studied for pectinase activity, orange peel gave maximum pectinase of 98.65 U/ml at 35 °C, pH 6, and 48 h of incubation (Govindaraji and Vuppu 2020). Mango peel utilization for pectinase production using Aspergillus foetidus gave the highest polygalacturonase and pectin lyase under SSF. The pectinase produced by this way gave the maximum mango juice clarification (92.5 ± 0.26%) at 40 °C and 150 min of incubation (Kumar and Sharma 2012). Similarly, Reddy and Saritha used mango fruit processing waste as a carbon source for pectinase production by using Enterobacter sp. under submerged fermentation (SmF) (Reddy and Saritha 2016). Grape skin and olive pomace were exploited to produce lignocellulosic enzymes, including pectinase. Two local species of Aspergillus, A. niger, and A. fumigatus were grown in solid media containing grape skin and olive pomace, and in some cases, media was supplemented with wheat (Sánchez et al. 2015). Orange peel was added in Czapeck media as a carbon source under SmF to produce pectinase from Aspergillus. They also showed the maximum pectinase yield (117.1 ± 3.4 μM/mL/min) at 30 °C and pH 5.5 after 4 days of incubation (Ahmed et al. 2016). In another study, Bacillus licheniformis produced 219 U/ml of pectinase under SmF and media supplemented with orange peels (Bibi et al. 2016). Similarly, orange peel and coconut fiber in 4:1 ratio were also used under SSF for pectinase production. In this condition, maximum pectinase (3315 U/gds) and pectin lyase (10.5 U/gds) were recorded at pH 4, moisture content of 60%, and in 4 days and 8 days of incubation, respectively (Kaur and Gupta 2017). Papaya is a tropical worldwide popular fruit having medicinal and nutritional values and contains pectin. Therefore, papaya peel has been used for pectin methylesterase production (246.83 U/gds) by exploiting Aspergillus tubingensis. The methylesterase thus produced was applied for pineapple juice clarification (Patidar et al. 2016).
Algal biomass
Algal biomass has also been used as a natural resource to produce pectinases. The presence of a significant amount of pectin in the middle lamella of the algal cell wall makes it applicable for this application. For example, biomass of charophyte green algae, Penium margaritaceum, has been studied in pectinase production (Domozych et al. 2014). In addition, brown or green algae such as Dictyopteris polypodioides, Sargassum wightii, Dictyopeteris divaricate, Ulva lactuca, and Codium tomentosum were used for pectinase production. Besides, the algal biomass can be used as the feedstock for other hydrolytic enzymes production. The green algal biomass from U. lactuca was observed to be the most suitable substrate than the rice husk and sugarcane bagasse for Bacillus licheniformis in producing pectinases under SmF (2457 ± 3.31 U/mg) compared to SSF (1432 ± 1.46 U/mg) (Pervez et al. 2017).
Pectinases production strategies
Fermentation strategies
In general, two fermentation techniques solid-state fermentation (SSF) and submerged state fermentation (SmF) are traditional methods of pectinase production. During fermentation, different parameters like growth medium, cultivation conditions, pH, temperature, aeration, moisture, salts, carbon source, nitrogen source, inoculum volume, inoculum age, type of strain, and inducers are considered for boosting pectinase production using microorganisms (Amin et al. 2017). However, the processes are tedious and time consuming.
Most of the enzyme manufacturing industries produce enzymes by using SmF because this process can easily be accessed, and the production is topped up (Oumer and Abate 2018). During SmF, there is uniform mixing of nutrient, better heat and mass transfer, and better diffusion of microorganisms. Therefore, for the commercial production of pectinase, SmF is generally used. However, the high cost of medium ingredients, low yield, high energy consumption, need of antifoaming agent to reduce the foam occurrence, and high effluent production, SSF is replacing SmF (Couto and Sanromán 2006; Garg et al. 2016; Patidar et al. 2018). To find out the economical and appropriate source of carbon for pectinase production, different agricultural wastes, including wheat bran, orange bagasse, sugarcane bagasse, and banana peel, are studied using SSF (Sharma et al. 2013). A little or no water is added to a solid substrate in SSF so that microbes, mostly fungi, grow as in their natural habitat and produce maximum pectinase. Also, microbes get sufficient nutrients from the substrate added, and no need for extra nutrients in SSF (John et al. 2020). Some of the pectinase produced following SmF and SSF with the optimum fermentation conditions are listed in Table 1.
Immobilization strategies
Immobilization of enzyme or microbes onto the solid carrier is another effective technique to enhance enzyme production and improve enzyme properties. Enzymes and whole cells are immobilized using various methods such as adsorption, covalent bonding, and entrapment. Several inorganic supports (bentonite, silica, ceramics, polyacrylamide, glass beads, etc.), organic supports (chitosan, proteins, alginate, agar–agar, etc.), and organic–inorganic hybrid (magnetic nanoparticles, silica–dialdehyde starch, etc.) are used for enzymes immobilization (Sánchez et al. 2015; Rehman et al. 2016; Hosseini et al. 2020). However, different types of carriers for immobilization need to have some unique properties like biocompatibility, low toxicity, biodegradability, and tailored surface chemistry. The different supporting materials for immobilization need to be stable, cheap, nontoxic, and not reactive in the reaction solution or mixture. The selection of immobilization process and supporting material are very important (Zdarta et al. 2018). The immobilization is advantageous to recover enzyme easily when it is in free form and has short time stability. Therefore, the different advantages of using immobilized enzymes or cells are they get proper attachment or confined in a specific region for their catalytic activities, can be repeatedly or continuously exploited, can be easily separated from the media, stability or tolerance of enzymes to temperature and pH are enhanced, minimize enzyme loss, enhance enzymatic properties, and decrease the product contamination, etc. (Rehman et al. 2016; Martín et al. 2019; Hosseini et al. 2020; John et al. 2020). The pectinase enzyme was immobilized onto the surface of magnetic nanoparticles by dextran polyaldehyde as a cross-linking agent and applied in apple juice clarification. The immobilized pectinase showed superior thermal stability than the free enzyme, 87% residual activity after seven cycles of recyclability, and rapid reduction of turbidity up to 74% (Sojitra et al. 2017). The polyaldehyde pullulan can be a potential alternative for the immobilization of pectinase on the glass beads (Hosseini et al. 2020). Similarly, pectinase immobilized in magnetic chitosan particles was exploited in the clarification of juices (Magro et al. 2019). Rehman et al. (2016) immobilized polygalacturonase by entrapping in calcium alginate, agar–agar, and polyacrylamide gel. The biocatalyst polygalacturonase produced from Streptomyces halstedii when immobilized in sodium alginate hydrolyzed orange pectin; it was reusable for 29 reactions with stable achievements (Ramírez-Tapias et al. 2017). Low-cost agrowaste like corn cob was also used as an inexpensive matrix support for immobilization of yeast (Geotrichum candidum) and has illustrated the increased production of pectinase compared to free yeast cell (Ejaz et al. 2018). Whole cell immobilization of Streptomyces sp. in polyurethane foam support enhanced the pectinase production by 32% (Kuhad et al. 2004). The functionalized nanoporous activated charcoal (FNAC) was used for pectinase immobilization produced from Aspergillus ibericus for citrus processing wastewater treatment and was successful to treat 94% pectin present in the wastewater (Mahesh et al. 2016). Furthermore, in another study, the pectin lyase from Schizophyllum commune entrapped and adsorbed in sodium alginate and chitosan respectively was found thermal stable and reusable up to 6 cycles. Also, the enzyme activity of immobilized enzyme was stimulated by Ca2+ and Ma2+ (Mehmood et al. 2019).
Genetic modification strategies/strain development
Microbial strain development or improvement is another technique/method for improving bacterial strain applied in the commercial development of fermentation processes to enhance bioproducts productions. The method implies the blending of classical techniques, biochemical engineering, and molecular genetics. The strains of bacteria are developed or improved by different processes like mutagenesis and molecular process, which impart mainly in changing the microbial DNA (Heerd et al. 2014). The microbial recombinant DNA technology tools such as gene cloning and metabolic engineering are applied to increase pectinase production and have been successfully applied in cloning and expressing the gene of interest for relevant enzymes production efficiently and effectively (Amin et al. 2019). The recombinant technology has resulted in significant increase about 14 and 400 folds production of polygalacturonase and pectin lyase using Penicillium griseoroseum under submerged fermentation (Gonçalves et al. 2012). Gene shuffling also helps to enhance the pectinase activity of bacteria; for example, two round of genomic shuffling in Bacillus subtilis increased the pectinase activity by 1.6 times. The alignment of gene sequences were replaced in three bases after shuffling which produced amino acid substitution resulting in enhancement of pectinase synthesis (Yu et al. 2019). Strain development also benefits from an economic point of view by decreasing the production cost and not increasing the capital expenditure (Parekh et al. 2000; Ademakinwa et al. 2017). Many factors influence bacterial strain development, such as the type of processes, methods, treatment duration, and microorganisms type (Munir et al. 2020). Chemicals such as N-methyl-N-nitro-N-nitrosoguanidine (NTG), hydroxylamine, and nitrous acid are commonly used chemical mutants. Ultraviolet (UV), X-ray, and gamma-ray radiation are physical mutants. UV radiation induces cross-links and pyrimidine dimerization in DNA. The intercalating agents, for example, ethidium bromide and acridine dyes, intercalate the base pairs between nucleotides resulting in frameshifts or loss of plasmids (Parekh et al. 2000; Heerd et al. 2014). Mutation can also produce constitutive expression of pectinase as in the study conducted by Alazi et al. (2019). The activator and repressor both were mutated, but the inducer independent expression for pectinase was observed in Aspergillus niger demonstrating the ability of producing pectinase independently to the substrate (Alazi et al. 2019). In another study, mutant strain of Penicillum occitanis produced by nitrous acid mutagenesis showed exceptionally high pectinases constitutively. The study illustrated the pectinases production by mutant stain did not depend on pectin, glycerol, or glucose as an inducer. However, the mutant strain showed pectinase mRNA transcripts in Northern blots analysis, and none of the transcript was detected in the parental strain (Ayadi et al. 2011).
Heerd et al. (2014) applied UV radiation and/or NTG for Aspergillus sojae strain development and studied their effects on pectinolytic activities. The study demonstrated the PG production was enhanced by strain development and could be applied industrially (Heerd et al. 2014). In another study, A. tamatii was treated with UV radiation, sodium azide, nitrous acid, and ethyl methane sulfonate for strains development. The study illustrated sodium azide–treated strain produced high polygalacturonase activity (Munir et al. 2020). Similarly, the strain enhanced the production of fructosyltransferase when chemicals such as ethidium bromide and ethyl methane sulfonate were used as mutagens (Ademakinwa et al. 2017). Thus, the strain development of microorganisms plays an important role in meeting the increasing demand for enzymes in different avenues of applications.
There are some studies which illustrated the success of recombinant technology in improving the properties like thermotolerance and catalytic efficiency further increasing pectinase production. Kun et al. (2021) developed the mutants of Aspergillus niger by constructing the changes in the chimeric transcription factors. They observed significantly higher pectinase production when the mutated chimeric transcription factor was expressed in A. niger. Also, A. niger was found to be resistant to hygromycin B (Kun et al. 2021). The recombinant technology has made possible to transfer gene of interest in other organisms and successfully express the interested enzyme or protein. For example, the TPG1 gene which is responsible for polygalacturonase production was identified in cold adapted Tetracladium sp., and when expressed in Pichia pastoris, it was observed that the recombinant polygalacturonase was highly active at low temperature (15 °C) (Carrasco et al. 2019). Similarly, the alkaline pectate lyase gene derived metagenomically when expressed in E. coli, the expressed pectate lyase demonstrated thermostability, alkaline stability, and high specific activity (Wang et al. 2014). Furthermore, the pectate lyase gene (pel4j4) from Dickeya dadantii was cloned and transformed into E. coli BL21. The expressed pectate lyase showed the enzyme activity of 204.4 IU/mL, stable at 45 °C and pH 8.5 to 10, and the activity was dependent on Ca2+ (Cheng et al. 2019). Furthermore, the pectate lyase gene from Paenibacillus polymyxa when cloned and expressed in E. coli, the recombinant pectate lyase showed optimal temperature and pH of 40 °C and 10 respectively, and potential for degrading the citrus pectin of approximate 230.2 kDa molecular weight was degraded into 24 kDa (Yuan et al. 2019). These characteristics of recombinant pectate lyase and pectinases directs to be applicable in various industries for facilitating in producing fruit juices and fermented beverages, bio-degumming, bio-scouring processes, and preparing of pharmacological active products, etc.
Classification of pectinases
Based on the action of pectinase on a substrate, the pectinase enzymes can be classified into three groups, protopectinase, pectinesterase, and depolymerase (hydrolase and transeliminase) (Garg et al. 2016; Oumer 2017; Patidar et al. 2018) which is shown in Fig. 1 and Table 2.
Protopectinase
This type of pectinase is generally present in unripe fruits and converts insoluble protopectin into a soluble form of pectin. Thus, protopectinase is supposed to be the first enzyme necessary for pectin degradation. Pectinosinase is alternately termed protopectinase (Tapre and Jain 2014; Patidar et al. 2018).
Pectin esterase (PE, de-esterase)
This pectinase is also known as pectin methyl hydrolase, pectin methylesterase, and pectase. PE is a carboxylic acid esterase that catalyzes the de-esterification of methyl ester linkage of galacturonan backbone of pectin to yield pectic acid and methanol (Jayani et al. 2005; Garg et al. 2016). It is reported that after the action of PE, the resulting pectin is then acted upon by PG and lyases. PEs are found in higher plant’s fruits, leaves, flowers, stems, and roots. They are believed to shift pH from acidic to alkaline conditions (Kavuthodi and Sebastian 2018; Nighojkar et al. 2019).
Depolymerizing enzyme
This class constitutes hydrolyzing enzymes and cleaving enzymes.
-
a.
Hydrolyzing enzymes (hydrolases) include polygalacturonase (PG) and polymethylgalacturonase (PMG). Both PG and PMG hydrolyze the pectin chain by acting on α 1 → 4 glycosidic bond in the presence of water molecule and produce polygalacturonate and polymethylgalacturonate, respectively (Jayani et al. 2005; Garg et al. 2016).
-
b.
Pectin transaminase (transeliminase) includes polygalacturonate lyase (PGL) and polymethyl galacturonate lyase (PMGL), which cleave α (1 → 4) glycosidic linkage by transelimination reaction producing unsaturated galacturonates. The breakage of the pectin chain is non-hydrolytic; the action is by eliminating H from C-5 and producing D4:5 unsaturated products (Tapre and Jain 2014).
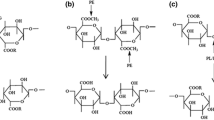
The depolymerizing enzymes, both hydrolases and transeliminases, can be exo and endo based on the site of pectinase action on the pectin chain. If pectinase acts randomly in a long chain of pectin, it is known as endo-pectinase, and if pectinase works at the reducing end of the chain terminally, it is exo-pectinase. The summary of different types of pectinases with their mode of action and final products is shown in Fig. 2. Pectinases are also classified as alkaline or acidic pectinase depending on the pH range in which pectinases operate. Most fungi produce acidic pectinase as they work at acidic pH, while most bacteria produce alkaline pectinase able to function at alkaline pH (Kavuthodi and Sebastian 2018; Nighojkar et al. 2019). Based on the application of temperature, pectinases are classified into psychrophilic, thermophilic, and mesophilic pectinase. Generally, most of the pectinase exploited in industries are mesophilic, which works in a wide temperature range between 20 and 50 °C. However, pectinases which show activity at 37 °C are not applicable in such industries that use low or high temperature processes. Besides, extremophiles can be the best alternatives (John et al. 2020). Due to the increasing demand for pectinase in various industries, there is a need to produce hyperactive, resisting a wide range of pH, and thermostable characteristics pectinase.
Mode of action of pectinases (a) polymethylgalacturonase and polygalacturonase, (b) pectin esterase, (c) pectin lyase and polygalacturonate lyase (adopted and modified from (Garg et al. 2016))
Applications of pectinases
Pectinases are economically very important due to their vast implications. The discovery of the pectinase enzyme brought a revolution in the economic and commercial sectors. Although it was used in making fruit juice and wine in the past, recently, its application is increasing continuously. The applications of pectinases in fruit juice industries, wine industries, paper and pulp industries, wastewater treatment, bioethanol production, extraction of DNA from a plant, and protoplast isolation from a plant are also significant. Furthermore, pectinases are employed in preparing animal feed, saccharification and liquefaction of biomass, retting and degumming of plant fiber, bio-scouring of cotton fiber, coffee and tea fermentation, and oil extraction (Kubra et al. 2017; Oumer 2017; Shrestha et al. 2021).
Pharmaceutical industries
The most substantial industrial enzymes, pectinases, have remarkable applications in the pharmaceutical industry. Humans are consuming a variety of dietary fiber foods of plant origin, which need to be properly digested in our digestive system. However, pectinase plays an important role in digesting the fermentable dietary fibers as well as improving the immune system. Many pharmaceutical products are produced from the fermentation of pectic substances present in fruits and vegetable peels using pectinase. The products thus produced are used as a fiber supplement for the treatment of diabetes and obesity (Satapathy et al. 2020). Moreover, plant essential oils are used for the treatment of various microbial infections, including wound healing and cancer. The organic solvent in oil extraction from different medicinal plants may damage some essential properties and lose the concentration of important phytochemicals. Thus, pectinase is applied in pharmaceutical industries to minimize such possible losses. The pectinase stimulates the liquefaction of cell wall components of the plant and destroys emulsifying properties of pectin to increase the product yields (Satapathy et al. 2020). Also, pectinase is used to produce enzyme-sensitive colon-specific tablets in the pharmaceutical industries for oral colon-specific drug delivery application. Generally, oral drugs have a short half-life and an absolute bioavailability and are destroyed by acidic pH in the stomach. This may need high doses or a large amount of drug administration, resulting in many side effects such as increasing toxic levels and gastrointestinal problems. Therefore, a controlled delivery system such as an oral colon-specific drug delivery system is appropriate where the drugs are not damaged by acidic gastric juice in the stomach and are shipped to the colon. Furthermore, the tablets are degraded by pectinase and attain an accurate controlled release of drug in the colon (Zhu et al. 2019).
Fruit juice industries
The fruit juice industries use pectinases, and other cell wall degrading enzymes such as cellulases and proteases to soften fruits, facilitate the extraction, increase the juice yield, and clarify the juice (Patidar et al. 2018; Verma et al. 2018; Nighojkar et al. 2019). The fruits, mainly tropical fruits, have more pectin and are pulpy (Tapre and Jain 2014). The mechanical grinding of fruits in juice extraction may contribute to jelly, viscous, and cloud-like appearance products due to the positively charged protein that gets coated around by negatively charged pectin. The addition of pectinases in fruit juice would result in degrading pectin and exposing the positively charged protein. The exposition of positively charged protein reduces the electrostatic repulsion between cloud particles and the formation of a more massive particle, which further settles down and gives clear juice (Tapre and Jain 2014). Also, the starch complex is responsible for the characteristic viscosity and turbidity of the juice. However, pectinases supplement breaks the glycosidic bonds present between the galacturonic acid monomers to increase the juice yield. In addition, pectinases decrease the water holding capacity of pectin, and reduce the viscosity and turbidity (Tapre and Jain 2014; Nighojkar et al. 2019; Shrestha et al. 2021). Furthermore, the enzymatic treatment clears the juice by breaking down the pectin, helps to settle down the suspended particles, and eliminates undesirable changes in color, smell, and stability. The pectinases are applicable in manufacturing fruit purees, peeling of fruits segments, and wine clarification. The implication of biocatalyst reduces the cost and increases the yield of products and is highly competitive than the other different established processes. It represents a simple alternative method for diversifying production, mainly in tropical fruit juice, and increasing market share (Tapre and Jain 2014; Verma et al. 2018).
Paper and pulp industry
In paper industries, the sheet formation step is the most crucial, and the presence of pectin in pulp reduces dewatering. In the past, the filter fiber used to have bigger holes so that water gets easily removed from paper, and peroxide bleaching reduces cationic demand (Reid and Ricard 2000; Samanta 2019). The use of chemical and bleaching treatment aids in retaining properties of paper, but these are not environmentally friendly; therefore, enzymatic treatment is becoming more popular. In paper manufacturing, pectinases are used to lower the cationic demand of pectin polymers and bleaching treatment of pulp. Pectinases depolymerize the long pectin chain and reduce the cationic need because pectin, more than hexamers, has cationic demand, whereas shorter than hexamers do not have cationic demand (Reid and Ricard 2000; Samanta 2019). The alkaline pectinases produced from Streptomyces sp., Bacillus sp., Erwinia carotovora, and some fungi are applied in making the paper more uniform, softer to touch, brighter, and to increase the pulp strength compared to the conventional soda-ash cooking method (Kubra et al. 2017; Samanta 2019).
Oil extraction
An organic solvent like hexane, which has the potential to cause cancer, was used in the past for the extraction of edible oil from oil-producing crops like rapeseed, coconut germ, sunflower seed, palm, and olives (Ortiz et al. 2017). Nowadays, pectinases and other cell wall degrading enzymes, cellulases, and hemicellulases are used to extract oil from plants, olive, sunflower, coconut, canola, and other oil seeds. During the grinding process, enzymes are added to degrade pectin in the middle lamellae of plant cells and promote the extraction of oils from the inner part of the seeds or germ layer, thus, enhancing oil release from different sources. The pectinases addition also increases the stability, yield, and rheological properties of oil and improves the level of vitamin E contents, polyphenols, and organoleptic quality of oil (Hoondal et al. 2002; Iconomou et al. 2010; Ortiz et al. 2017). Another study illustrated pectinases from bacteria facilitated the oil extraction from sesame seeds increasing the yield and clarity, and decreasing the relative viscosity of oil. In this study, the bacteria were isolated from the forest soil (Shrestha et al. 2021).
Textile industries
In textile industries, pectinases are used with other enzymes like amylase, lipase, cellulase, and hemicellulase to remove sizing agents from cotton safely and eco-friendly (Hoondal et al. 2002; Bristi et al. 2019). The enzymes are specific; the action is quick and saves energy, cost, raw material, water, and energy; thus, the enzymes are replacing the harsh, costly, and environment polluting chemical method for scouring of cotton fiber in textile industries (Bristi et al. 2019). Pectinases treatment helps to decrease the fabric weight and wettability. The addition of enzymes is an eco-friendly process that maintains the quality, whiteness, and brightness of the textile, like the conventional method, and lowers the release of eco-toxic agents (Vigneswaran et al. 2012; Singh et al. 2020).
Bio-scouring of cotton
Bio-scouring means removing undesirable non-cellulosic contaminants like minerals, pectin, natural colorants, fats, waxes, protein, and water-soluble compounds. These impurities can be removed using enzymes but not with chemicals. For example, pectinase is used for bio-scouring of cotton without any harmful side effects (Kubra et al. 2017) but improves the dyeing properties, water absorption capacity, texture of cotton (Vigneswaran et al. 2012), and lowers the weight. This biological scouring method decreases total dissolved solids (TDS) value but increases biological oxygen demand amounts of bio-scouring effluent (Aggarwal et al. 2020).
Degumming and retting fiber
Degumming is the procedure of removing heavily coated gum from outside the xylem, phloem, or pericarp of the plant fiber like ramie and sun hemp before its use for textile making. The enzymatic processing of fiber is energy conservative, eco-friendly, non-toxic, non-biodegradable, and non-polluting. The combination of chemical and enzymatic treatment in fiber reduces chemical and energy consumption (Iconomou et al. 2010; Chamani et al. 2012). Ramie fiber is the best natural fiber but contains pectic substances and hemicellulosic material; thus, pectinases and xylanases are applied for effective degumming in textile. This green enzymatic process prevents the use of the hot alkaline solution, high energy consumption, and environmental pollution (Singh et al. 2020). In addition to this, pectinases improve the malleability and good separation of blast fiber from decorticated ramie fiber, producing better quality yarn (Banik and Ghosh 2008).
Coffee, cocoa, and tea fermentation
Pectinases are used to remove the mucilaginous coat from coffee beans and accelerate coffee’s, cocoa, and tea fermentation process. The characteristics of tea depend on oxidation products like theaflavins, thearubigins, and other inherent components. Generally, non-volatile compounds are present in tea leaves, such as polyphenols, flavonols and flavonol glycosides, flavones, and phenolic acids. Also, amino acids, chlorophyll, carbohydrates, other pigments, organic acids, caffeine, enzymes, vitamins, and minerals, etc. are found in tea leaves (Chaturvedula and Prakash 2011). Pectinases decrease foam formation in the instant coffee/tea powders, remove the thick layer consisting of pectic substances from coffee beans, accelerate the fermentation of coffee/tea, and develop chocolate flavor in cocoa fermentation (Samanta 2019). The concentration of pectinases should be maintained to get maximum efficiency. Also, the enzyme must be added in a proper level because an excess of enzyme lowers the glaze and quality of tea leaves, decreases the color, flavor, aroma, and increases the rate of spoilage of tea leaves (Chaturvedula and Prakash 2011; Sharma et al. 2013; Samanta 2019). A study illustrated that pectinase from Bacillus tequilensis effectively removed mucilage from the coffee beans (Koshy and De 2019).
Wine industries
Pectinases containing low pectin methylesterase are used not only in the extraction of wine. Still, they are also used to increase juice yield and accelerate filtration. The main problem in winemaking is a cloudiness that makes filtration of wine difficult due to the presence of pectin. The addition of pectinase hydrolyzes pectin and removes the cloudiness present in wine. Also, pectinase enhances the aroma and taste of the wine (Garg et al. 2016; Rollero et al. 2018). However, the phenolic compound is a secondary metabolite; the enzymatic action intensifies and stabilizes the color of wine by increasing the phenolic compound of wine (Busse-Valverde et al. 2011). The immobilized pectinase has been used recently for decreasing the viscosity of grape must in winemaking and facilitating in clearing and filtering wine (Martín et al. 2019). Pectinase is applied as a biocatalyst in winemaking because it enhances the extraction of compounds and improves the properties, volatile flavor, and aroma of wine (Rollero et al. 2018; Jiang et al. 2020).
Protoplast preparation
Protoplast preparation is the initial and important step in producing a new plant with specific and interesting traits/characteristics. Protoplast from different plant leaves such as maize, Arabidopsis, tobacco, wheat, barley, and rice has shown a significant effect in genetic modification, plant physiology, and development. Protoplasts can be isolated from different sources, either mechanically or enzymatically. The mechanical process may cause more breakage and result in more osmotic shrinkage. Thus, enzymatic protoplast preparation is beneficial compared to the mechanical process (Chamani et al. 2012). The protoplast isolated from plant tissues has revealed high transformation efficacy with low maintenance and holds their cell identity. The protoplast transient expression systems have contributed to elucidating intracellular signaling mechanisms in a plant. The studies in plant protoplast systems provide a framework for fundamental plant analysis. Enzymes like pectinases are useful to extract protoplast for plant tissue culture by which new genetic traits are induced in plant cells. Pectinases, in combination with cellulase, is used for the efficient isolation of protoplast (Solís et al. 1996). An enzyme mixture of cellulase, crude pectinase, and chitinase is also used for maximizing protoplast yield. A high output of protoplast can be obtained by adding pectinase, cellulase, and macerozyme (Shen et al. 2017). In another study, 4% cellulase and 1% pectinase treatment produced the highest number of viable protoplast and illustrated the best combination of enzymes for protoplast isolation from Lilium (Chamani et al., 2012).
Purification and detection of plant viruses
According to the types of viruses, several viral purification systems can be selected for use. In certain conditions, a mixture of enzymes such as alkaline pectinase and cellulase is employed for the viral purification process (Sharma et al. 2013). The cell wall degrading enzymes can be exploited to liberate virus from plant tissues generally when the virus is constrained to the phloem (Hoondal et al. 2002). In a study, tobacco necrotic dwarf virus and potato leaf roll virus transmitted by aphids were purified using enzymes. The plant tissue macerating commercial enzyme named Driselase mainly contains pectinase and cellulase. The study revealed the enzymatic treatment increased the purification yield and is limited to a phloem-related virus (Takanami and Kubo 1979). The pectinase facilitates detecting hepatitis A virus and norovirus from different fresh and frozen berries and vegetables (Butot et al. 2007).
Wastewater treatment
Wastewater produced from vegetables and fruit processing industries, especially fruit juice industries or citrus processing industries, contains pectinaceous material that needs treatment before they are integrated into the environment. The physical and chemical treatments like physical dewatering, chemical coagulation, chemical hydrolysis, and methanogenesis are time-consuming, expensive, and hazardous to the environment and health (Hoondal et al. 2002). Therefore, biological enzymatic treatment has become the best alternative for sewage water treatment. Pectinases can quickly degrade pectic substances present in wastewater, which, in turn, helps in the decomposition of pectin residue by activated sludge treatment. The microbes may not have decomposed this wastewater during the activated sludge treatment. Thus, nowadays, alkalophilic microorganisms producing pectinases are used to treat wastewater and increase biological oxygen demand (Mahesh et al. 2016). The pectinases can remove oil and grease from kitchen wastewater too, and the process is both cost-effective and eco-friendly. Besides, pectinases help to decrease biological oxygen demand and remove grease and oil from wastewater (Kamaruddin et al. 2019). Tobacco wastewater can be the source for culturing pectinase-producing microbes like Rhizopus oryzae. The pectinase made from R. oryzae, in turn, hydrolyze pectin–containing biomass to acquire fermentable sugars, which has possibilities for profitable biofuels production (Zheng et al. 2017). The wastewater produced from domestic, industrial, and agricultural water activities contains different organic and inorganic substances, thus polluting the environment. However, thus produced wastewater can be used as a carbon or nitrogen source for the growth of microbes for reutilization purposes (Zheng et al. 2017). Immobilized pectinases on nanoporous activated carbon has been studied for treating pectin–containing wastewater, and the study revealed 94% of pectin was treated from wastewater (Mahesh et al. 2016).
Animal and poultry feed
An animal rumen contains some bacteria, fungi, and protozoa, which produces pectinolytic enzymes and plays an essential role in digestion. Lachnospira multiparus is a common bacterium found in rumen. This ruminant bacterium produces pectin lyase and pectin methylesterase, which are helpful in the breakdown of pectin present in food. The supplement of enzymes helps complete organic matter breakdowns in the rumen (Murad and Azzaz 2011). Pectinases when supplemented in animal feed production improves the feed quality by lowering the viscosity and boosting the nutrients absorptivity. Similarly, enzymes reduce the fiber content of the feed and enhance the liberation of nutrients trapped in fibers (Jayani et al. 2005). Some animals cannot lyse pectin present in plants, so the addition of pectinases in the feed helps the captivation of nutrients by liberating the nutrients after the breakdown of non-degradable fibers or releasing nutrients blocked by these fibers and decreases the fecal amount. Pectinases reduce feed viscosity, which directly intensifies the absorption of the nutrients and reduces animal defecation, and finally increases the weight of poultry (Garg et al. 2016; Abdulla et al. 2017; Bhardwaj et al. 2017).
Recycling of wastepaper
The recycling of paper products is increasing recently, and deinking of the used paper is a necessary step. Enzymatic deinking improves brightness, reduces chemical consumption, improves drainage, and lowers residual ink of paper. However, excess use of the enzyme during deinking may result in fiber loss by more depolymerization and high biological oxygen demand in effluents (Pathak et al. 2010). The enzymatic deinking process is less polluting, energy-saving, and friendly; gives better performance to achieve the desired deinked pulp properties; and results in lowering disposal problems. Contrary to the enzymatic process, the chemical deinking process uses many chemicals that are harmful to the environment. Pectinase alters bonds near the ink particle and removes the ink from the surface washed out by washing or floatation (Pathak et al. 2010). Pectinase, in combination with xylanase, has been used for deinking of wastepaper to reduce the effects of harmful chemicals to 40% and improve the level of physical and optical properties, enhancing the quality of paper sheets (Singh et al. 2019). The xylano-pectinolytic enzyme, when applied in the deinking of wastepaper from school to reuse, decreased the use of chemicals by 50%, biological oxygen demand 20.15%, and chemical oxygen demand 22.64% compared to the conventional chemical method. Also, this enzymatic deinking method was successful in attaining the same properties of paper like whiteness and brightness. Thus, this approach was noticed to be cost-effective, environmentally friendly, and safe for recycling wastepaper from school (Singh et al. 2012).
Hydrolysis of biomass and bioethanol production
Different polysaccharide degrading enzymes like pectinase, hemicellulases, and cellulases are used in the saccharification of plant cell wall polysaccharides, including pectic substances, into simple sugars, which are used in bioethanol production. These enzymes are used in the pretreatment of various feedstocks for bioethanol production enhancement. The improvement in glucose yield, ethanol concentration, and productivity was attained by treating the feedstocks with pectinases (Chen et al. 2012). The enzyme cocktail containing high pectinase activity saccharifies various biomasses effectively and aid in bioethanol production (Wang et al. 2019; Mihajlovski et al. 2020). The rye bran as a substrate achieved the maximal enzymatic activities for cellulase, amylase, xylanase, and pectinase by Streptomyces fulvissimus. Thus, S. fulvissimus was exploited for enzymatic degradation and saccharification of different agricultural biomass, including corn stover, cotton material, horsetail waste, and yellow gentian waste. The bioethanol was successfully produced by employing Saccharomyces cerevisiae, horsetail waste, and treated cotton waste after enzymatic hydrolysis of wastes (Mihajlovski et al. 2020). Similarly, Sethi et al. (2016) obtained maximum pectinase activity by Aspergillus terreus by hydrolyzing banana peel under both SSF and SmF. Bioethanol was also produced by utilizing S. cerevisiae and different agro-waste biomass (Sethi et al. 2016).
Enhance antimicrobial activity
The antibacterial activity of the plant, Emblica officinalis, was enhanced when treated with pectinase. The study demonstrated the thermostable pectinase–producing bacteria isolated from hot water springs by using inexpensive agricultural residues as macronutrients promoted the antibacterial activity of Emblica officinalis (Sarsar and Pathak 2019). The isozyme of pectinase associated with polygalacturonase activity from Aspergillus niger exhibited hydrolysis of chitosan into chitooligosaccharides and monomers. Thus, formed chitooligosaccharides and monomers mixture revealed antibacterial effects towards Bacillus cereus and E. coli (Kittur et al., 2005). Pectic heteropolysaccharides produced from the action of pectinase demonstrated antibacterial activity. The antibacterial activity was enhanced significantly when combined with lactic acid, tea polyphenols, nisin, and sodium acetate. Due to this property, the pectic oligosaccharides are used as a food preservative or food additive to increase the self-life of food or inhibit the growth of the undesirable microorganisms in food (Li et al., 2013). The pectic oligosaccharides produced from an orange can inhibit the adherence and invasion of Campylobacter jejuni to Caco-2 cells to some extent. Thus, pectinase-induced pectic oligosaccharides might be useful as alternatives in foodborne pathogen control (Ganan et al., 2010).
Concrete crack healing
Recently, the study has found the pectinase application in the construction area and in the production of reinforced natural fiber composites. The natural fibers retted with the pectinase, either pectate lyases or polygalacturonases, retain the strength of fibers with variable fineness. This important eco-friendly property is useful in the development of natural polymer composites and is used in packing, construction, and automobile applications (Sisti et al. 2018). The incorporation of natural fibers in composites enhances the thermal stability, tensile strength, elastic characteristics, flexural strength, and compressive strength of the composites. The addition of oil palm fibers in cement composites proved to increase setting time and reduced the workability of fresh cement, decreasing the cost (Osofero et al. 2018). In addition, the pectinase produced from spore-forming microbes that are resistant to high pH can heal the concrete crack. Those microbes in cracks form calcite complex and precipitate calcium carbonate in the presence of water providing compatibility and compressive strength to concrete. When pectinases get incorporated as a catalyst or mineral precursor, the biological healing process prevents various environmental and health problems. The method is very cost-effective and environmentally friendly. Inorganic materials like silica fume, epoxy resin, and fly ash, when used for crack healing, may cause concrete thermal expansion (Govindaraji and Vuppu 2020).
Conclusions
Microbial pectinases are prominently growing enzymes that have humongous applications in different avenues and illustrate the need for a green and economical process for maximum pectinase production. This study attempts to enclose all the recent applications so that researchers can have the direction to further studies. However, the primary consideration is the stability of the enzyme in a wide range of industrial environmental conditions and to make the process cost-effective. The stability of enzymes over a wide range of temperature, and pH is the most crucial factor that gives an additional advantage to a microbial strain. So, new microbes with high pectinase activity, stable over a wide range of temperature and pH for a more extended period, along with their cost-effective production, need to be emphasized. Immobilization and re-immobilization of pectinases onto low-cost material can have great potential for making the process more cost-effective; hence, further research should be focused on this area as well. More powerful and versatile pectinase enzymes are needed to be developed through protein engineering and recombinant DNA technologies and discover the combined effects of different enzymes. New and exciting enzymes which drastically decrease the production cost for specific applications are beneficial that in many areas, lower energy consumption, and enhance the quality of products.
References
Abdollahzadeh R, Pazhang M, Najavand S, Fallahzadeh-Mamaghani V, Amani-Ghadim AR (2020) Screening of pectinase-producing bacteria from farmlands and optimization of enzyme production from selected strain by RSM. Folia Microbiol (praha) 65:705–719. https://doi.org/10.1007/s12223-020-00776-7
Abdulla JM, Rose SP, Mackenzie AM, Pirgozliev VR (2017) Feeding value of field beans (Vicia faba L. var. minor) with and without enzyme containing tannase, pectinase and xylanase activities for broilers. Arch Anim Nutr 71:150–164. https://doi.org/10.1080/1745039X.2017.1283823
Ademakinwa AN, Ayinla ZA, Agboola FK (2017) Strain improvement and statistical optimization as a combined strategy for improving fructosyltransferase production by Aureobasidium pullulans NAC8. J Genet Eng Biotechnol 15:345–358. https://doi.org/10.1016/j.jgeb.2017.06.012
Aggarwal R, Dutta T, Sheikh J (2020) Extraction of pectinase from Candida isolated from textile mill effluent and its application in bio-scouring of cotton. Sustain Chem Pharm 17:100291. https://doi.org/10.1016/j.scp.2020.100291
Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A (2016) Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci 9:148–154. https://doi.org/10.1016/j.jrras.2015.11.003
Alazi E, Niu J, Otto SB, Arentshorst M, Pham TTM, Tsang A, Ram AFJ (2019) W361R mutation in GaaR, the regulator of D-galacturonic acid-responsive genes, leads to constitutive production of pectinases in Aspergillus niger. Microbiologyopen 8:1–12. https://doi.org/10.1002/mbo3.732
Amin F, Bhatti HN, Bilal M (2019) Recent advances in the production strategies of microbial pectinases—a review. Int J Biol Macromol 122:1017–1026. https://doi.org/10.1016/j.ijbiomac.2018.09.048
Amin F, Bhatti HN, Bilal M, Asgher M (2017) Multiple parameter optimizations for enhanced biosynthesis of exo-polygalacturonase enzyme and its application in fruit juice clarification. Int J Food Eng 13:16. https://doi.org/10.1515/ijfe-2016-0256
Anand G, Yadav S, Yadav D (2017) Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech 7:1–8. https://doi.org/10.1007/s13205-017-0760-3
Ayadi M, Trigui S, Trigui-Lahiani H, Hadj-Taïeb N, Jaoua M, Gargouri A (2011) Constitutive over-expression of pectinases in Penicillium occitanis CT1 mutant is transcriptionally regulated. Biotechnol Lett 33:1139–1144. https://doi.org/10.1007/s10529-011-0546-3
Aydìn Z, Akbuǧa J (1996) Preparation and evaluation of pectin beads. Int J Pharm 137:133–136. https://doi.org/10.1016/0378-5173(95)04458-2
Banik S, Ghosh SN (2008) Pectinolytic activity of microorganisms in piling of jute. Indian J Fibre Text Res 33:151–156
Belda I, Conchillo LB, Ruiz J, Navascués E, Marquina D, Santos A (2016) Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int J Food Microbiol 223:1–8. https://doi.org/10.1016/j.ijfoodmicro.2016.02.003
Bhardwaj V, Degrassi G, Bhardwaj RK (2017) Microbial pectinases and their applications in industries: a review. Int Res J Eng Technol 4:829–836
Bibi N, Ali S, Tabassum R (2016) Statistical optimization of pectinase biosynthesis from orange peel by Bacillus licheniformis using submerged fermentation. Waste Biomass Valorization 7:467–481. https://doi.org/10.1007/s12649-015-9470-4
Bristi U, Pias AK, Lavlu FH (2019) A Sustainable process by bio- scouring for cotton knitted fabric suitable for next generation. J Text Eng Fash Technol 5:41–48. https://doi.org/10.15406/jteft.2019.05.00179
Busse-Valverde N, Gómez-Plaza E, López-Roca JM, Gil-Muñoz R, Bautista-Ortín AB (2011) The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J Agric Food Chem 59:5450–5455. https://doi.org/10.1021/jf2002188
Butot S, Putallaz T, Sánchez G (2007) Procedure for rapid concentration and detection of enteric viruses from berries and vegetables. Appl Environ Microbiol 73:186–192. https://doi.org/10.1128/AEM.01248-06
Carrasco M, Rozas JM, Alcaíno J, Cifuentes V, Baeza M (2019) Pectinase secreted by psychrotolerant fungi: identification, molecular characterization and heterologous expression of a cold-active polygalacturonase from Tetracladium sp. Microb Cell Fact 18:1–11. https://doi.org/10.1186/s12934-019-1092-2
Chamani E, Tahami SK, Zare N, Zakaria RA, Mohebodini M, Joyce D (2012) Effect of different cellulase and pectinase enzyme treatments on protoplast isolation and viability in Lilium ledebeourii Bioss. Not Bot Horti Agrobot Cluj-Napoca 40:123–128. https://doi.org/10.15835/nbha4028055
Chambin O, Dupuis G, Champion D, Voilley A, Pourcelot Y (2006) Colon-specific drug delivery: influence of solution reticulation properties upon pectin beads performance. Int J Pharm 321:86–93. https://doi.org/10.1016/j.ijpharm.2006.05.015
Chaturvedula VSP, Prakash I (2011) The aroma, taste, color and bioactive constituents of tea. J Med Plants Res 5:2110–2124
Chen J, Liu W, Liu CM, Li T, Liang RH, Luo SJ (2015) Pectin modifications: a review. Crit Rev Food Sci Nutr 55:1684–1698. https://doi.org/10.1080/10408398.2012.718722
Chen Q, Jin Y, Zhang G, Fang Y, Xiao Y, Zhao H (2012) Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 5:3019–3032. https://doi.org/10.3390/en5083019
Cheng L, Duan S, Zheng K, Feng X, Yang Q, Liu Z, Liu Z, Peng Y (2019) An alkaline pectate lyase D from Dickeya dadantii DCE-01: clone, expression, characterization, and potential application in ramie bio-degumming. Text Res J 89:2075–2083. https://doi.org/10.1177/0040517518790971
Chiliveri SR, Koti S, Linga VR 2016 Retting and degumming of natural fibers by pectinolytic enzymes produced from Bacillus tequilensis SV11-UV37 using solid state fermentation Springerplus 5https://doi.org/10.1186/s40064-016-2173-x
Couto SR, Sanromán MÁ (2006) Application of solid-state fermentation to food industry-a review. J Food Eng 76:291–302. https://doi.org/10.1016/j.jfoodeng.2005.05.022
Dasari PK (2020) Parametric optimizations for pectinase production by Aspergillus awamori. GSC Biol Pharm Sci 12:093–098. https://doi.org/10.30574/gscbps.2020.12.2.0247
Demir N, Nadaroglu H, Demir Y, Isik C, Taskin E, Adiguzel A, Gulluce M (2014) Purification and characterization of an alkaline pectin lyase produced by a newly isolated Brevibacillus borstelensis (p35) and its applications in fruit juice and oil extraction. Eur Food Res Technol 239:127–135. https://doi.org/10.1007/s00217-014-2198-8
Domozych DS, Sørensen I, Popper ZA, Ochs J, Andreas A, Fangel JU, Pielach A, Sacks C, Brechka H, Ruisi-Besares P, Willats WGT, Rose JKC (2014) Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol 165:105–118. https://doi.org/10.1104/pp.114.236257
Ejaz U, Ahmed A, Sohail M (2018) Statistical optimization of immobilization of yeast cells on corncob for pectinase production. Biocatal Agric Biotechnol 14:450–456. https://doi.org/10.1016/j.bcab.2018.04.011
Ganan M, Collins M, Rastall R, Hotchkiss AT, Chau HK, Carrascosa AV, Martinez-Rodriguez AJ (2010) Inhibition by pectic oligosaccharides of the invasion of undifferentiated and differentiated Caco-2 cells by Campylobacter jejuni. Int J Food Microbiol 137:181–185. https://doi.org/10.1016/j.ijfoodmicro.2009.12.007
Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R (2016) Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech 6:1–13. https://doi.org/10.1007/s13205-016-0371-4
Giacobbe S, Pepe O, Ventorino V, Birolo L, Vinciguerra R, Faraco V (2014) Enzyme from Paenibacillus xylanolyticus. 9:4873–4887
Gonçalves DB, Teixeira JA, Bazzolli DMS, de Queiroz MV, de Araújo EF (2012) Use of response surface methodology to optimize production of pectinases by recombinant Penicillium griseoroseum T20. Biocatal Agric Biotechnol 1:140–146. https://doi.org/10.1016/j.bcab.2011.09.002
Govindaraji PK, Vuppu S (2020) Characterisation of pectin and optimization of pectinase enzyme from novel Streptomyces fumigatiscleroticus VIT-SP4 for drug delivery and concrete crack-healing applications: an eco-friendly approach. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2020.07.024
Guo F, Li X, Zhao J, Li G, Gao P, Han X (2019) Optimizing culture conditions by statistical approach to enhance production of pectinase from Bacillus sp. Y1. Biomed Res Int 2019:10. https://doi.org/10.1155/2019/8146948
Habrylo O, Evangelista DE, Castilho PV, Pelloux J, Henrique-Silva F (2018) The pectinases from Sphenophorus levis: potential for biotechnological applications. Int J Biol Macromol 112:499–508. https://doi.org/10.1016/j.ijbiomac.2018.01.172
Haile M, Kang WH (2019) Isolation, identification, and characterization of pectinolytic yeasts for starter culture in coffee fermentation. Microorganisms 7:16. https://doi.org/10.3390/microorganisms7100401
Handa S, Sharma N, Pathania S (2016) Multiple parameter optimization for maximization of pectinase production by Rhizopus sp. C4 under solid state fermentation. Fermentation 2:9. https://doi.org/10.3390/fermentation2020010
Harholt J, Suttangkakul A, Scheller HV (2010) Biosynthesis of pectin. Plant Physiol 153:384–395. https://doi.org/10.1104/pp.110.156588
Heerd D, Tari C, Fernández-Lahore M (2014) Microbial strain improvement for enhanced polygalacturonase production by Aspergillus sojae. Appl Microbiol Biotechnol 98:7471–7481. https://doi.org/10.1007/s00253-014-5657-z
Hoondal G, Tiwari R, Tewari R, Dahiya N, Beg Q (2002) Microbial alkaline pectinases and their industrial applications: a review. Appl Microbiol Biotechnol 59:409–418. https://doi.org/10.1007/s00253-002-1061-1
Hosseini SS, Khodaiyan F, Seyed SM, Kennedy JF, Azimi SZ (2020) A health-friendly strategy for covalent-bonded immobilization of pectinase on the functionalized glass beads. Food Bioprocess Technol. https://doi.org/10.1007/s11947-020-02524-8
Iconomou D, Arapoglou D, Israilides C (2010) Improvement of phenolic antioxidants and quality characteristics of virgin olive oil with the addition of enzymes and nitrogen during olive paste processing. Grasas Aceites 61:303–311. https://doi.org/10.3989/gya.064809
Jackson CL, Dreaden TM, Theobald LK, Tran NM, Beal TL, Eid M, Gao MY, Shirley RB, Stoffel MT, Kumar MV, Mohnen D (2007) Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology 17:805–819. https://doi.org/10.1093/glycob/cwm054
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Jiang X, Lu Y, Liu SQ (2020) Effects of pectinase treatment on the physicochemical and oenological properties of red dragon fruit wine fermented with Torulaspora delbrueckii. Lwt 132:109929. https://doi.org/10.1016/j.lwt.2020.109929
John J, Kaimal KKS, Smith ML, Rahman PKSM, Chellam PV (2020) Advances in upstream and downstream strategies of pectinase bioprocessing: a review. Int J Biol Macromol 162:1086–1099. https://doi.org/10.1016/j.ijbiomac.2020.06.224
Kamaruddin MA, Ibrahim MH, Thung LM, Emmanuel MI, Niza NM, Shadi AMH, Norashiddin FA (2019) Sustainable synthesis of pectinolytic enzymes from citrus and Musa acuminata peels for biochemical oxygen demand and grease removal by batch protocol. Appl Water Sci 9:1–10. https://doi.org/10.1007/s13201-019-0948-2
Karthik JL, Kumar G, Rao KVB (2011) Screening of pectinase producing microorganisms from agricultural waste dump soil. Asian J Biochem Pharm Res 1:329–337
Kaur S, Kaur HP, Tb P, Bharti E (2016) Production and optimization of pectinase by Bacillus sp. isolated from vegetable waste soil. Indo Am J Pharm Res 6:4185–4190
Kaur SJ, Gupta VK (2017) Production of pectinolytic enzymes pectinase and pectin lyase by Bacillus subtilis SAV-21 in solid state fermentation. Ann Microbiol 67:333–342. https://doi.org/10.1007/s13213-017-1264-4
Kavuthodi B, Sebastian D (2018) Review on bacterial production of alkaline pectinase with special emphasis on Bacillus species. Biosci Biotechnol Res Commun 11:18–30. https://doi.org/10.21786/bbrc/11.1/4
Kavuthodi B, Thomas S, Sebastian D (2015) Co-production of pectinase and biosurfactant by the newly isolated strain Bacillus subtilis BKDS1. Br Microbiol Res J 10:1–12. https://doi.org/10.9734/bmrj/2015/19627
KC S, Upadhyaya J, Joshi DR, Lekhak B, Chaudhary DK, Pant BR, Bajgai TR, Dhital R, Khanal S, Koirala N, Raghavan V 2020 Production, characterization, and industrial application of pectinase enzyme isolated from fungal strains Fermentation 6https://doi.org/10.3390/FERMENTATION6020059
Khan M, Nakkeeran E, Umesh-Kumar S (2013) Potential application of pectinase in developing functional foods. Annu Rev Food Sci Technol 4:21–34. https://doi.org/10.1146/annurev-food-030212-182525
Kittur FS, Vishu Kumar AB, Varadaraj MC, Tharanathan RN (2005) Chitooligosaccharides - preparation with the aid of pectinase isozyme from Aspergillus niger and their antibacterial activity. Carbohydr Res 340:1239–1245. https://doi.org/10.1016/j.carres.2005.02.005
Kohn R (1982) Binding of toxiccation of pectin, its oligomeric fragments and plant tissues. Carbohydr Polym 2:273–275
Koshy M, De S (2019) Effect of Bacillus tequilensis SALBT crude extract with pectinase activity on demucilation of coffee beans and juice clarification. J Basic Microbiol 59:1185–1194. https://doi.org/10.1002/jobm.201900321
Kubra KT, Ali S, Walait M, Sundus H (2017) Potential applications of pectinases in food, agricultural and environmental sectors. J Pharm Chem Biol Sci 6:23–34
Kuhad RC, Kapoor M, Rustagi R (2004) Enhanced production of an alkaline pectinase from Streptomyces sp. RCK-SC by whole-cell immobilization and solid-state cultivation. World J Microbiol Biotechnol 20:257–263. https://doi.org/10.1023/B:WIBI.0000023833.15866.45
Kumar A, Sharma R (2012) Production of alkaline pectinase by bacteria ( Cocci sps.) isolated from decomposing fruit materials. Enzyme 4:1–5
Kun RS, Garrigues S, Di Falco M, Tsang A, de Vries RP (2021) The chimeric GaaR-XlnR transcription factor induces pectinolytic activities in the presence of D-xylose in Aspergillus niger. Appl Microbiol Biotechnol 105:5553–5564. https://doi.org/10.1007/s00253-021-11428-2
Lara-Espinoza C, Carvajal-Millán E, Balandrán-Quintana R, López-Franco Y, Rascón-Chu A 2018 Pectin and pectin-based composite materials: beyond food texture Molecules 23https://doi.org/10.3390/molecules23040942
Li S, Li T, Zhu R, Wang N, Song Y, Wang S, Guo M (2013) Antibacterial action of haw pectic oligosaccharides. Int J Food Prop 16:706–712. https://doi.org/10.1080/10942912.2011.565904
Magro LD, de Moura KS, Backes BE, de Menezes EW, Benvenutti EV, Nicolodi S, Klein MP, Fernandez-Lafuente R, Rodrigues RC (2019) Immobilization of pectinase on chitosan-magnetic particles: influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnol Reports 24:e00373. https://doi.org/10.1016/j.btre.2019.e00373
Mahesh M, Arivizhivendhan KV, Maharaja P, Boopathy R, Hamsavathani V, Sekaran G (2016) Production, purification and immobilization of pectinase from Aspergillus ibericus onto functionalized nanoporous activated carbon (FNAC) and its application on treatment of pectin containing wastewater. J Mol Catal B Enzym 133:43–54. https://doi.org/10.1016/j.molcatb.2016.07.012
Martín MC, López OV, Ciolino AE, Morata VI, Villar MA, Ninago MD (2019) Immobilization of enological pectinase in calcium alginate hydrogels: a potential biocatalyst for winemaking. Biocatal Agric Biotechnol 18:101091. https://doi.org/10.1016/j.bcab.2019.101091
Mehmood T, Saman T, Irfan M, Anwar F, Ikram MS, Tabassam Q (2019) Pectinase production from Schizophyllum commune through central composite design using citrus waste and its immobilization for industrial exploitation. Waste Biomass Valorization 10:2527–2536. https://doi.org/10.1007/s12649-018-0279-9
Mellinas C, Ramos M, Jiménez A, Garrigós MC 2020 Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications Materials (basel) 13https://doi.org/10.3390/ma13030673
Mihajlovski K, Buntić A, Milić M, Rajilić-Stojanović M, Dimitrijević-Branković S 2020 From agricultural waste to biofuel: enzymatic potential of a bacterial isolate Streptomyces fulvissimus CKS7 for bioethanol production Waste and Biomass Valorization 10https://doi.org/10.1007/s12649-020-00960-3
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277. https://doi.org/10.1016/j.pbi.2008.03.006
Munir M, Abdullah R, Ul Haq I, Kaleem A, Iqtedar M, Naz S (2020) Strain improvement by random mutagenesis of Aspergillus Tamarii RMLC-10 for improved biosynthesis of polygalacturonase. Pakistan J Bot 52:1809–1813. https://doi.org/10.30848/PJB2020-5(29)
Murad HA, Azzaz HH (2011) Microbial pectinases and ruminant nutrition. Res J Microbiol 6:246–269. https://doi.org/10.3923/jm.2011.246.269
Nighojkar A, Patidar MK, Nighojkar S (2019) Pectinases: production and applications for fruit juice beverages. Elsevier Inc.
Ortiz GE, Ponce-Mora MC, Noseda DG, Cazabat G, Saravalli C, López MC, Gil GP, Blasco M, Albertó EO (2017) Pectinase production by Aspergillus giganteus in solid-state fermentation: optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J Ind Microbiol Biotechnol 44:197–211. https://doi.org/10.1007/s10295-016-1873-0
Osofero AI, Momoh E, Salih M, Koliopoulos T (2018) Innovative use of agricultural wastes for eco-friendly construction. Emerg Environ Technol Heal Prot 1:96–115
Oumer OJ (2017) Pectinase: substrate, production and their biotechnological applications. Int J Environ Agric Biotechnol 2:1007–1014. https://doi.org/10.22161/ijeab/2.3.1
Oumer OJ, Abate D (2018) Comparative studies of pectinase production by Bacillus subtilis strain Btk 27 in submerged and solid-state fermentations. Biomed Res Int 2018:10. https://doi.org/10.1155/2018/1514795
Pancerz M, Kruk J, Łukasiewicz M, Witek M, Kucharek M, Jaschik J, Ptaszek A (2021) Red currant pectin: the physicochemical characteristic of pectin solutions in dilute and semi dilute regimes. Food Hydrocoll 113:106420. https://doi.org/10.1016/j.foodhyd.2020.106420
Paniagua C, Posé S, Morris VJ, Kirby AR, Quesada MA, Mercado JA (2014) Fruit softening and pectin disassembly: an overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann Bot 114:1375–1383. https://doi.org/10.1093/aob/mcu149
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301. https://doi.org/10.1007/s002530000403
Pathak P, Bhardwaj NK, K SA (2010) Enzymatic deinking of office waste paper: an overview. IPPTA J 22:1–6
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2016) Papaya peel valorization for production of acidic pectin methylesterase by Aspergillus tubingensis and its application for fruit juice clarification. Biocatal Agric Biotechnol 6:58–67. https://doi.org/10.1016/j.bcab.2016.02.008
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2018) Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: a review. 3 Biotech 8:1–24. https://doi.org/10.1007/s13205-018-1220-4
Pauchet Y, Kirsch R, Giraud S, Vogel H, Heckel DG (2014) Identification and characterization of plant cell wall degrading enzymes from three glycoside hydrolase families in the cerambycid beetle Apriona japonica. Insect Biochem Mol Biol 49:1–13. https://doi.org/10.1016/j.ibmb.2014.03.004
Paudel YP, Lin C, Shen Z, Qin W (2015) Characterization of pectin depolymerising exo polygalacturonase by Bacillus sp. HD2 isolated from the gut of Apis mellifera L. Microbiol Discov 3:2. https://doi.org/10.7243/2052-6180-3-2
Pedrolli DB, Monteiro AC, Gomes E, Carmona EC (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol J 3:9–18. https://doi.org/10.2174/1874070700903010009
Pereira GS, Cipriani M, Wisbeck E, Souza O, Strapazzon JO, Gern RMM (2017) Onion juice waste for production of Pleurotus sajor-caju and pectinases. Food Bioprod Process 106:11–18. https://doi.org/10.1016/j.fbp.2017.08.006
Pervez S, Shahid F, Aman A, Qader SAU (2017) Algal biomass: a sustainable, economical and renewable approach for microbial production of pectinolytic enzymes using submerged and solid state fermentation techniques. Biocatal Biotransformation 35:442–449. https://doi.org/10.1080/10242422.2017.1364731
Picot-Allain MCN, Ramasawmy B, Emmambux MN (2020) Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: a review. Food Rev Int 00:1–31. https://doi.org/10.1080/87559129.2020.1733008
Polizeli MLT de M, de Lima Damásio AR, Maller A, Cabral H, Polizeli AM, Rai M (2016) Pectinases produced by microorganisms: Properties and applications. Fungal Enzym 316–340
Ramírez-Tapias YA, Lapasset Laumann AS, Britos CN, Rivero CW, Trelles JA (2017) Saccharification of citrus wastes by immobilized polygalacturonase in an improved alginate matrix. 3 Biotech 7:3–8. https://doi.org/10.1007/s13205-017-1010-4
Reddy MPC, Saritha KV (2016) Effects of the culture media optimization on pectinase production by Enterobacter sp. PSTB-1. 3 Biotech 6:1–11. https://doi.org/10.1007/s13205-016-0502-y
Rehman HU, Aman A, Nawaz MA, Karim A, Ghani M, Baloch AH, Qader SAU (2016) Immobilization of pectin depolymerising polygalacturonase using different polymers. Int J Biol Macromol 82:127–133. https://doi.org/10.1016/j.ijbiomac.2015.10.012
Reid I, Ricard M (2000) Pectinase in papermaking: solving retention problems in mechanical pulps bleached with hydrogen peroxide. Enzyme Microb Technol 26:115–123. https://doi.org/10.1016/S0141-0229(99)00131-3
Rollero S, Bloem A, Ortiz-Julien A, Camarasa C, Divol B (2018) Fermentation performances and aroma production of non-conventional wine yeasts are influenced by nitrogen preferences. FEMS Yeast Res 18:1–11. https://doi.org/10.1093/femsyr/foy055
Ropartz D, Ralet M-C (2020) Pectin structure. In: Kongtogiorgos V (ed) Pectin: technological and physiological properties. Springer 17–36
Samanta S (2019) Microbial pectinases: a review on molecular and biotechnological perspectives. J Microbiol Biotechnol Food Sci 9:248–266. https://doi.org/10.15414/jmbfs.2019.9.2.248-266
Sánchez SR, Sánchez GI, Arévalo-Villena M, Pérez AB (2015) Production and immobilization of enzymes by solid-state fermentation of agroindustrial waste. Bioprocess Biosyst Eng 38:587–593. https://doi.org/10.1007/s00449-014-1298-y
Sarsar MS, Pathak AP (2019) Thermostable pectinase mediated enhanced antimicrobial activity of Emblica officinalis: a novel application. Indian J Geo-Marine Sci 48:1404–1410
Satapathy S, Rout JR, Kerry RG, Thatoi H, Sahoo SL (2020) Biochemical prospects of various microbial pectinase and pectin: an approachable concept in pharmaceutical bioprocessing. Front Nutr 7:1–17. https://doi.org/10.3389/fnut.2020.00117
Sayah MY, Chabir R, Benyahia H, Kandri YR, Chahdi FO, Touzani H, Errachidi F (2016) Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS One 11:1–16. https://doi.org/10.1371/journal.pone.0161751
Sethi B, Satpathy A, Tripathy S, Parida S, Singdevsachan SK, Behera B (2016) Production of ethanol and clarification of apple juice by pectinase enzyme produced from Aspergillus terreus NCFT 4269.10. Int J Biol Res 4:67. https://doi.org/10.14419/ijbr.v4i1.6134
Sharma N, Rathore M, Sharma M (2013) Microbial pectinase: sources, characterization and applications. Rev Environ Sci Biotechnol 12:45–60. https://doi.org/10.1007/s11157-012-9276-9
Shen Y, Meng D, McGrouther K, Zhang J, Cheng L (2017) Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1. Plant Methods 13:1–10. https://doi.org/10.1186/s13007-017-0193-3
Shrestha S, Khatiwada JR, Zhang X, Chio C, Kognou ALM, Chen F, Han S, Chen X, Qin W (2021) Screening and molecular identification of novel pectinolytic bacteria from forest soil. Fermentation 7:40. https://doi.org/10.3390/fermentation7010040
Shrestha S, Mokale AL, Zhang J, Qin W (2020) Different facets of lignocellulosic biomass including pectin and its perspectives. Waste Biomass Valorization. https://doi.org/10.1007/s12649-020-01305-w
Singh A, Kaur A, Yadav RD, Mahajan R (2019) An efficient eco-friendly approach for recycling of newspaper waste. 3 Biotech 9:1–7. https://doi.org/10.1007/s13205-019-1590-2
Singh A, Varghese LM, Battan B, Patra AK, Mandhan RP, Mahajan R (2020) Eco-friendly scouring of ramie fibers using crude xylano-pectinolytic enzymes for textile purpose. Environ Sci Pollut Res 27:6701–6710. https://doi.org/10.1007/s11356-019-07424-9
Singh A, Yadav RD, Kaur A, Mahajan R (2012) An ecofriendly cost effective enzymatic methodology for deinking of school waste paper. Bioresour Technol 120:322–327. https://doi.org/10.1016/j.biortech.2012.06.050
Sisti L, Totaro G, Vannini M, Celli A (2018) Retting process as a pretreatment of natural fibers for the development of polymer composites. In Lignocellulosic composite materials. Springer, Cham.Springer International Publishing
Sojitra UV, Nadar SS, Rathod VK (2017) Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr Polym 157:677–685. https://doi.org/10.1016/j.carbpol.2016.10.018
Solís S, Flores ME, Huitrón C (1996) Protoplasts from pectinolytic fungi: isolation, regeneration and pectinolytic enzyme production. Lett Appl Microbiol 23:36–42. https://doi.org/10.1111/j.1472-765X.1996.tb00024.x
Takanami Y, Kubo S (1979) Enzyme-assisted purification of two phloem-limited plant viruses: tobacco necrotic dwarf and potato leafroll. J Gen Virol 44:153–159. https://doi.org/10.1099/0022-1317-44-1-153
Takcı HAM, Turkmen FU (2016) Extracellular pectinase production and purification from a newly isolated Bacillus subtilis strain. Int J Food Prop 19:2443–2450. https://doi.org/10.1080/10942912.2015.1123270
Tapre AR, Jain RK (2014) Pectinases: Enzymes for fruit processing industry. Int Food Res J 21:447–453
Taskin M (2013) Co-production of tannase and pectinase by free and immobilized cells of the yeast Rhodotorula glutinis MP-10 isolated from tannin-rich persimmon (Diospyros kaki L.) fruits. Bioprocess Biosyst Eng 36:165–172. https://doi.org/10.1007/s00449-012-0771-8
Thakur S, Chaudhary J, Kumar V, Thakur VK (2019) Progress in pectin based hydrogels for water purification: trends and challenges. J Environ Manage 238:210–223. https://doi.org/10.1016/j.jenvman.2019.03.002
Verma H, Narnoliya LK, Jadaun JS (2018) Pectinase: a useful tool in fruit processing industries. Nutr Food Sci Int J 5:4. https://doi.org/10.19080/nfsij.2018.05.555673
Vigneswaran C, Anbumani N, Ananthasubramanian M, Rajendran R (2012) Prediction and process optimization of pectinolytic reaction on organic cotton fabrics for bioscouring with alkaline pectinase. Indian J Fibre Text Res 37:183–190
Vityazev FV, Fedyuneva MI, Golovchenko VV, Patova OA, Ipatova EU, Durnev EA, Martinson EA, Litvinets SG (2017) Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr Polym 157:9–20. https://doi.org/10.1016/j.carbpol.2016.09.048
Wang H, Li X, Ma Y, Song J (2014) Characterization and high-level expression of a metagenome-derived alkaline pectate lyase in recombinant Escherichia coli. Process Biochem 49:69–76. https://doi.org/10.1016/j.procbio.2013.10.001
Wang J, Chio C, Chen X, Su E, Cao F, Jin Y, Qin W (2019) Efficient saccharification of agave biomass using Aspergillus niger produced low-cost enzyme cocktail with hyperactive pectinase activity. Bioresour Technol 272:26–33. https://doi.org/10.1016/j.biortech.2018.09.069
Yamada Y, Koibuchi M, Miyamoto K, Ueda J, Uheda E (2015) Breakdown of middle lamella pectin by {filled circle}OH during rapid abscission in Azolla. Plant Cell Environ 38:1555–1564. https://doi.org/10.1111/pce.12505
Yang JS, Mu TH, Ma MM (2018) Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem 244:197–205. http://dx.doi.org/10.1016/j.foodchem.2017.10.059
Yang JY, Li J, Wang M, Zou XG, Peng B, Yin YL, Deng ZY (2019) A novel aqueous extraction for Camellia oil by emulsified oil: a frozen/thawed method. Eur J Lipid Sci Technol 121:1–11. https://doi.org/10.1002/ejlt.201800431
Yu P, Wang X, Ren Q, Huang X, Yan T (2019) Genome shuffling for improving the activity of alkaline pectinase in Bacillus subtilis FS105 and its molecular mechanism. World J Microbiol Biotechnol 35:1–10. https://doi.org/10.1007/s11274-019-2749-z
Yu P, Xu C (2018) Production optimization, purification and characterization of a heat-tolerant acidic pectinase from Bacillus sp. ZJ1407. Int J Biol Macromol 108:972–980. https://doi.org/10.1016/j.ijbiomac.2017.11.012
Yuan Y, Zhang XY, Zhao Y, Zhang H, Zhou YF, Gao J (2019) A novel pl9 pectate lyase from Paenibacillus polymyxa kf-1: cloning, expression, and its application in pectin degradation. Int J Mol Sci 20:1–17. https://doi.org/10.3390/ijms20123060
Zdarta J, Meyer AS, Jesionowski T, Pinelo M 2018 A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility Catalysts 8https://doi.org/10.3390/catal8020092
Zhang G, Li S, Xu Y, Wang J, Wang F, Xin Y, Shen Z, Zhang H, Ma M, Liu H (2019) Production of alkaline pectinase: a case study investigating the use of tobacco stalk with the newly isolated strain Bacillus tequilensis CAS-MEI-2-33. BMC Biotechnol 19:1–11. https://doi.org/10.1186/s12896-019-0526-6
Zheng Y-X, Wang Y-L, Pan J, Zhang J-R, Dai Y, Chen K-Y (2017) Semi-continuous production of high-activity pectinases by immobilized Rhizopus oryzae using tobacco wastewater as substrate and their utilization in the hydrolysis of pectin-containing lignocellulosic biomass at high solid content. Bioresour Technol 241:1138–1144. https://doi.org/10.1016/j.biortech.2017.06.066
Zhu W, Han C, Dong Y, Jian B (2019) Enzyme-responsive mechanism based on multi-walled carbon nanotubes and pectin complex tablets for oral colon-specific drug delivery system. J Radioanal Nucl Chem 320:503–512. https://doi.org/10.1007/s10967-019-06501-0
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada Funding (RGPIN-2017–05366) to Wensheng Qin and Ontario Graduate Scholarship to Sarita Shrestha.
Author information
Authors and Affiliations
Contributions
SS did the major contribution in designing and writing the manuscript. WQ did proofread, and all authors helped in writing and editing. All authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics approval
This review does not encompass any studies with animal or human participants by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrestha, S., Rahman, M.S. & Qin, W. New insights in pectinase production development and industrial applications. Appl Microbiol Biotechnol 105, 9069–9087 (2021). https://doi.org/10.1007/s00253-021-11705-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11705-0