Abstract
Flaxseed has gained significant interest as a source of edible oil that is rich in omega-3 fatty acids, high content of flaxseed proteins and lignans that are known to be therapeutic. Low oxidative stability of flaxseed oil necessitates the use of extraction technologies that are advanced and economically viable than the currently used cold press extraction. This work compares the yield and quality of the flaxseed oil obtained by individually optimized supercritical carbon dioxide extraction (SCE), three-phase partitioning (TPP), solvent extraction and the reported values of cold press extraction. The yields of oil obtained were comparable for SCE (30.03% w/w), TPP (22.46% w/w), ultrasonic pre-treated TPP (27.05% w/w), enzyme-pre-treated TPP (26.24% w/w) and reported value of 25.50% w/w in commercial screw-press expeller but lower than solvent extraction (41.53% w/w). Amongst the techniques evaluated, enzyme-pre-treated TPP using Accellerase® is recommended due to excellent protein recovery of 86.62%, better oil quality (iodine value, peroxide value, acid value and 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity) and a potential of being industrially scalable.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flaxseeds (Linum usitatissimum L.) are one of the most important oilseeds as they contain 38–44% of oil rich in omega-3 fatty acids and linolenic acid (Oomah 2001), 1–5% lignans that possess very high antioxidant activity (Almario and Karakas 2013), 10–31% of good quality proteins and around 28% dietary fibre. Besides applications as an edible oil, flaxseed oil also finds applications in manufacturing of paints, dyes and animal feed (Shim et al. 2014). Regular consumption of flaxseed is associated with numerous therapeutic benefits such as reduction in serum triglycerides and cholesterol, anti-inflammatory action and anti-cancer activity (Almario and Karakas 2013). The major functional class of compounds imparting therapeutic effects are a combination of constituents present therein such as polyunsaturated fatty acids, the lignan complex, polysaccharides, flaxseed proteins and cyclolinopeptides (Almario and Karakas 2013; Shim et al. 2014).
Flaxseed proteins and protein hydrolysates have the ability to reduce hypertension due to its similarity with soy protein hydrolysate in terms of concentration and composition of amino acids (Shim et al. 2014). Considering the nutritional as well as therapeutic effect of both oil and protein from flaxseeds (Oomah 2001; Shim et al. 2014) along with its agronomics (Bozan and Temelli 2002; Pradhan et al. 2010), it is worthwhile to explore techniques that can simultaneously separate protein and oil in a short time without damaging the quality of both the constituents.
Although solvent extraction of flaxseed gives maximum oil extraction, it results in considerable deterioration of the same (Bozan and Temelli 2002; Pradhan et al. 2010). Hence, mechanical pressing or cold pressing of flaxseed is generally practised commercially. This however gives lower yields of oil but of better quality (Pradhan et al. 2010). Supercritical carbon dioxide extraction (SCE) is an eco-friendly technique (Kagliwal et al. 2012) that has been demonstrated to be useful in extracting edible oils from numerous sources viz. sunflower, coriander, tomato, peanut, almond, fennel and grape (Reverchon and Marrone 2001); wheat germ (Piras et al. 2009); palm (Akanda et al. 2012); rapeseed (Cvjetko et al. 2012); and tea seed (Feng et al. 2015). This technique has also been used for extracting flaxseed oil of quality that is comparable to mechanical pressing (Bozan and Temelli 2002; Pradhan et al. 2010). The use of carbon dioxide and its recyclability make SCE an economical and safe (Pradhan et al. 2010) process and hence a method of choice for flaxseed oil extraction.
Three-phase partitioning (TPP) follows principles of green chemistry and requires three components to be mixed, i.e. an aqueous slurry (sample), t-butanol and ammonium sulphate, where the t-butanol used is completely recyclable, making the process eco-friendly (Alfonsi et al. 2008; Harde and Singhal 2012). This technique enables separation of oils in upper t-butanol layer and hydrophilic substances in aqueous ammonium sulphate lower layer with proteins in the middle layer in a single step (Dennison and Lovrien 1997; Mulchandani et al. 2015; Vidhate and Singhal 2013). Due to intricate interactions that are ionic, kosmotropic, osmotic and colloidal in nature, t-butanol which is generally soluble in aqueous phase becomes insoluble due to ammonium sulphate resulting in formation of three layers, where proteins float above lowest aqueous layer as a result of increased buoyancy (Dennison and Lovrien 1997; Kiss et al. 1998). TPP has been successfully used in extraction of various oils from soybean (Sharma et al. 2002), jatropa (Shah et al. 2004), Garcinia indica (Vidhate and Singhal 2013), Crotalaria juncea (Dutta et al. 2015), oleogeneous materials such as turmeric oleoresin (Kurmudle et al. 2011), ginger oleoresin (Varakumar et al. 2017) and to separate rice bran oil from protein (Phongthai and Rawdkuen 2015). It has also been used for separation and purification of various enzymes such as β-amylase from Abrus precatorius (Sagu et al. 2015), peroxidase from Citrus sinenses (Vetal and Rathod 2015), zingibain from Zingiber officinale (Gagaoua et al. 2015) and serratiopeptidase from Serratia marcescens (Pakhale and Bhagwat 2016).
This work is a comparative study of techniques such as solvent extraction, SCE and TPP on the extraction yield of flaxseed oil and further quality evaluation of the same. Response surface methodology (RSM) was used for process optimization of both SCE and TPP to get maximum possible yield. Oil quality has been examined by testing it for the level of unsaturation (iodine value), rancidity (peroxide value), acid value and antioxidant activity. An overall comparison has been drawn on selecting the most appropriate extraction method that would be ideal for commercial use.
Materials and Methods
Materials and Equipment
Brown variety of flaxseed was procured from the local retailer in Matunga, Mumbai, India. Carbon dioxide cylinders were supplied by Bombay Carbon Dioxide Gas Company, Mumbai, India. A laboratory-scale supercritical carbon dioxide extraction system Speed™ SFE-2 by Applied Separations Inc., PA, USA, was used to optimize SCE. Along with the instrument, accessories like filling material (Spe-ed matrix), separating material (Spe-ed Wool), metal column (5 mL) and collection vials were also used. Ammonium sulphate, t-butanol, ethanol, petroleum ether and all other chemicals used were purchased from S.D. Fine Chemicals Limited, Mumbai, India. Enzyme sample (Accellerase® 1500, activity 2400–2800 carboxymethyl cellulose (CMC) U g−1) was procured from Genencor International, Mumbai, India.
Sample Preparation
Flaxseed was ground to fine powder in a laboratory-scale grinder for 40 s. Grinding was discontinuous with a gap after every 10 s. This was done to ensure reduced clogging and loss of oil. Ground flax seed was passed through a sieve of mesh size 0.425 mm, and the resultant powder was stored at −19 °C until use. Proximate analysis of the flaxseed powder was carried out prior to all the extractions.
Soxhlet Extraction
The standard Association of Official Analytical Chemists and Horwitz (2000) protocol was followed. A thimble containing weighed amount of ground flaxseed was kept in the Soxhlet extraction chamber. Petroleum ether (boiling point, 60 to 80 °C) was introduced from extractor to boiling flask attached at the bottom (volume of solvent was enough to run 1 and a half extraction cycles). A condenser was attached at the top and extraction was carried out at 80 °C for 13 h to ensure complete extraction of oil. After the extraction process, solvent was evaporated in a vacuum evaporator (Rotavapor ® R-100, BUCHI, India), and the yield of oil was calculated gravimetrically. Oil obtained by this method was considered to be the total oil content of flaxseed. All further optimizations and yield calculations were performed on the basis of this total oil content.
Supercritical Carbon Dioxide Extraction
RSM consisting of Box-Behnken design (Kagliwal et al. 2012; Montgomery 2009) was used to design the experiments for optimization of SCE. The design of 3 factors, each at 3 levels, had 17 runs with 5 centre point replicates. The three factors under consideration were pressure, temperature and time. The levels at which these factors were varied were 200–400 bar, 40–60 °C and 60–180 min, which were arrived at by performing initial trial and error experiments.
One gram of ground flaxseed was added in the extrac-tion column (5 mL). Spe-ed wool was added to both ends of column and the column was closed. The filled column was then placed in an oven where temperature was controlled throughout the extraction process. System setup was then pressurized using pneumatic pump in static mode. Once the pressure reached the desired value, dynamic mode was started by opening outlet. Flow rate was kept constant throughout the extraction at 200 mL min−1 carbon dioxide. After the desired time of extraction, system was depressurised and flushed with carbon dioxide and solvent (for representational image, please see Fig. S1 in the supplementary file). The response was gravimetric yield of flaxseed oil on basis of total oil. All trials except central point replicates were done in triplicates.
Three-Phase Partitioning
Flaxseed slurry was prepared in water by adding flaxseed powder. It was found that higher concentrations (> 5% (w/v)) resulted in increased viscosity of the slurry. Five percent (w/v) were found to be optimum. One-factor-at-a-time experiments were carried out to analyse optimum ranges from which the design of experiments was planned. Three factors optimized were ammonium sulphate concentration, solvent to slurry ratio and pH of the system. One-factor-at-a-time experiments included ammonium sulphate concentration (20 to 50% w/v), solvent to slurry ratio (0.5:1 to 2.5:1) and pH (3–7). The desired ranges were 30–40% w/v for ammonium sulphate concentration, 1:1 to 2:1 for solvent to slurry ratio and 4–7 for pH. With the desired ranges, a central composite design (CCD) for RSM study (Kar et al. 2015; Montgomery 2009) was formed of 3 factors and 5 levels with 20 runs (6 centre point replicates). The response was gravimetric yield of flaxseed oil on the basis of total oil.
A 5% (w/v) slurry was prepared and pH, ammonium sulphate and t-butanol were added to the mixture according to CCD. The mixture was then extracted for 1 h with constant stirring after which it was allowed to stand for another hour to let the three phases separate. This experimentation was carried out at room temperature (30 ± 2 °C). After separation of the three phases in a separating funnel, the lower aqueous layer was removed and the top two layers were centrifuged at 7298×g for 30 min. After centrifugation, t-butanol layer on the top containing lipophilic compounds (Mulchandani et al. 2015) was separated by gentle decanting. The recovered solvent was then subjected to evaporation to remove solvent. The middle layer of RSM-optimized TPP was estimated for the protein content by Kjeldhal method (Baker 1961) after dialysis to remove ammonium sulphate.
Ultrasound Pre-treatment
Flaxseed slurry was subjected to ultrasound pre-treatment using a probe sonicator (ultrasonic horn with single frequency of 22 kHz, maximum input power of 120 W and probe diameter 1.2 cm; M/s Dakshin Pvt. Ltd., Mumbai, India). Twenty millilitres of flaxseed slurry (5% w/v) was subjected to ultrasound pre-treatment in a 100-mL beaker. About 0.1–0.2 cm of probe depth was immersed in the slurry. The temperature was not maintained and the increase in temperature was monitored, which was between 60 and 70 °C. One-factor-at-a-time approach was employed for optimization by varying power output (30, 50 and 70 W), duty cycle (30, 50 and 70%) and time (5, 10 and 15 min). After pre-treatment, the slurry was subjected to extraction by RSM-optimized TPP parameters at room temperature.
Enzymatic Pre-treatment
For enzymatic pre-treatment, Accellerase® 1500 was used. The activity of this enzyme was calculated in CMC units by 3,5-dinitrosalicylic acid (DNSA) method (Miller 1959) using CMC as substrate as described by Zhang et al. (2009). The activity of Accellerase® 1500 used for experiments was found to be 2180 CMC U g−1. One-factor-at-a-time model was used to optimize enzymatic pre-treatment. The enzyme concentration was varied as 1, 3, 5 and 7% (v/v) and incubated for 1 h. After optimizing the enzyme concentration, time of incubation was varied as 15, 30, 60 and 90 min.
Enzyme was added at varied concentrations to slurry prepared in citrate buffer (pH 4–4.5) and subjected to stirring at 30 ± 2 °C at optimized pH of TPP extraction. Since the optimum pH for TPP extraction and enzyme activity were similar (4.0–4.5 and 4.19, respectively), this parameter was not considered for optimization. After the pre-treatment, the slurry was subjected to TPP extraction at room temperature.
Analysis of Oil Extracted by Different Techniques
These analyses included iodine value, peroxide value (Association of Official Analytical Chemists and Horwitz 2000), acid value (ISI 1986) and antioxidant activity (Siger et al. 2008). Iodine, peroxide and acid value are titrimetric methods that provide quantification of the level of unsaturation, rancidity and free fatty acids present, respectively, in oil. The antioxidant activity was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay in which the red colour of the stable DPPH changes to yellow after scavenging free radicals and which was quantified spectrophotometrically at 517 nm.
Statistical Analysis
All experiments except the central point replicates of both the RSM studies were done in triplicates in order to get average and standard deviation (SD). Design Expert 7.0 (State Ease, Minneapolis, MN, USA) was used for RSM experiments, analysis of variance (ANOVA) and obtaining regression model. ANOVA of the data amongst different extraction techniques was carried out by using IBM-SPSS software with significance level of p < 0.05.
Results and Discussion
Proximate Analysis and Soxhlet Extraction
Moisture content of ground flaxseed was found to be 6%, whilst that of oil after 13 h of Soxhlet extraction was 41.53 ± 1.01% (w/w) oil. This was considered as the amount of total oil. Further oil yields are stated in terms of percentage extraction based on this value of total oil. Flaxseed oil recovery reported for solvent extraction using petroleum ether after RSM optimization was 33.14% (w/w) oil (Ondrejovič et al. 2011). Protein content was estimated by Kjeldahl method and was found to be 20.71 ± 0.67% (w/w). Ash content was found to be 3.72 ± 0.02% (w/w). Hence, the remaining dietary fibre and soluble sugars contributed about 28.04% (w/w). Gopalan et al. (1989) found 6.5% moisture, 37.1% fats, 20.3% proteins, 28.9% carbohydrates and 2.4% ash in flaxseeds cultivated in India. These proximate constituents in flaxseed can vary greatly from region to region depending upon soil, irrigation, climatic conditions and the type of cultivar used (Shim et al. 2014).
Optimization of Supercritical Carbon Dioxide Extraction
After performing Box-Behnken design (Table S1 in the supplementary file), a quadratic polynomial function was obtained which was as follows:
where A, B and C represent the pressure (bar), temperature (°C) and time (min), respectively. Regression coefficient (R 2) value of the model was found to be 0.94, suggesting an accuracy of 94% for the model. Coefficient of variation of the model was 2.78, revealing its reliability. Model was found to be significant, and the most significant interaction was found to be AC (i.e. between pressure and time) (Fig. 1) with p < 0.05 that was detrimental to SCE optimization (Table S2 and Fig. S2 in the supplementary file). Thus, the optimal parameters for SCE after performing RSM were found to be 316 bar (pressure), 59.3 °C (temperature) and 131 min (time). This condition yielded 72.30 ± 0.44% (w/w) of total oil, which was very close to the predicted value of 72.70% (w/w) of total oil by the software. Bozan and Temelli (2002) have reported a flaxseed oil yield of 66% (w/w) of total oil after one-factor-at-a-time optimization of SCE with parameters of 550 bar, 70 °C and 180 min. Pradhan et al. (2010) have reported a flaxseed oil yield of 90% (w/w) of total oil with SCE parameters of 300 bar, 50 °C and 180 min. These difference in yields are attributed to different SCE parameters adopted (Harde et al. 2013; Kagliwal et al. 2011; Lang 2001).
Optimization of Three-Phase Partitioning
One-factor-at-a-time approach was used to find out the desired ranges for RSM experiments. The ranges that were finalized for CCD RSM experiments were 30 to 40% for ammonium sulphate concentration, 1:1 to 2:1 for solvent to slurry ratio and 4 to 7 for pH (please refer to Table S3 in the supplementary file for TPP CCD and responses obtained). After performing CCD, the following quadratic polynomial function was obtained:
where A, B and C represent the ammonium sulphate concentration (w/v), solvent to slurry ratio and pH, respectively. The R 2 value of the model was found to be 0.95, indicating a 95% accuracy of the same. The coefficient of variation of the model was 3.88, revealing its reliability. Model was found to be significant, and the most significant interaction was found to be AC (i.e. between ammonium sulphate concentration and pH) (Fig. 2) with p < 0.05 that was important to TPP optimization (Table S4 and Fig. S3 in the supplementary file). Thus, the optimal parameters for TPP after performing RSM were found to be 38.23 (w/v) ammonium sulphate concentration, a solvent:slurry ratio of 1.41:1 and a pH of 4.19. This condition yielded 54.09 ± 0.86% (w/w) of total oil, which was very close to the predicted value of 54.32% (w/w) of total oil by the software.
TPP is an extraction technique where simultaneous extraction of fats and proteins can be achieved (Mulchandani et al. 2015; Phongthai and Rawdkuen 2015) due to its unique chemical interactions as mentioned in the “Introduction” section of this paper. Flaxseeds have a tough seed structure (Shim et al. 2014). Grinding of flaxseed ruptures this structure and disrupts the cells which enhance oil extraction by Soxhlet, SCE and TPP (Bozan and Temelli 2002; Chisti and Moo-Young 1986; Harde et al. 2013; Ondrejovič et al. 2011). However, most of the oil vacuoles that are present inside the flaxseed plant cell do not rupture during grinding. The intracellularly entrapped flaxseed oil in the vacuoles can be readily extracted if a cell disruption protocol is followed prior to extraction (Domínguez et al. 1994; Kar and Singhal 2015; Mulchandani et al. 2015). Most of the cell disruption protocols require the formation of a slurry (Chisti and Moo-Young 1986), which cannot be utilized in Soxhlet and SCE unless it is dried. This however increases one step in unit operation, whereas TPP can be readily used as it requires the formation of slurry. Hence, it was decided to evaluate pre-treatment techniques prior to TPP in order to achieve cellular disruption and as reported in the literature (Chougle et al. 2014; Harde and Singhal 2012).
Optimization of Ultrasound and Enzymatic Pre-treatment Prior to Three-Phase Partitioning
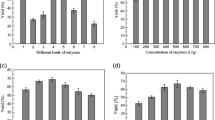
The % w/w yield of total oil increased with an increase in power output (Fig. 3a). An increase in the power output generated heat in the slurry and increased the temperature. A power output of 70 W was found to be optimum as it gave an increased yield, and the resultant temperature, checked immediately after treatment, was 68 °C. For optimization of power output, duty cycle and time were kept constant at 50% and 10 min, respectively. It was observed (Fig. 3b) that increasing duty cycle increased the oil yield up to 50%, beyond which no further increase in oil yield (constant parameters 70 W and 10 min) was observed. It was seen that 10 min of sonication treatment at 70-W power with 50% duty cycle was optimum to get a maximum yield of 65.13 ± 1.76% of total flaxseed oil post-TPP.
It was observed that the yield of oil increased with an increase in enzyme concentration from 1 to 3% (v/v), beyond which there was no further increase in oil yield (Fig. 4a). Hence, 3% enzyme concentration was considered to be optimum. Using the optimum concentration of enzyme, time was optimized. A 60 min of enzymatic pre-treatment was found to be sufficient (Fig. 4b). The optimized parameters for the enzymatic pre-treatment were therefore 3% (v/v) enzyme in slurry and 60 min of incubation, which yielded 63.18 ± 0.82% (w/w) of total oil.
Accellerase® 1500 (Genencor, India) used in this study consisted of cellulase and β-glucosidase. Sixty percent of total fibre of flaxseed are insoluble, of which a major portion is cellulose. The fibre network is held together by long-chained cellulosic microfibrils which form a secondary cell wall in oilseeds. In flaxseeds, oil is present in the intracellular vacuoles that are attached to other biomolecules, and its extraction is increased by the hydrolytic action of carbohydrases (Domínguez et al. 1994). Use of Accellerase® 1500 helped in hydrolysing the cellulose and disintegrating the structure of seed tissues/cells, which in turn increased the exposure of solvent with oil and hence facilitated extraction.
Which is a Better Method for Extraction?
Iodine value represents the amount of unsaturation in oil. It was observed that ultrasound pre-treated TPP oil had the least iodine value and oil obtained from SCE had the highest (Table 1). No significant difference was found in Soxhlet, TPP and enzyme pre-treated oil. This suggests that majority of polyunsaturated fatty acids were extracted in SCE. Peroxide value represents the extent of primary oxidation (rancidity) that has taken place in oil. It was observed that oil extracted by Soxhlet method had the highest peroxide value, whereas ultrasonication pre-treated TPP showed similar peroxide value as that of Soxhlet (Table 1). This could be due to the high temperature during Soxhlet extraction and the heat generated during sonication. Peroxide value of commercial screw-press expeller was the lowest, whilst SCE, TPP and enzyme pre-treated TPP demonstrated peroxide value similar to commercial screw-press expeller. Acid value represents the number of free fatty acids that are present in the sample. Commercial screw-press expeller showed the lowest acid value, whereas ultrasound pre-treated TPP showed the highest; other methods showed more or less similar acid values. Chemical extraction techniques may result in breaking of ester bonds, which increase free fatty acid and hence show higher acid value when compared to mechanical extraction (Rodrigues et al. 2007). It has been reported that flaxseed contains high content of antioxidants, namely phenolic acids and lignans (Wanasundara et al. 1997). It was observed that TPP-extracted oil had similar antioxidant activity as that of solvent extracted and SCE flaxseed oil (Table 1). Oil obtained after enzyme pre-treated TPP had lower and ultrasonic pre-treated had the lowest antioxidant activity than that obtained by other techniques of extraction. During pre-treatment, some loss of antioxidant activity may be taking place.
The oil yield followed the order of Soxhlet > SCE > ultrasound pre-treated = enzyme pre-treated TPP = commercial screw-press expeller > TPP (Table 1). A recent study published by Tan et al. (2016) found enzyme-assisted TPP (cellulase/proteinase/pectinase in ratio of 1:1:1) to be better than TPP, Soxhlet extraction and cold press method with a flaxseed oil yield of around 75% (enzyme assisted TPP > Soxhlet extraction > cold press method > TPP). The difference in the trend observed in this work and as reported above may be due to use of different enzyme combinations for enzyme-assisted TPP used in this study (Accellerase® = cellulase and β-glucosidase) and by Tan et al. (2016) (cellulase/proteinase/pectinase).
In addition to extraction of oil, proteins were also simultaneously extracted in the middle layer of TPP (Fig. S4 in the supplementary file). From RSM-optimized TPP, 17.94% (w/w) of flaxseed protein were extracted from a total flaxseed protein of 20.71% (w/w), which amounted to 86.62% recovery of the same. Flaxseed protein is used in feed as well as non-feed industrial use. Recently, many therapeutic peptides have also been discovered in flaxseed protein, making it an area that has tremendous scope for commercial exploitation and research (Shim et al. 2014).
When compared to Soxhlet, SCE and commercial screw-press expeller, TPP has some advantages. TPP is environment friendly as it uses the principles of green chemistry and the t-butanol used can be recycled (Mulchandani et al. 2015). The melting point of t-butanol is 25 °C. Flaxseed oil can be easily separated by simple decantation below 25 °C, making TPP a very cost-effective, unique and industrially scalable extraction method. None of the other methods studied herein have the advantage of simultaneous separation and extraction of proteins and fat. Hence, preference could be given to the enzymatic pre-treated TPP as it is economical and energy efficient when compared to ultrasound pre-treatment TPP. In case of enzymatic pre-treatment TPP, the enzyme can be re-used by immobilization approach (Chaudhari et al. 2015).
Conclusion
Various techniques were used to extract flaxseed oil, where the quantity and quality of flaxseed oil were measured. Solvent extraction gave the highest oil yield. Oil extracted by SCE showed the best quality in terms of unsaturation and antioxidant activity. Enzymatic pre-treated TPP is a promising approach for extraction of oil from flaxseed as it simultaneously separates protein and oil. An added advantage is that the reagents and enzyme used in the enzymatic pre-treated TPP may have the potential to be recycled. The oil yield by enzymatic pre-treated TPP was comparable to commercial screw-press expeller, and quality of oil was also appreciable. Enzymatic pre-treated TPP can become a cost-effective industrial method of oil extraction if proper scale-up is performed.
References
Akanda, M. J. H., Sarker, M. Z. I., Ferdosh, S., Manap, M. Y. A., Ab Rahman, N. N. N., & Ab Kadir, M. O. (2012). Applications of supercritical fluid extraction (SFE) of palm oil and oil from natural sources. Molecules, 17(12), 1764–1794. doi:10.3390/molecules17021764.
Alfonsi, K., Colberg, J., Dunn, P. J., Fevig, T., Jennings, S., Johnson, T. A., et al. (2008). Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chemistry, 10(1), 31. doi:10.1039/b711717e.
Almario, R. U., & Karakas, S. E. (2013). Lignan content of the flaxseed influences its biological effects in healthy men and women. Journal of the American College of Nutrition, 32(3), 194–199. doi:10.1080/07315724.2013.791147.
Association of Official Analytical Chemists, & Horwitz, W. (2000). Official methods of analysis of AOAC international. AOAC international. Arlington, Va: AOAC International.
Baker, P. (1961). The micro-Kjeldahl determination of nitrogen an investigation of the effects of added salt and catalysts. Talanta, 8(2–3), 57–71. doi:10.1016/0039-9140(61)80040-4.
Bozan, B., & Temelli, F. (2002). Supercritical CO2 extraction of flaxseed. Journal of the American Oil Chemists’ Society, 79(3), 231–235. doi:10.1007/s11746-002-0466-x.
Chaudhari, S.A., Kar, J. R., & Singhal, R. S. (2015). Immobilization of proteins in alginate: functional properties and applications. Current Organic Chemistry, 19, 1732–1754. doi:10.2174/1385272819666150429232110.
Chisti, Y., & Moo-Young, M. (1986). Disruption of microbial cells for intracellular products. Enzyme and Microbial Technology, 8(4), 194–204. doi:10.1016/0141-0229(86)90087-6.
Chougle, J. A., Singhal, R. S., & Baik, O. D. (2014). Recovery of astaxanthin from Paracoccus NBRC 101723 using ultrasound-assisted three phase partitioning (UA-TPP). Separation Science and Technology, 49(6), 811–818. doi:10.1080/01496395.2013.872146.
Cvjetko, M., Jokić, S., Lepojević, Ž., Vidović, S., Marić, B., & Radojčić Redovniković, I. (2012). Optimization of the supercritical CO2 extraction of oil from rapeseed using response surface methodology. Food Technology and Biotechnology, 50(2), 208–215.
Dennison, C., & Lovrien, R. (1997). Three phase partitioning: concentration and purification of proteins. Protein Expression and Purification, 11(2), 149–161. doi:10.1006/prep.1997.0779.
Domínguez, H., Núñez, M. J., & Lema, J. M. (1994). Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: a review. Food Chemistry, 49(3), 271–286. doi:10.1016/0308-8146(94)90172-4.
Dutta, R., Sarkar, U., & Mukherjee, A. (2015). Process optimization for the extraction of oil from Crotalaria juncea using three phase partitioning. Industrial Crops and Products, 71, 89–96. doi:10.1016/j.indcrop.2015.03.024.
Feng, J., Lei, H., & Ge, F. (2015). Modeling of the extraction process of tea seed oil with supercritical carbon dioxide. Brazilian Journal of Chemical Engineering, 32(4), 941–947. doi:10.1590/0104-6632.20150324s20140252.
Gagaoua, M., Hoggas, N., & Hafid, K. (2015). Three phase partitioning of zingibain, a milk-clotting enzyme from Zingiber officinale roscoe rhizomes. International Journal of Biological Macromolecules, 73, 245–252. doi:10.1016/j.ijbiomac.2014.10.069.
Gopalan, C., Sastri, B. V. R., & Balasubramanian, S. C. (1989). Nutritive value of Indian foods. National Institute of Nutrition, Indian Council of Medical Research. https://books.google.co.in/books?id=biFBAAAAYAAJ
Harde, S. M., Kagliwal, L. D., Singhal, R. S., & Patravale, V. B. (2013). Supercritical fluid extraction of forskolin from Coleus forskohlii roots. Journal of Food Engineering, 117(4), 443–449. doi:10.1016/j.jfoodeng.2012.12.012.
Harde, S. M., & Singhal, R. S. (2012). Extraction of forskolin from Coleus forskohlii roots using three phase partitioning. Separation and Purification Technology, 96, 20–25. doi:10.1016/j.seppur.2012.05.017.
ISI (1986) Methods of sampling and test for oils and fats. IS: 543 Bureau of Indian. Standards. Manak Bhawan, New Delhi.
Kagliwal, L. D., Patil, S. C., Pol, A. S., Singhal, R. S., & Patravale, V. B. (2011). Separation of bioactives from seabuckthorn seeds by supercritical carbon dioxide extraction methodology through solubility parameter approach. Separation and Purification Technology, 80(3), 533–540. doi:10.1016/j.seppur.2011.06.008.
Kagliwal, L. D., Pol, A. S., Patil, S. C., Singhal, R. S., & Patravale, V. B. (2012). Antioxidant-rich extract from dehydrated seabuckthorn berries by supercritical carbon dioxide extraction. Food and Bioprocess Technology, 5(7), 2768–2776. doi:10.1007/s11947-011-0613-8.
Kar, J. R., Hallsworth, J. E., & Singhal, R. S. (2015). Fermentative production of glycine betaine and trehalose from acid whey using Actinopolyspora halophila (MTCC 263). Environmental Technology & Innovation, 3, 68–76. doi:10.1016/j.eti.2015.02.001.
Kar, J. R., & Singhal, R. S. (2015). Investigations on ideal mode of cell disruption in extremely halophilic Actinopolyspora halophila (MTCC 263) for efficient release of glycine betaine and trehalose. Biotechnology Reports, 5, 89–97. doi:10.1016/j.btre.2014.12.005.
Kiss, é., Szamos, J., Tamás, B., & Borbás, R. (1998). Interfacial behavior of proteins in three-phase partitioning using salt-containing water/tert-butanol systems. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 142(2–3), 295–302. doi:10.1016/S0927-7757(98)00361-6.
Kurmudle, N. N., Bankar, S. B., Bajaj, I. B., Bule, M. V., & Singhal, R. S. (2011). Enzyme-assisted three phase partitioning: a novel approach for extraction of turmeric oleoresin. Process Biochemistry, 46(1), 423–426. doi:10.1016/j.procbio.2010.09.010.
Lang, Q. (2001). Supercritical fluid extraction in herbal and natural product studies—a practical review. Talanta, 53(4), 771–782. doi:10.1016/S0039-9140(00)00557-9.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. doi:10.1021/ac60147a030.
Montgomery, D. C. (2009). Introduction to statistical quality control (6th ed.). Hoboken, N.J: Wiley.
Mulchandani, K., Kar, J. R., & Singhal, R. S. (2015). Extraction of lipids from Chlorella saccharophila using high-pressure homogenization followed by three phase partitioning. Applied Biochemistry and Biotechnology, 176, 1613–1626. doi:10.1007/s12010-015-1665-4.
Ondrejovič, M., Chmelová, D., & Maliar, T. (2011). Response surface methodology for optimization of the extraction of flax (Linum usitatissimum) seed oil. Potravinarstvo, 5(4). doi:10.5219/168.
Oomah, B. D. (2001). Flaxseed as a functional food source. Journal of the Science of Food and Agriculture, 81(9), 889–894. doi:10.1002/jsfa.898.
Pakhale, S. V., & Bhagwat, S. S. (2016). Purification of serratiopeptidase from Serratia marcescens NRRL B 23112 using ultrasound assisted three phase partitioning. Ultrasonics Sonochemistry, 31, 532–538. doi:10.1016/j.ultsonch.2016.01.037.
Phongthai, S., & Rawdkuen, S. (2015). Preparation of rice bran protein isolates using three-phase partitioning and its properties. Food and Applied Bioscience Journal, 3(2), 137–149.
Piras, A., Rosa, A., Falconieri, D., Porcedda, S., Dessì, M. A., & Marongiu, B. (2009). Extraction of oil from wheat germ by supercritical CO2. Molecules, 14(7), 2573–2581. doi:10.3390/molecules14072573.
Pradhan, R. C., Meda, V., Rout, P. K., Naik, S., & Dalai, A. K. (2010). Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. Journal of Food Engineering, 98(4), 393–397. doi:10.1016/j.jfoodeng.2009.11.021.
Reverchon, E., & Marrone, C. (2001). Modeling and simulation of the supercritical CO2 extraction of vegetable oils. The Journal of Supercritical Fluids, 19(2), 161–175. doi:10.1016/S0896-8446(00)00093-0.
Rodrigues, C. E. C., Goncalves, C. B., Batista, E., & Meirelles, A. J. A. (2007). Deacidification of vegetable oils by solvent extraction. Recent Patents on Engineering, 1(1), 95–102. doi:10.2174/187221207779814699.
Sagu, S. T., Nso, E. J., Homann, T., Kapseu, C., & Rawel, H. M. (2015). Extraction and purification of beta-amylase from stems of Abrus precatorius by three phase partitioning. Food Chemistry, 183, 144–153. doi:10.1016/j.foodchem.2015.03.028.
Shah, S., Sharma, A., & Gupta, M. N. (2004). Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Industrial Crops and Products, 20(3), 275–279. doi:10.1016/j.indcrop.2003.10.010.
Sharma, A., Khare, S. K., & Gupta, M. N. (2002). Three phase partitioning for extraction of oil from soybean. Bioresource Technology, 85(3), 327–329. doi:10.1016/S0960-8524(02)00138-4.
Shim, Y. Y., Gui, B., Arnison, P. G., Wang, Y., & Reaney, M. J. T. (2014). Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends in Food Science & Technology, 38(1), 5–20. doi:10.1016/j.tifs.2014.03.011.
Siger, A., Nogala-Kalucka, M., & Lampart-Szczapa, E. (2008). The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. Journal of Food Lipids, 15(2), 137–149. doi:10.1111/j.1745-4522.2007.00107.x.
Tan, Z., Yang, Z., Yi, Y., Wang, H., Zhou, W., Li, F., & Wang, C. (2016). Extraction of oil from flaxseed (Linum usitatissimum L.) using enzyme-assisted three-phase partitioning. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-016-2068-x.
Varakumar, S., Umesh, K. V., & Singhal, R. S. (2017). Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme-assisted three phase partitioning. Food Chemistry, 216, 27–36. doi:10.1016/j.foodchem.2016.07.180.
Vetal, M. D., & Rathod, V. K. (2015). Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinenses). Food and Bioproducts Processing, 94, 284–289. doi:10.1016/j.fbp.2014.03.007.
Vidhate, G. S., & Singhal, R. S. (2013). Extraction of cocoa butter alternative from kokum (Garcinia indica) kernel by three phase partitioning. Journal of Food Engineering, 117(4), 464–466. doi:10.1016/j.jfoodeng.2012.10.051.
Wanasundara, P. K. J. P. D., Shahidi, F., & Shukla, V. K. S. (1997). Endogenous antioxidants from oilseeds and edible oils. Food Reviews International, 13(2), 225–292. doi:10.1080/87559129709541106.
Zhang, Y. H. P., Hong, J., & Ye, X. (2009). Cellulase assays. In J. R. Mielenz (Ed.), Biofuels (Vol. 581, pp. 213–231). Totowa, NJ: Humana Press http://springerlink.bibliotecabuap.elogim.com/10.1007/978-1-60761-214-8_14. Accessed 7 September 2015.
Acknowledgements
First author Nikhil G Kulkarni is grateful to Technical Education Quality Improvement Programme (TEQIP), Government of India, and assisted by World Bank for their financial support in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulkarni, N.G., Kar, J.R. & Singhal, R.S. Extraction of Flaxseed Oil: A Comparative Study of Three-Phase Partitioning and Supercritical Carbon Dioxide Using Response Surface Methodology. Food Bioprocess Technol 10, 940–948 (2017). https://doi.org/10.1007/s11947-017-1877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1877-4