Abstract
Background and aims
Rhizodeposited-carbon (C) plays an important role in regulating soil C concentrations and turnover, however, the distribution of rhizodeposited-C into different soil organic carbon (SOC) pools and how regulated by nitrogen (N) fertilization still remains elusive.
Methods
We applied five N fertilization rates (0, 10, 20, 40, and 60 mg N kg−1 soil) to rice (Oryza sativa L.) with continuously labeled 13CO2 for 18 days, to measure 13C allocation into plant tissues and soil C fractions.
Results
Relative to the unfertilized controls, the ratio of 13C in plant aboveground shoot /belowground root increased as a result of N fertilization, and the contribution of rhizodeposited-C to SOC was increased by N fertilization, presumably due to the relatively high root biomass and exudates. Also, N fertilization increased 13C incorporation into large aggregates (0.25–2.0 mm) and the humic acid fraction. Biological C immobilization might occur and preserve rhizodeposition following high rates of N addition, which regulates rhizodeposits and C cycling, thus determining the stabilization of rhizodeposits in the different SOC pools.
Conclusion
Rhizodeposited-C from rice plants and its distribution within SOC pools strongly depend upon N fertilization, thus determines C sequestration potential from the rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant carbon (C) is the primary source of soil organic carbon (SOC), delivered to the soil as aboveground shoot residues and belowground rhizodeposits, which drive C cycling in terrestrial ecosystems (Ge et al. 2015; Weintraub et al. 2007). Rhizodeposited-C accounts for approximately 11% of all net photo-assimilates, mainly comprising water-soluble compounds with low molecular weight, e.g. organic acids, and insoluble compounds with greater molecular weight, such as mucilage (Jones et al. 2009; Kuzyakov and Schneckenberger 2004). Rhizodeposited-C strongly influences C sequestration in soil by affecting the mineralization and formation of SOC (Kuzyakov et al. 2003). Enhancing C sequestration and stabilization is especially important in paddy soils, due to its large contributions (up to 0.52 Gt of CO2 equivalents) to atmosphere C (Ge et al. 2012, 2015). It was reported that C transferred remaining belowground and rhizodeposition were 1065 kg C ha−1 and 200 kg C ha−1 for flooded rice, respectively (Lu et al. 2002b). Therefore, understanding rhizodeposited-C flow and its ecological implications for paddy soils requires investigation into the fate of this C within SOC pools.
Isotopic labeling allow to distinguish rhizodeposited C released by roots from the C already present in soil (SOM, microbial biomass etc.) (Kuzyakov et al. 2003). Pulse labeling, which label period is much shorter than the plant growth and C flow process, allows to investigate the dynamics of C fluxes in the plant organs (shoots, leafs, roots, etc.) and in soil compartments (e.g. microbial biomass, DOM, SOM pools, etc.). Based on the dynamics of the 14C or 13C in any of the pools after the pulse labeling, the rates of the inputs and outputs of root released C in the respective pool can be calculated. The alternative is the labeling period corresponding to the whole duration of the process under study, termed as continuous labeling, which allows direct estimation of total C input by roots into the soil over the whole growth period.
The fractionation of SOC based on physically defined fractions play various roles in understanding SOC stabilization (Six et al. 2002). Studies on the distribution of rhizodeposited-C are crucial for understanding its C sequestration effects as the spatial arrangement largely determines SOC bio-accessibility to microorganisms (Brookes et al. 2017; Schmidt et al. 2011; von Lützow et al. 2007). The dynamics of soil aggregates has gained increasing attention because of their role in C sequestration thus maintaining SOC levels. It is estimated that 90% of surface SOC is associated with aggregates, and 20–40% of this large subset is intra-microaggregate SOC (Carter 2002; Six et al. 2002). The formation of microaggregates through biotic glues and abiotic clay flocculation makes them more stable, with longer turnover times than macroaggregates formed by more transient biogenic processes (Rillig and Mummey 2006; Six et al. 2002).
Nitrogen (N) is one of the most limiting nutrients for plants, and its availability can affect the quantity and quality of rhizodeposition (Atere et al. 2017; Ge et al. 2015). A recent review found conflicting results and different methodological approaches among existing studies concerning N effects on the fate of rhizodeposition (Bowsher et al. 2017). For example, Ge et al. (2015) found enhanced assimilation of rice C into SOC at high rates of N fertilizer in paddy ecosystems, while, with barley, the opposite results were observed. More photosynthesis-derived C in barley systems was incorporated into SOC without N fertilization (Johansson 1992). Although previous research has gained knowledge of N fertilization effects on the above/belowground distribution and fate of plant-assimilated C in soils, whether N promotes deposition of photosynthesis-derived C into different soil organic C pools, e.g. aggregates still remains largely unknown (Ge et al. 2015).
Thus, we performed 13C-CO2 continuous labeling (18 days) of rice plants grown in a paddy system under a range of N fertilization rates during the entire tillering stage during the rice vegetative growth period, to quantify rice rhizodeposited-C inputs into the aboveground plant, belowground root and SOM fractions (by both physically separated aggregation and as chemical extraction approaches), at different N fertilization rates. We hypothesized that (1) N fertilization increased total plant biomass and, as a result, increased belowground C allocation through the release of C from rice roots into the soil, (2) the distribution of rhizodeposited-C in particle-size and humus fractions was shifted by N fertilization.
Materials and methods
Soil
Soil samples were collected from the Ap horizon (0–20 cm) from a rice field (113°19′52″E, 28°33′04″N, 80 m a.s.l.) in the subtropical region of China, with a mean annual temperature of 17.5 °C; rainfall of 1300 mm; sunshine of 1663 h; and frost-free period of 274 days per year (Shen et al. 2014). Soil samples were then sieved (<4 mm) and the coarse plant residues were removed. The soil was classified as an Hydragric Anthrosol (Eutric, Siltic, Gleyic), Ge et al. (2017) developed from a granite parent material after subtropical weathering (Ge et al. 2017). The soil texture was 7.5% clay, 68.4% silt, and 24.1% sand. The soil contained 18.1 g kg−1 organic C, 1.8 g kg−1 total N, and 0.4 g kg−1 total P, and had a pH of 5.6 (1:2.5, soil to water ratio).
Experimental layout
Nitrogen fertilizer as (NH4)2SO4 was applied to the soils with five levels: N-free (N0); 10 mg N kg−1 soil (N10); 20 mg N kg−1 soil (N20); 40 mg N kg−1 soil (N40); and 60 mg N kg−1 soil (N60). The soils were then placed into plastic pots (17.2 cm diameter and 16.7 cm height) with NaH2PO4 (20 mg P kg−1 soil) and KCl (80 mg K kg−1 soil) and deionized water, which was maintained throughout the rice growing season at 3- to 5-cm above the soil surface. Three replicates for each N fertilization regime resulted in 6 pots: 3 pots were used for continuous 13C labeling (see below) and 3 pots were used as controls without labeling. Soils were sprayed with NaH2PO4 and KCl (20 mg P kg−1 soil and 80 mg K kg−1 soil, respectively), and placed into plastic pots with 20 cm diameter and 5 cm height. Sufficient deionized water was added to maintain a 1 to 2 cm depth of water covering the soil surface throughout the rice cultivation period. More information about the experimental design is given by Ge et al. (2017).
13CO2 continuous labeling
Rice plants were subjected to 13CO2 continuous labeling between May 14th and 31st, 2013, which was during the rice vegetative growth period (including the entire tillering stage). During the labeling period, plants were transferred to an automatically controlled gas-tight growth chamber system (area 110 cm × 250 cm, height 180 cm). Growth chambers were placed in a rice field so that environmental conditions would be identical for labeled plants and unlabeled controls. Pot surfaces were covered with black plastic sheets to prevent algal photosynthesis in the flood water so that only the rice shoots were be exposed to 13CO2. The 13CO2 (99 atom% 13C) was supplied during rice growth, and the pots were watered every few days to maintain a water depth of 3–5 cm above the soil surface until harvest. Irrigation water was introduced through a nylon tube (inner diameter, 5 mm) connected to each pot. More information about 13CO2 continuous labeling is given by Ge et al. (2017).
Rice growth conditions and sampling
The temperature and CO2 concentrations in the growth chamber were measured and controlled according to our previous study (Ge et al. 2012, 2017). The rice plants were harvested and soils were sampled after 18 days of continuous 13C labeling. Shoots were cut off at the stem base to separate the components of root, shoot, and soil. Soil adhering to the roots was removed by gentle agitation with 0.01 M CaCl2 (pH 6.2) for 1 min and thorough washing with water, then air-dried for aggregate size separation and SOM fractionation. Then, the shoots and roots were dried in an oven at 70 °C for 72 h, weighed, and pulverized.
Aggregate size fractionation
Aggregate size fractionation was performed by a wet sieving method according to Gale et al. (2000). Briefly, soil was sieved <8 mm and gently crumbled manually to approximately 2-mm pieces. Soil sample (100 g) was transferred to two sieves (< 0.25 and < 0.053 mm) and shaken for 5 min. Subsequently, macro-aggregates (2–0.25 mm) and micro-aggregates (0.25–0.053 mm) were collected from the <0.25 mm and < 0.053 mm sieves, respectively. The remaining material that passed through the <0.053 mm sieve was classified as silt and clay (< 0.053 mm). All size fractions were dried at 70 °C, weighed, and stored for 13C analysis.
Chemical fractionation of soil organic matter
The extraction of SOC pools from air-dried soil was performed using the methodology suggested by the International Humic Substances Society (IHSS), using NaOH-Na4P2O7·10H2O (0.1 M, pH = 13) as the extraction agent. Three fractions were separated from 5 g soil samples based on their solubility in alkaline and acid solutions: (a) alkali- and acid-extractable fulvic acids (FAs); (b) alkali-extractable, acid non-extractable humic acids (HAs); and (3) alkali and acid non-extractable humin (Hu).
Measurement of Ctot and δ13C
The C contents in the soils (including aggregate size fractions), shoots, roots, FA, and HA extracts were analyzed using an automated C/N analyzer (vario MAX; Elementar Analysensysteme GmbH, Germany). The stable C isotope ratios (13C/12C) of shoots, soils, FA, and HA extract samples were measured using an isotope ratio mass spectrometer (MAT253) coupled with an elemental analyzer (FLASH 2000; Thermo Fisher Scientific, USA). The natural 13C abundance is expressed as parts per thousand relative to an international standard Peedee Belemnite (PDB) using delta units (δ‰).
Calculations of rice-derived C (13C) amount in soil
The 13C incorporation into plant and soil samples was calculated according to the following equation:
where (Atomic13C%)s,L and (Atomic13C%)s,UL are the atomic 13C% in labeled and unlabeled samples, respectively, and Cs is the total C content of the samples.
The 13C incorporation into shoots, roots, and soil pools was expressed as a percentage of 13C recovery. The total 13C recovery after sampling was calculated as the sum of 13C in the shoots, roots, and soil (Tian et al. 2012).
Statistics
All data are expressed as the mean of three replicates ± standard error. Differences between means were evaluated using least significant difference analysis at the 5% level and were calculated using a one-way analysis of variance (ANOVA) and SPSS (Version 10.5, SPSS Inc., Chicago, IL, USA). Correlation analyses between 13C-SOC in physical fractionation (>0.25; 0.25–0.053 mm; <0.053 mm) and chemical fractionation pools (FA, HA and Hu) and N fertilization rates were conducted using SPSS 10.5 (SPSS Inc., Chicago, IL), with significance defined at p < 0.05.
Results
13C incorporation into plant shoot, root and soil
Total 13C incorporated into shoots, roots, and soils increased linearly with N fertilization rate (p < 0.01; Fig. 1). The amount of 13C incorporated into shoots (range, 44.0 to 157.6 mg pot−1) was approximately 3.2 to 5.2 fold higher than that in roots and soil. The highest ratio of 13C-aboveground to 13C-belowground was observed in the N10 treatment, and the ratios gradually decreased from 2.4 (N10) to 1.9 (N60).
Net 13C assimilation (mg pot−1) by rice plants and the ratio of 13C distributed into above- to below-ground depending on N fertilization rates during the 18-d continuous 13CO2 labeling period. Error bars represent standard errors of the means (n = 3). (Net 13C assimilation by rice plants is the sum of 13C content in roots, shoots and soils measured immediately after the day 18 of continuous 13CO2 -labeling)
13C incorporation in the aggregate-size classes and SOM fractions
The size classes were fractionated by sieving as macro-aggregates (2.00–0.25 mm), micro-aggregates (0.25–0.053 mm), and silt and clay (<0.053 mm), which represented, on average, 45%, 39%, and 16% of the bulk soil, respectively. N fertilization did not affect aggregate size distribution (data not shown), but it did affect the 13C incorporated into aggregate classes (macro-aggregates, micro-aggregates, and silt and clay (p < 0.01; Fig. 2). The total 13C incorporated into coarse sand was increased by 494% in the N60 treatment compared with that in N0. The 13C incorporated into fine sand, silt and clay was enhanced approximate 2.5 and 3.0 folds, respectively, when the N fertilization rate was increased from 0 to 60 mg N mg−1 soil. The 13C distribution into both FA and Hu increased exponentially with N fertilizer addition (p < 0.05; Fig. 3), which ranged from 3.2 to 8.4 mg kg−1 soil and ranged from 2.2 to 8.1 mg kg−1 soil, respectively (Fig. 3). The 13C content of HA lineally increased with N fertilization rate, however, it was only 6% of that in FA and Hu.
13C content belowground (sum of 13C in soil and root) recovery in organic matter fractions (alkali- and acid-extractable fulvic acids (FA); alkali-extractable, acid non-extractable humic acids (HA), and alkali and acid non-extractable humin (Hu)) depending on N fertilization rates. Error bars represent standard errors of the means (n = 3)
Rice rhizodeposition-derived C in SOM fraction per root
To provide some simple tools for assessment of rice rhizodeposition, we calculated the ratio of rice-derived C (13C) amount in soil. The contribution of rice rhizodeposits to 13C assimilation in the coarse sand fraction significantly increased with increasing N fertilization rate, while the largest ratios of 13C assimilation in the fine sand, silt and clay fractions were observed in the N10 treatment. The rhizodeposits derived C in the SOM fractions were relatively higher than in the soil aggregates and increased with increasing N fertilizer rates. The incorporation of rhizodeposits derived C into the FA fraction increased from N0 to N20 treatment, and then decreased at higher rates. With the increase in N fertilizer application rate the amount of rhizodeposits derived C assimilated into HA was decreased, and was less than 10% of that in FA. Nitrogen fertilization significantly increased the amounts of rhizodeposits incorporated into Hu, and the highest amount of rhizodeposits derived C in Hu was 7.2 mg kg−1 soil at the N40 rate (p < 0.05; Fig. 4).
13C recovery belowground
The N fertilization rate affected the 13C recovery from the different aggregate classes (Fig. 5). The 13C in the macro-aggregates was greater than that in the other size fractions and increased with the level of N fertilization. In contrast, the 13C in micro-aggregates and silt and clay tended to decrease exponentially with N application rate (Fig. 5). The 13C in the three SOM fractions (FA, HA and Hu) decreased with N fertilizer addition (p < 0.05; Fig. 5). The 13C content was greater (p < 0.05) in the FA fractions than from the Hu and HA fractions, regardless of the N fertilization rate. The 13C recovery from the FA fraction of the N60 treatment was 7% lower than with other N treatments, which were not different from each other (Fig. 5).
Discussion
Effect of N fertilization on allocation of assimilates in aggregates
Although changes in plant rhizodeposition induced by N fertilizer have been widely reported, its distribution into different soil aggregate fractions remains poorly understood. Early studies reported that the stabilization of rhizodeposited-C in aggregate size fractions in the rice-soil system mainly depends on water management, with more 14C distribution in macroaggregates in the non-flooded treatment, while there were no significant differences under flooded and alternate water regime after 45-day periods of 14CO2 labeling (Tian et al. 2012). This study revealed that N fertilizer increased the incorporation of assimilates (13C) into macroaggregates but not into microaggregates, silt, or clay fractions (Fig. 3). Because most of the roots are located between and within large macro-aggregates and the root biomass was strongly increased by N fertilization (Fig. 1), the formation of macro-aggregates from micro-aggregates, silt, and clay particles was stimulated, leading to the observed decrease in the incorporation of new C into the latter fractions. Also, it is likely that the roots-associated symbionts promote soil aggregation. The entangling of fine roots and fungal hyphae might be involved in the stabilization of the macro-aggregates (Jastrow et al. 2007; Rillig and Mummey 2006).
Paddy soils are regularly flooded and intermittently irrigated, which lead to unique properties and have two consequences, including: 1) water between macro-aggregates (in large pores) has much greater O2 exchange with the atmosphere than water in small pores and micro-aggregates. The relatively high O2 availability leads to preferential root allocation between and within macro-aggregates, and 2) regular flooding changes the oxidizing agents in paddy fields from O2 to low-molecular-weight organic acids (e.g., acetate), which caused the formation of complexes and stabilize the root-derived organic matter(Six et al. 2002; Yuan et al. 2016).
Allocation of rice rhizodepositions within SOM chemical fractions
Although few studies have evaluated the distribution of photosynthesized C within the soil density fractions (Comeau et al. 2013; Qiao et al. 2017) or particle size factions using isotopic techniques with either 14C (Tian et al. 2012) or 13C (Atere et al. 2017; Mwafulirwa et al. 2016), little information is available on the translocation of plant-assimilated C into different chemical fractions, especially with different N fertilizer rates. Previous incubation experiments showed that the greatest amount of 13C was derived from rice rhizodeposition within the Hu soil extract fractions (Lu et al. 2002a). In the present study, however, more 13C assimilates were incorporated into the FA fractions than the Hu and HA fractions, regardless of the N fertilizer rate (Fig. 3). Lu et al. (2002a) used 13CO2 pulse-labeling and this might explain the differences in the 13C distribution among soil extract fractions between their study and the present study which used 13CO2 continuous-labeling. It should be noted that the Hu fraction contains not only humic substances but also particulate organic matter, such as root debris. Therefore, if plant residues are not completely removed from the soil before fractionation, the remained 13C-labeled plant in soil might lead to an over-estimation of Hu. The FA and HA, in contrast, are not affected by plant residues remaining in the soil at the time of extraction.
Direct N effects on root biomass released rhizodeposition content
Nitrogen fertilization affects the partitioning and allocation of plant photosynthates (Atere et al. 2017). A recent review also examined N effects on rhizodeposition in soils, and found that N additions increased nearly all rhizodeposited-C pools per plant (Bowsher et al. 2017; Xu et al. 2018). The observed greater rhizodeposition occurs in rice plants grown under high rates of N addition might be due to the enhanced plant root biomass and exudates (Tian et al. 2012). Our previous studies also confirmed that rice shoot and root biomass increased with N fertilizer rate (Ge et al. 2015). N additions increased nearly all rhizodeposition-C pools when expressed on a per plant basis, while no rhizodeposition-C pools were significantly altered when expressed per unit root mass (Bowsher et al. 2017). This is consistent with our results that the N fertilizer effects on rhizodeposition were not apparent (apart from the course fraction, Fig. 4) when expressed as per unit root mass, indicating the larger root biomass due to higher N inputs is the main reason explaining the observed higher rhizodepositd C. The N increased root biomass can be the direct and first driving force for C sequestration in traditionally flooded paddy soil, via the pathway of physical transfer (Cotrufo et al. 2015).
Indirect microbial mechanisms
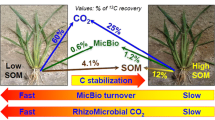
Apart from the enhanced root biomass, N addition might affect soil microbial processes by changing soil C:N ratios. It was reported that the amended N exerted certain effects of nutrients availability to soil microbial biomass and enzymes, thus further modifying organic C turnover in soil (Li et al. 2017; Zhu et al. 2018). Based on the 14C labeling within 16 days, it was estimated that 62% of total rhizodeposition was mineralized, 7% was incorporated into microbial biomass C (MBC) and 31% was recovered in the soil (Pausch et al. 2013). Much smaller photosysthesized 13C inputs (0.15–0.94%) were incorporated into MBC in the early pulse labeling periods and 0.18–0.75% still remained in the end (Lu et al. 2002a), suggesting 1) the important role of the biomass pool in regulating rhizodeposition turnover in soil, and 2) that agriculture practices and other managements influence the microbial biomass pool size (Li et al. 2013; Zhang et al. 2016; Wei et al. 2017), which might further affect the fate of rhizodeposited-C. The N fertilization caused allocation of photosynthates belowground which might be regulated by microbial pool size, which determines the distribution of rhizodeposits within soil organic C pools. How N addition adjusts the microbial utilization of rhizodeposited C, determines its distribution in soil organic C fractions spatially and temporally is the key question towards understanding its C sequestration potential (Fig. 6).
Nitrogen enhanced rhizodeposition alters C availability to microbes which, in turn, switch substrate type from more recalcitrant SOM to root released C, which lead to less allocation within soil aggregates or respiratory processes associated with N acquisition (Jones et al. 2013; Philippot et al. 2013). When labile rhizodeposits are preferentially utilized by microbes, rhizodeposition also retards the decomposition of more recalcitrant native SOM (Johnson et al. 2006; Zhu et al. 2016). However, due to the reduction in rhizodeposition in the later stage of plant growth, irrespective of N addition, the accumuled rhizodeposited-C and N within the microbial biomass pool might be released and finally incorporated into SOM (Jones et al. 2013). To maintain the large biomass size, a switch in microbial substrate utilization (from relative available C to more recalcitrant SOM) and microbial community (r-strategist to K-strategist) might occur after rhizodeposited-C decreased at late stages (Jones et al. 2013; Luo et al. 2011; Zhu et al. 2017). Our previous study found that 60 mg N kg−1 soil resulted in a lower 14C recovery in MBC, than addition of 0, 10, 20, or 40 mg N kg−1 soil (Ge et al. 2015), suggesting the dominant microbial group might use SOM, instead of rhizodeposited-C. High N addition was also reported to increase the 13C incorporation into fungal PLFAs (Ge et al. 2017), which, being regarded as oligotrophic, was able to adapt to the low substrate C inputs and utilize more recalcitrant C (SOC) as a substrate (Fontaine et al. 2011; Luo et al. 2017a), thus mediate relative long-term sequestration of carbon in soil (Luo et al. 2017b).
We believe that, compared with low N treatments, plant-microbe interactions in the soil with high N additions are more complex, involving microbial community changes (bacteria to fungi) and ecology strategist shift (r-strategist to K-strategist), which further mediate decomposition of organic C (rhizoseposits and SOC) and stabilization of SOM. Also, the decomposition of rhizodeposition and its induced SOM turnover not only depends on the amount but also its form in the rhizodeposits (Cheng et al. 2014). However, we do not know the N-mediated quality of root released C and the microbial process within spatial (aggregates fractions) and temporal scales (growth stages) (Philippot et al. 2013). This needs further research by adopting DNA-SIP to examine functional microbial groups within soil aggregates during different plant growth stages (Table 1).
Conclusions
Nitrogen fertilization affected rice (Oryza sativa L.) growth and its rhizodeposition distribution into soil. The ratio of 13C in shoot, root and SOM was enhanced with higher N fertilization rate. Also, N addition with higher rates resulted in higher 13C incorporation into soil fractions (physically separated aggregations and chemical extraction forms), and the 13C incorporation into macroaggregates was positively correlated with N application rate. Relative to the lower N, the higher application rate also increased 13C incorporation into the humic acid fraction. This might be due to the relatively high root biomass, or microbial immobilization that lead to an accumulation of rhizodeposition in the microbial biomass pool, which was released at a later stage to contribute to the stabilization within SOM pools. We, therefore, conclude that sufficient N fertilization enhances rice rhizodeposition and leads to SOM stabilization in the paddy soil.
Abbreviations
- SOC :
-
Soil organic carbon
- TN :
-
Soil total nitrogen
- DOC :
-
Dissolved organic carbon
- MBC :
-
Microbial biomass carbon
References
Atere CT, Ge T, Zhu Z, Tong C, Jones DL, Shibistova O, Guggenberger G, Wu J (2017) Rice rhizodeposition and carbon stabilisation in paddy soil are regulated via drying-rewetting cycles and nitrogen fertilisation. Biol Fertil Soils 53:407–417. https://doi.org/10.1007/s00374-017-1190-4
Bowsher AW, Evans S, Tiemann LK, Friesen ML (2017) Effects of soil nitrogen availability on rhizodeposition in plants: a review. Plant Soil 423:1–27. https://doi.org/10.1007/s11104-017-3497-1
Brookes PC, Chen Y, Chen L, Qiu G, Luo Y, Xu J (2017) Is the rate of mineralization of soil organic carbon under microbiological control? Soil Biol Biochem 112:127–139. https://doi.org/10.1016/j.soilbio.2017.05.003
Carter MR (2002) Soil quality for sustainable land management. Agron J 94:38–47
Cheng W, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44. https://doi.org/10.1111/nph.12440
Comeau LP, Lemke RL, Knight JD, Bedard-Haughn A (2013) Carbon input from 13C-labeled crops in four soil organic matter fractions. Biol Fertil Soils 49:1179–1188. https://doi.org/10.1007/s00374-013-0816-4
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix Michelle L, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. https://doi.org/10.1038/ngeo2520
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Gale WJ, Cambardella CA, Bailey TB (2000) Root-derived carbon and the formation and stabilization of aggregates. Soil Sci Soc Am J 64:201–207. https://doi.org/10.2136/sssaj2000.641201x
Ge T, Yuan H, Zhu H, Wu X, Nie S, Liu C, Tong C, Wu J, Brookes P (2012) Biological carbon assimilation and dynamics in a flooded rice – soil system. Soil Biol Biochem 48:39–46. https://doi.org/10.1016/J.SOILBIO.2012.01.009
Ge T, Liu C, Yuan H, Zhao Z, Wu X, Zhu Z, Brookes P, Wu J (2015) Tracking the photosynthesized carbon input into soil organic carbon pools in a rice soil fertilized with nitrogen. Plant Soil 392:17–25
Ge T, Li B, Zhu Z, Hu Y, Yuan H, Dorodnikov M, Jones DL, Wu J, Kuzyakov Y (2017) Rice rhizodeposition and its utilization by microbial groups depends on N fertilization. Biol Fertil Soils 53:37–48. https://doi.org/10.1007/s00374-016-1155-z
Jastrow JD, Amonette JE, Bailey VL (2007) Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Chang 80:5–23. https://doi.org/10.1007/s10584-006-9178-3
Johansson G (1992) Below-ground carbon distribution in barley (Hordeum vulgare L.) with and without nitrogen fertilization. Plant Soil 144:93–99
Johnson JMF, Allmaras RR, Reicosky DC (2006) Estimating source carbon from crop residues, roots and Rhizodeposits using the National Grain-Yield Database. Agron J 98:622–636
Jones DL, Clode PL, Kilburn MR, Stockdale EA, Murphy DV (2013) Competition between plant and bacterial cells at the microscale regulates the dynamics of nitrogen acquisition in wheat (Triticum aestivum). New Phytol 200:796–807. https://doi.org/10.1111/nph.12405
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Kuzyakov Y, Leinweber P, Sapronov D, Eckhardt K-U (2003) Qualitative assessment of rhizodeposits in non-sterile soil by analytical pyrolysis. J Plant Nutr Soil Sci 166:719–723. https://doi.org/10.1002/jpln.200320363
Kuzyakov Y, Schneckenberger K (2004) Review of estimation of plant rhizodeposition and their contribution to soil organic matter formation. Arch Agron Soil Sci 50:115–132. https://doi.org/10.1080/03650340310001627658
Li Y, Li Y, Chang SX, Liang X, Qin H, Chen J, Xu Q (2017) Linking soil fungal community structure and function to soil organic carbon chemical composition in intensively managed subtropical bamboo forests. Soil Biol Biochem 107:19–31. https://doi.org/10.1016/j.soilbio.2016.12.024
Li Y, Zhang J, Chang SX, Jiang P, Zhou G, Fu S, Yan E, Wu J, Lin L (2013) Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For Ecol Manag 303:121–130. https://doi.org/10.1016/j.foreco.2013.04.021
Lu Y, Watanabe A, Kimura M (2002a) Contribution of plant-derived carbon to soil microbial biomass dynamics in a paddy rice microcosm. Biol Fertil Soils 36:136–142
Lu Y, Watanabe A, Kimura M (2002b) Input and distribution of photosynthesized carbon in a flooded rice soil. Glob Biogeochem Cycles 16:31–38. https://doi.org/10.1029/2002gb001864
Luo Y, Dungait JAJ, Zhao X, Brookes PC, Durenkamp M, Li G, Lin Q (2017a) Pyrolysis temperature during biochar production alters its subsequent utilization by microorganisms in an acid arable soil. Land Degrad Dev 4:1–7. https://doi.org/10.1002/ldr.2846
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314. https://doi.org/10.1016/j.soilbio.2011.07.020
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017b) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35. https://doi.org/10.1016/j.soilbio.2016.12.006
Mwafulirwa L, Baggs EM, Russell J, George T, Morley N, Sim A, de la Fuente Cantó C, Paterson E (2016) Barley genotype influences stabilization of rhizodeposition-derived C and soil organic matter mineralization. Soil Biol Biochem 95:60–69. https://doi.org/10.1016/j.soilbio.2015.12.011
Pausch J, Tian J, Riederer M, Kuzyakov Y (2013) Estimation of rhizodeposition at field scale: upscaling of a 14 C labeling study. Plant Soil 364:273–285
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nature Review Microbiology 11:789–799. https://doi.org/10.1038/nrmicro3109
Qiao Y, Miao S, Han X, Yue S, Tang C (2017) Improving soil nutrient availability increases carbon rhizodeposition under maize and soybean in Mollisols. Sci Total Environ 603-604:416–424
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53. https://doi.org/10.1111/j.1469-8137.2006.01750.x
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DA, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Shen JL, Tang H, Liu JY, Wang C, Li Y, Ge TD, Jones DL, Wu JS (2014) Contrasting effects of straw and straw-derived biochar amendments on greenhouse gas emissions within double rice cropping systems. Agiculture Ecosystems & Environment 188:264–274. https://doi.org/10.1016/j.agee.2014.03.002
Six J, Conant R, Paul E, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176
Tian J, Pausch J, Fan M, Li X, Tang Q, Kuzyakov Y (2012) Allocation and dynamics of assimilated carbon in rice-soil system depending on water management. Plant Soil 363:273–285. https://doi.org/10.1007/s11104-012-1327-z
von Lützow M, Kögel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207. https://doi.org/10.1016/j.soilbio.2007.03.007
Weintraub MN, Scott-Denton LE, Schmidt SK, Monson RK (2007) The effects of tree Rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine Forest ecosystem. Oecologia 154:327–338. https://doi.org/10.1007/s00442-007-0804-1
Wei X, Hu Y, Peng P, Zhu Z, Cornelius TA, O’Donnell AG, Wu J, Ge T (2017) Effect of P stoichiometry on the abundance of nitrogen-cycle genes in phosphorus-limited paddy soil. Biology and Fertility of Soils 53:767–776. https://doi.org/10.1007/s0037
Xu Q, Wang X, Tang C (2018) The effects of elevated CO2 and nitrogen availability on rhizosphere priming of soil organic matter under wheat and white lupin. Plant Soil 425:375–387. https://doi.org/10.1007/s11104-018-3601-1
Yuan H, Zhu Z, Liu S, Ge T, Jing H, Li B, Liu Q, Lynn T, Wu J, Kuzyakov Y (2016) Microbial utilization of rice root exudates: 13C labeling and PLFA composition. Biol Fertil Soils 52(5):615–627
Zhang K, Chen L, Li Y, Brookes PC, Xu J, Luo Y (2016) The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol Fertil Soils 53:77–87. https://doi.org/10.1007/s00374-016-1154-0
Zhu Z, Ge T, Liu S, Hu Y, Ye R, Xiao M, Tong C, Kuzyakov Y, Wu J (2018) Rice rhizodeposits affect organic matter priming in paddy soil: the role of N fertilization and plant growth for enzyme activities, CO2 and CH4 emissions. Soil Biol Biochem 116:369–377. https://doi.org/10.1016/j.soilbio.2017.11.001
Zhu Z, Ge T, Hu Y, Zhou P, Wang T, Shibistova O, Guggenberger G, Su Y, Wu J (2017) Fate of rice shoot and root residues, rhizodeposits, and microbial assimilated carbon in paddy soil - part 2: turnover and microbial utilization. Plant Soil 416:243–257
Zhu Z, Zeng G, Ge T, Hu Y, Tong C, Shibistova O, He X, Wang J, Guggenberger G, Wu J (2016) Fate of rice shoot and root residues, rhizodeposits, and microbe-assimilated carbon in paddy soil - part 1: decomposition and priming effect. Biogeosciences 13:4481–4489
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (41522107; 31470629; 41671233), the Youth Innovation Team Project of Institute of Subtropical Agriculture, Chinese Academy of Sciences [grant number, 2017QNCXTD_GTD] and Royal Society Newton Advanced Fellowship (NA150182). We especially thank the Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Xinhua He.
Rights and permissions
About this article
Cite this article
Luo, Y., Zhu, Z., Liu, S. et al. Nitrogen fertilization increases rice rhizodeposition and its stabilization in soil aggregates and the humus fraction`. Plant Soil 445, 125–135 (2019). https://doi.org/10.1007/s11104-018-3833-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3833-0