Abstract
Microwave plasma technology is revolutionizing reaction engineering fields such as methane dry reforming, chemical synthesis, biomass conversion, and waste treatment. Microwave generated plasma offers sustainable, cleaner and efficient operations compared to conventional methods. Microwave plasma reactions are more efficient when integrated with catalysts. In this article, a thorough categorization and comparison of microwave plasma-assisted catalytic reactions are presented, while highlighting their contribution to an energy efficient and sustainable future in chemical processing. An introduction on commercial applications of microwave plasma technology is also presented to emphasize its advantages in modern industries. Microwave irradiation can be used as a source of heat or plasma. The addition of heterogeneous catalyst to either microwave heated or microwave enhanced plasma systems can lead to complex pathways in reaction systems. A final section in this article is dedicated to comprehend this complexity in chemical reactions occurring in microwave heated and microwave plasma-enhanced catalytic systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma, a distinct state of matter, is made of electron, ions, and neutral gas molecules. Since plasmas have a low degree of ionization, the density of charged particles is much lower than that of neutral species. At low pressure, the lighter electrons and some ions quickly accelerate to higher velocities than the heavier neutral molecules. Hence, the kinetic energy of electrons is much higher than the bulk neutrals causing non-equilibrium plasma. Since non-equilibrium plasma does not raise the temperature of the bulk gas, it is termed as cold or non-thermal plasma. On the contrary, when the pressure is high enough to excite the charged particles and the neutrals almost equally, the plasma is termed as equilibrium. At increased pressure conditions, the electron, ions as well as neutral molecules are highly agitated causing an increase in gas temperature and hence, equilibrium plasma is termed as hot or thermal plasma [1]. Since thermal plasma can produce high heat, it is prevalent in the incineration of waste and toxic materials [2]. The nonthermal or cold plasma technology is extensively used in industrial applications such as coating, surface modifications, and etching [3].
Sources of plasma vary depending upon the type of electrode and excitation or energy source. Plasma generators are qualified and quantified based on breakdown voltage, electron energy, and electron density. Non-thermal plasmas are created either at low or at atmospheric pressure. Low-pressure plasma discharges require cost-intensive process chambers to maintain a vacuum. In contrast, non-thermal plasmas generated at atmospheric pressure do not require such arrangements and hence are more advantageous [4]. Nonthermal atmospheric plasma generators are classified based on the source as corona discharge, atmospheric pressure plasma jet, dielectric barrier discharge, atmospheric glow micro hollow cathode discharge, and microwave discharges [5].
It is interesting to note that microwaves without plasma generation can be used as an energy source in a catalytic reaction system and is a very well researched area. Microwave irradiations are generated using electromagnetic waves at frequencies higher than 300 MHz. Microwave plasma or discharge, the subject of this review, is formed when a gas is ionized under this high energy source. It can be generated under a wide range of power and system’s pressure using a variety of gases [6, 7]. Microwave plasma offers several advantages over other non-thermal plasma sources as shown in Fig. 1. The microwave plasma density is reported to reach higher than 1013 activated species per cm3. The gas temperature is known to reach more than 1000 K. These advantages gives an edge to microwave generated plasma in many industrial applications such as etching, layering, and disinfection [8]. Since high pressure conditions can intensify electron and ion collision pushing the plasma state to equilibrium state leading to high gas temperature, microwave plasma can be put into thermal plasma category if generated at high pressure [9].
The role of catalysts in chemical reaction technology is well known since the early 1800 s [10]. Catalytic processes or reactions carried out in microwave-assisted plasma systems is termed as microwave plasma-assisted catalytic technology or reactions. This technology has brought a paradigm shift in catalytic reactions by offering reaction mechanisms with lower energy pathways and improved yield [11]. Free electrons in the plasma collide with gas molecules imparting excitation energies higher than the bond dissociation energy. This helps in breaking of strong covalent bonds and formation of new chemical species at mild reaction conditions [1, 12]. It is now extensively investigated in areas such as removal of volatile organic compounds [13, 14], waste gas treatment [15], and reforming technologies [16]. Microwave plasma reactors help in easy integration of catalysts because of their electrode free designs, which is a major advantage over other nonthermal or thermal plasma generators [17].
There are three main terminologies used throughout this review article. First, microwave heated catalytic reactions- the reactions where microwave acts as a source of thermal energy in the presence of a catalyst. Second, microwave plasma reactions- the reactions that occur under gas plasmas ignited by microwave. Third, microwave plasma-assisted or enhanced catalytic reaction- the reactions occurring in the gas plasma generated by microwave in the presence of catalysts. The article begins with an overview of commercial applications of microwave plasma to emphasize its current applications. The following section exclusively discusses microwave plasma-enhanced catalytic reactions. This section is broadly classified into methane conversion to C2 and hydrogen, methane dehydroaromatization, carbon dioxide utilization, and ammonia synthesis highlighting the advancements in the past decade. The final section is categorized into three distinct systems: Microwave heated catalysis, microwave plasma, and microwave plasma-enhanced catalysis. The interaction of plasma species and microwaves on catalyst surface can be best understood by this categorization.
Commercial Applications of Microwave Plasma Technology
Plasma technology has spanned and found its place in many industrial applications in the last few decades. Microwave plasma specifically finds application in food and packaging industries, microelectromechanical systems (MEMS), and chemical processing. Figure 2 shows the present and future applications of microwave plasma technology in a nutshell. The following section highlights some established technologies utilizing microwave plasma.
Microwave Plasma in Food Industry
Microwave plasma technology is widely used in the food industries for packaging, in barrier coating for food preservation, and disinfections. It is used in surface treatment to remove undesired organic impurities and in substrate deposition [18, 19]. Microwave plasma UV lamps (MPUVL) is a device used in disinfecting food before their packaging and sealing in a thermos-fill-seal (TFFS) system. The UV rays produced kill the contaminating bacteria on packaging material before the food items are packaged in a vacuum sealing chamber. The integration of disinfection and sealing in a single device is useful for large scale food disinfection and packaging [20, 21]. MPUVL offers several advantages over conventional UV lamps [22, 23]:
- (1)
Electrodes in the conventional UV lamps have to be replaced periodically but MPUVL does not require electrodes. This increases the lifetime of a UV lamp.
- (2)
The UV radiation can be pulsed at different modulation frequencies and intensities.
- (3)
MPUVL is 95% efficient in converting all microwave energy into UV radiations.
- (4)
The disinfection time is only 12 s while electrode UV lamps need 20 s to 3 min to warm up.
- (5)
MPUVL has very low residual radiation energy and thus has almost instant shut off capability. This prevents overheating of heat-sensitive materials near the lamps.
Microwave Plasma in Microelectromechanical Systems (MEMS)
MEMS are used to manufacture microscopic devices from materials such as silicon, polymers, metals, and ceramics. Chemical vapor deposition (CVD) and plasma etching are two of the main manufacturing processes of these devices that require microwave plasma. Microwave plasma-assisted CVD (MPCVD) is commercially used to produce high quality diamonds [24, 25]. In a typical microwave-plasma CVD system, microwaves triggers breakage of C–H bonds in methane (CH4) forming hydrogen gas plasma. The free carbon radicals thus deposited create crystal diamonds and ultrafine diamond films [26]. Another significant application of plasma-enhanced CVD is layering of multi-crystalline silicon solar cells with silicon nitride (Si3N4). Plasma generates uniform layers of Si3N4- which provides reflective surfaces to solar cell wafers [27]. Similarly, plasma-etching systems are highly commercialized and readily available in various forms and capabilities. In such systems, gas plasmas, usually made of oxygen react with the unwanted material on the substrate’s surface. The volatile products hence formed are purged out from the system leaving behind a clean and scratch-free surface [28, 29].
Microwave Plasma in Chemical Processing
Reaction technologies assisted by various plasma sources are now regarded as a sustainable alternative to many conventional chemical processes. Microwave plasma alone is well studied in many chemical applications such as reforming, waste gas treatments, and pyrolysis [30]. An interesting example of industrial application is biomass gasification by Plasma2Energy. It is a medium scale gasification plant producing 1830 m3 ethanol and 253 m3 of diesel fuel annually utilizing only 20% of generated energy [31]. RMX technology manufactures carbon fiber in a low-pressure microwave plasma reactor. This small reactor (0.05 m diameter and 4 m in length) having only one-third of residence time of the conventional reactor reduced energy requirement by 75% and manufacturing cost by 25% [32].
Microwave plasma has established its advantages in some commercial processes but chiefly remains unexplored in many important chemical processes such as reforming and ammonia synthesis. It is still at a fundamental stage of research and development. This review article summarizes chemical processes driven by microwave plasma in three main categories: methane reforming & dehydroaromatization, carbon-dioxide utilization, and chemical synthesis of ammonia & organic compounds. Commercial scale-up of these processes face many challenges such as reliability, reproducibility, and energy efficiency. This paper evaluates these challenges in every category by extensively reviewing published reports in this area.
Microwave Plasma-Enhanced Catalytic Reactions in Methane (CH4) Utilization
The high methane content in natural gas makes it a viable fuel and energy source. It is now widely used in power plants to generate electricity, hydrogen production by commercial processes such as steam methane reforming (SMR), industrial processes such as ammonia production, and synthesis of aromatic compounds [33,34,35,36]. All traditional methods that utilize and reform methane release carbon dioxide, toxic gases, and undesired side products. Since the early 1900 s, using catalysts to convert methane to useful products turned out to be advantageous in terms of product yield and overall process efficiency. However, these conventional methods require extreme process conditions making them consume more energy [37].
Microwave plasma-enhanced catalytic conversion of methane to value added products aims to eliminate these drawbacks associated with conventional processes. The subsequent sections analyze most recent publications that addressee such issues.
Microwave Plasma-Enhanced Catalytic Conversion of Methane to C2 Hydrocarbons and Hydrogen
The earliest report on microwave plasma-enhanced catalytic conversion of methane to ethylene and ethane establish a 52% methane conversion (maximum) with a selectivity of 25% ethylene, 25% acetylene and 50% ethane. Power required for maximum conversion was 60 W while the theoretical power needed for 100% conversion was calculated as 3.294 W, so the calculated energy efficiency (3.294/60) was only 5.5%. Coking occurs in all the reaction scenarios either on the wall or catalyst surface, which was minimized using lower power and higher flow rates. As for the postulated mechanism behind product formation, interaction of microwave plasma over the Ni surface leads to H-atoms abstraction from CH4 forming theorized CH, CH2, and CH3 radicals. The free CHx radicals lead to the synthesis of ethane, ethylene, and acetylene which are valuable hydrocarbons. Hydrogen atoms abstracted recombine to form molecular hydrogen. The selective conversion of methane to ethylene and acetylene were influenced by reactor pressure, the power, and the feed flow rate [38]. Figure 3 represents the theorized reaction pathway of CH4 conversion to desired products over Ni- catalyst under microwave plasma.
The conversion of methane is theorized to form hydrocarbon radicals which then recombine into ethane, ethylene, and acetylene [38]
Cho et al. investigated the oxidative coupling of methane (OCM) over a transitional metal-promoted zeolite (ZSM-5) catalyst and achieved a 54.9% methane conversion to ethane, ethylene, and acetylene under vacuum. Most importantly, they observed that CH4 conversion increased by 20% in the presence of O2 plasma and the selectivities of acetylene and ethylene improved considerably over ethane when compared to the non-oxidative coupling of CH4 under similar conditions. The increase in conversion can be ascribed to the formation of metastable oxygen under the influence of microwave plasma. The first electronic state of atomic oxygen, O (1D) and second electronic state of molecular oxygen, O2 (1∑) is postulated to play an integral role in the activation of methane which enhanced its conversion to C2 products. In the presence of a catalyst, the CH4 conversion is increased by 10% and acetylene selectivity is improved indicating that the catalyst promotes unsaturation in this reaction [39]. Figure 4 represents the postulated mechanism behind OCM over metal promoted ZSM-5. The excited state oxygen species formed under the influence of plasma enhance the activation of methane. These activated methane radicals recombine over the catalyst surface to form C=C and C≡C products.
Postulated reaction mechanism in oxidative coupling of methane in plasma assisted catalysis reaction. Radical formation and recombination over ZSM-5 catalyst surface. Formation of 1st electronic state of atomic oxygen \({\text{O}}\left( {1_{D} } \right) \&\) 2nd electronic state of molecular oxygen O2\(\left( {1_{\varSigma } } \right)\) due to microwave plasma [39]
Introducing oxygen in methane dehydrogenation does reduce carbon deposition on the catalyst surface, but the yield of C2 products decreases. Nagazoe et al. introduced a new microwave plasma catalysis reaction scheme where the catalyst was placed next to the plasma reaction zone. In this low-pressure system, acetylene and hydrogen were the major products from the plasma zone. The presence of solid catalyst (Pt/Al2O3) facilitated the hydrogenation of the plasma zone products to yield ethylene. The percentage of ethylene in product stream decreased as the distance (ξ) between the catalyst position and plasma region increased producing a maximum yield of 78% at ξ = 0. The catalyst bed temperature at this point was reported 785 K [40]. High yield of ethylene can be obtained when the catalyst is placed in the plasma region manifesting the combined effects of plasma and catalysis.
It is evident that coking in microwave enhanced plasma systems immediately deactivates the catalyst surface. But the alternative solutions such as introducing oxygen into the system, delays coking and improves product selectivity.
Steam reforming of methane contributes to almost half of the total hydrogen production worldwide [41]. However, this multistep reaction requires high temperature and pressure to enhance hydrogen yield producing carbon dioxide, a major greenhouse gas [42]. Microwave plasma-enhanced catalytic steam reformation of methane can be a better alternative.
Wang et al. performed a single-stage steam reformation of methane in a microwave plasma reactor under atmospheric pressure. The authors observed a high selectivity (95.2%) for hydrogen and maximum methane conversion at 91.6%. Ni/Al2O3 catalyst placed at the discharge zone was rapidly deactivated due to nano-carbon deposition on its surface. At higher H2O/CH4 molar ratios, the selectivity of carbon and byproducts such as C2H2 decreased. The specific energy consumption (SEC) is defined as the energy consumed in electron volt (eV) per molecule of H2 formed. A maximum SEC of 13.8 eV/molecule-H2 was observed for H2O/CH4 ratio 1.0 [43]. Utilizing optical emission spectrometer, an in situ analysis tool helped identify activated species in the discharge region. Although the role of catalyst in this reaction could not be ascertained due to rapid coking, the low energy efficiency could be due to the high electrical power consumption in generating plasma.
Microwave Plasma-Enhanced Catalytic Dehydroaromatization of Methane
Methane dehydroaromatization (DHA) is beneficial in effective utilization of natural gas by producing important aromatics such as benzene and toluene [45]. Direct conversion of methane to aromatics in the absence of oxygen is thermodynamically unfavorable at temperatures lower than 500 °C and the equilibrium CH4 conversion is only about 12% at 700 °C [46]. Microwave enhanced plasma can be beneficial in improving selectivity and conversion in methane DHA. Heintze and Magureanu reported a 5.25% yield of benzene (C6H6) by using a nickel-carbon fiber catalyst, which was higher than a 0.9% yield by traditional molybdenum zeolite catalyst loaded in a fixed bed reactor. The selectivity of acetylene (C2H2) was highest (> 40%) for the maximum methane conversion (70%). As the reaction progressed, coking reduced the selectivity of C2H2 while that of C6H6 increased and reached to a maximum of 30%. The coke possibly catalyzed CHx dehydrogenation and its subsequent aromatization [44]. The role of microwave plasma in overcoming thermodynamic limitations remain unclear due to the missing temperature analysis of the reaction.
In conclusion, methane conversion to C2 products, hydrogen and aromatics in microwave enhanced plasma catalytic reactions are certainly advantageous over conventional methods in terms of feed conversion and product selectivities. However, catalyst coking and high power usage remain major drawbacks of this technology. Table 1 categorize and compares all the methane utilization reactions presented in Sect. 3 based on products formed, conversion rate, energy efficiency, and major contribution.
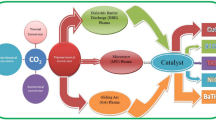
Microwave Plasma-Enhanced Catalysis in Carbon Dioxide (CO2) Utilization
Carbon dioxide emitted from fossil fuel combustion and other industrial processes make a 65% contribution to global greenhouse gas emission [47]. This adverse environmental effect has fueled technology to utilize carbon dioxide gas and minimize its release to the atmosphere. It is captured, separated, sequestrated [48] and converted to useful products such as organic compounds [49] and hydrocarbon fuels [50].
CO2 is a highly stable molecule and requires high temperature and pressure conditions to break down in most processes. Nonthermal plasma technology is known to provide the required dissociation and ionization energy for CO2 splitting at nominal gas temperatures and moderate pressure [51]. Since the decomposition of CO2 into CO & O2 in a non-equilibrium plasma occurs due to vibrational excitation of gas molecules, it is necessary to measure the energy efficiency of these processes. Interestingly, microwave plasma is shown to have the highest energy efficiency at medium pressure (50–200 Torr) conditions [52]. The presence of catalyst in non-thermal plasma reactions improves selectivity and reaction rates while providing the energy needed for endothermic bond dissociation of carbon dioxide [53]. The subsequent paragraph compares the effectiveness of microwave plasma-enhanced catalytic decomposition of CO2 in terms of catalyst activity, energy efficiency, conversion, and product selectivity.
Spencer and Gallimore investigated the efficiency in conversion of carbon dioxide to carbon monoxide (CO) and oxygen (O2) in a microwave plasma-enhanced catalytic reactor. Energy efficiency was defined as the ratio of the theoretical enthalpy of dissociation (2.9 eV/molecule) to the actual energy consumption of producing one CO molecule in the plasma. The actual energy consumption to produce CO was calculated by the ratio of specific energy input to the output mass flow rate of CO. Specific energy input was defined as the ratio of power consumed to CO2 mass flow rate into the reactor system. Based on these definitions, energy efficiency in the plasma catalyst system was always lower than the pure plasma system. While a trade-off between energy efficiency (20%) and conversion (45%) was established in pure plasma reactor, the plasma catalyst system could not offer any advantages to the reaction [54]. Perhaps, Rh/TiO2 catalyst was promoting reverse reactions leading to more unconverted CO2 in reactor outlet. Chen et al., using the same definition of energy efficiency, achieved better results by decomposing carbon dioxide over NiO supported TiO2 catalyst in a pulsed surface-wave microwave plasma. The catalyst, when calcined in the microwave plasma with CO2 and Argon (Ar), showed a significant increase in surface area. Raman spectroscopic analysis indicated the presence of a higher concentration of oxygen vacancies for Ar-plasma treated NiO/TiO2 catalyst and showed no carbon deposition. CO2 conversion (42%) and energy efficiency (17.2%) was highest for the catalyst pretreated in Ar- plasma. The plasma treatment created oxygen deficiency on catalyst surface enhancing the photocatalytic dissociation of CO2 [55]. The improvement in energy efficiency can be accredited to catalyst pretreatment in plasma. Comparison based on conversion, energy efficiency and major highlight of above two research works is shown in Table 2.
CO2 splitting in microwave plasmas has the best energy efficiencies in low and medium pressure conditions [56, 57]. Additionally, microwave plasma-enhanced catalytic decomposition of CO2 has lower energy efficiency than non-catalytic systems [58,59,60]. Since atmospheric pressure conditions are most favorable to catalytic systems, it is necessary to investigate the factors behind low energy efficiency in microwave plasma-enhanced catalytic decomposition of CO2. There may be two main factors at play. First, low and medium pressure conditions employed in non-catalytic reactions increase CO2 dissociation improving the energy efficiency. Second, the presence of a catalyst in the atmospheric pressure conditions may favor the recombination of CO and O2 decreasing CO2 conversion [61].
An efficient carbon-dioxide utilization can improve process economy by making value-added product from CO2 at competitive cost. In recent years, non-thermal plasma technology especially microwave has shown a growing interest in CO2 utilization. While energy efficiency seems to be the main bottleneck of this technology, innovations in catalyst design and the optimal trade-off between power consumption and gas conversion may help overcome the current challenge.
Microwave Plasma-Enhanced Catalytic Reactions in Ammonia (NH3) Synthesis
Ammonia is widely used in manufacturing nitrogen-based fertilizers and value-added chemicals. The Haber–Bosch process is the only commercial large-scale production of NH3 [62]. It has high temperature and pressure requirements leading to severe energy consumption and CO2 emissions. Non-thermal plasma catalysis, as an alternative to the conventional process, offers several advantages. Nitrogen (N2) chemisorption on the catalyst surface is a crucial step in the catalytic synthesis of ammonia. This process is enhanced substantially when N2 is atomized by using non-conventional energy sources such as non-thermal plasma [63]. Additionally, the interaction of highly energetic ions and electrons on the catalyst surface significantly improves reaction kinetics enabling ammonia synthesis at normal temperature and pressure [64]. This section summarizes articles that demonstrate ammonia production in microwave plasma-enhanced catalytic systems, highlighting the major contributions and hurdles in achieving better energy efficiency.
The earliest investigation on the catalytic synthesis of NH3 under microwave plasma reported a detailed study on synergetic effects of driving frequency and catalysts. Microwave plasma had 50% more NH3 yield than radio frequency (RF) plasma under similar conditions suggesting the presence of the higher number of free NH radicals in microwave plasma. Placement of iron (Fe) wires in plasma downstream doubled NH3 yield indicating the significant role of catalyst in adsorption of NH radicals [65]. The most recent work on NH3 synthesis from CH4 to N2 by our group raise important questions on the role of microwave irradiation and microwave plasma in catalytic reactions.
As shown in Fig. 5, the highest (80%) CH4 conversion was observed under microwave plasma on a Co/ɣAl2O3 catalyst. This conversion was similar to the reaction carried under microwave irradiation over Fe promoted Co/ɣAl2O3. The NH3 yield was around 37% more in case of microwave heated catalytic reaction over Fe promoted Co/ɣAl2O3. The results indicate that microwave irradiation without plasma can be as beneficial as microwave enhanced plasma in improving conversion and product yield in a catalytic reaction. Additionally, the presence of Fe led to carbon nanotubes formation on catalyst surface, a value-added by-product [66].
a NH3 production in mole NH3 per gram catalyst per second; b % methane conversion [66]
Although there are a good number of research on the catalytic synthesis of NH3 utilizing many forms of plasma sources, such as micro gap discharges [67], and dielectric barrier discharges [68,69,70], NH3 synthesis using microwave enhanced-plasmas is an area less investigated. Despite the advantages such as improved NH3 yield, microwave plasma-enhanced and microwave heated catalytic synthesis of ammonia have high energy requirements. To develop this process, efforts must be made to understand the synergy between plasma and catalyst. A comprehensive analysis of reaction kinetics through extensive experimentations, modeling and process optimization may improve the energy consumptions.
Reaction Mechanism
Despite all the extensive research on microwave enhanced plasma catalytic reactions, barriers such as high energy consumption and rapid coking prevent its full scale commercialization. The key to transforming a laboratory scale reaction to a large-scale industrial process is to make their establishment economical. Understanding the factors that impact engineering scalability becomes important. To achieve that, we need to have a comprehensive knowledge of the interaction between the catalyst and reactants in the presence of microwave plasma. It is important to note that the studies on catalyst effect in microwave plasma reactors are few due to limitations of in situ instrumentations. However, there are substantial investigations on reaction pathways under microwave enhanced plasma (non-catalytic) and microwave heated catalytic systems. To understand the complex interactions of reactive gases with catalysts under microwave plasma, it is advisable, to begin with comprehending the catalyst surface activation under microwaves without plasma, then the role of microwave plasma alone and finally, understanding the synergy between catalyst and microwave plasma. Hence, the reaction systems are categorized into microwave heated catalytic, microwave plasma, and microwave plasma-enhanced catalytic systems.
Microwave Heated Catalytic Systems
Microwave is electromagnetic radiation in the frequency range of 0.3–300 GHz. The heating of materials under microwave irradiation is a combination of three phenomena: (1) conduction loss, (2) dielectric, (3) magnetic loss. Conduction loss heating usually occurs by thermal activation of electrons and enhanced by the presence of defects in solid materials. Dielectric heating is caused by a change in direction of molecules’ electric dipole by the microwave’s electric field and applies only to polar compounds. Solid materials such as transition metal oxides that possess magnetic properties exhibit magnetic loss heating.
Heterogeneous catalysts exhibit a complex microwave absorption process originating from dielectric losses which lead to surface heating. The loss mechanisms can be described by two processes: dipolar or Debye loss and charge carrier processes. Dipole moment on a catalyst surface or in bulk material may originate due to vacancies and defects in the crystalline structure. The frictional interaction of the rotational motion of the dipole with the oscillation of microwave irradiation cause dipole losses [71]. Since the dipole may be localized, selective heating around the dipole occurs on the solid surface. Charge carrier process occurs either by conduction loss or space charge recombination.
Free electrons within the solid material oscillate with applied electromagnetic field creating an alternating current. The inherent resistance of the material to this current creates conduction loss which eventually heats the material. The electric field of the microwave also creates charge separation within the catalyst due to the movement of electrons and ions. The separated charges have the tendency to recombine against material resistances and other barriers. Charge recombination or relaxation is out of phase with the applied field causing energy loss and subsequent heating of the catalyst. The space charge recombination and dipolar or Debye loss on heterogeneous catalyst surface are explained graphically in Fig. 6 [72].
Space-charge and Debye or dipolar loss mechanisms for microwaves interacting with a catalyst surface for selective heating & bond activation of reactant molecules [72]
In addition to dielectric heating, the catalyst can be designed to interact with the microwave through other mechanisms. For example, metal dopants, like iron, can be added to catalytic sites. These ferromagnetic species can couple with the magnetic component of the microwave field, putting additional energy into the reaction [73].
Our research group has been exploring microwave catalysis in natural gas conversion. In our recent publication, ethane conversion was the highest under ambient pressure and relatively low temperature when compared to conventional heating. Table 3 compares ethane conversion in a microwave (MW) heated reaction to a fixed bead reactor heated conventionally over various catalysts [74].
The most accepted explanation behind the significant improvement in microwave heated catalytic reactions compared to conventionally heated systems is the hot-spot formation due to the microwave thermal effects. In such reactions, the reactants or products would be susceptible to selective bond activation due to localized heating (hot spots) around the dipoles, which in turn can enhance reaction rates. Mochizuki et al. created a carbon-filled zeolite which enhanced catalytic dehydration of alcohols under microwave irradiation in comparison to conventional heating. This enhancement was due to non-equilibrium local heating of the core carbon due to the difference in dielectric losses of carbon and zeolite [75].
However, explanation of rate enhancement due to hot spot formation is disputed. It has been argued, specifically for steam-carbon and Boudouard reactions, that hot spot formation should promote endothermic shift, which was not observed in these reactions [72]. It has been hypothesized that microwave irradiation shifts the equilibrium position by altering the forward and reverse reaction rates. The space charge mechanism, as discussed before, can potentially accelerate surface reactions with gaseous intermediates pushing the overall reaction forward. Additionally, the selective coupling of the microwave field with polar intermediate species on the surface of the catalyst can accelerate forward reactions [72, 76].
Microwave irradiation has been shown to change path-dependent thermodynamic properties suggesting that it significantly changes reaction pathways in heterogeneous catalysis [77, 78]. Figure 7 illustrates the reaction approach specifically for methane and ethane conversion to aromatics, acetylene, and ethylene [74]. The postulated decrease in activation energy can be explained by the changes in internal energy of reactant molecules absorbing a part of microwave irradiation while the rest is used up in heating the catalytic bed [79]. Xu et al. reported that the improvement in microwave catalytic decomposition of NO to N2 and O2 over conventional heating was due to the lowering of its activation energy. The hot spot formation could not explain the improved reaction efficiency. The NO conversion were much lower at the simulated hot spot temperatures than at the reaction temperatures [80]. Essentially, microwave energy can be selectively delivered to the interface between active sites and reaction intermediates without losing energy to the surrounding environment, therefore potentially improving energy efficiency.
Illustration of microwave irradiation in the conversion of natural gas over metal-promoted zeolite [74]
A project funded by DOE ARPA-E is underway where a scalable, cost-effective catalytic process of ammonia synthesis is developed by using microwave excitation under mild reaction conditions. In this research project, an interdisciplinary team consisting West Virginia University, National Energy Technology Laboratory, Florida State University, and two industrial partners have demonstrated that ammonia synthesis can be carried out at 200–300 °C and ambient pressure under microwave irradiation. Other than selective activation of dinitrogen to metastable radicals, the most obvious advantage that microwave irradiation affords in driving a heterogeneously catalyzed reaction is the ability to locally heat the catalytic sites. Many industrial processes utilizing heterogeneous catalysts are high-temperature processes where both components of a reaction (i.e., catalyst and medium) are heated to the temperature required for the reaction to occur. Results shown in Fig. 8 shows that, using Ruthenium (Ru) catalysts, nitrogen conversion of 3.5 mol% can be achieved under microwave irradiation. Instead of continuously supplying microwave energy, one of the energy-saving approaches is to operate microwave catalytic reactor system in pulsing mode. Such a pulsing configuration ensures the catalyst and reaction intermediate absorb microwave energy only when it is needed. The improvement in ammonia yield as the energy source is changed from continuous mode to pulsed mode is represented in Fig. 8. The nitrogen and hydrogen conversion increase simultaneously. This transformational ammonia synthesis process integrates system elements of electromagnetic sensitive catalysts and microwave reactor design. Taking advantages of microwave heating, catalytic ammonia synthesis undergoes a new reaction pathway. The barrier for the initial dissociation of the dinitrogen is decoupled from the bonding energy of the intermediates [81].
Local hot spot formation may or may not play a role in heterogeneous catalytic reaction acceleration, it causes a temperature gradient between the solid catalyst and gaseous reactants [82]. This difference in temperature distribution can lead to unwanted homogenous reactions in the gas phase adversely affecting the selectivity of desired products. For example, the low selectivity of isobutene from oxidative dehydrogenation of isobutane using CO2 in a microwave heated catalytic reaction is due to homogenous gas phase reactions that form methane, ethylene, and butane. This can be avoided by ensuring the least temperature gradient between the solid catalyst and gaseous reactants [83].
It is important to note that microwave irradiation has shown a profound impact on catalyzed and non-catalyzed gas–solid reactions. In particular, it has been demonstrated that microwave-specific effects can manifest themselves through the enhancement of reaction rates, equilibrium shifts, and the distribution of products. Despite the obvious advantages, commercialization of microwave heated catalytic processes would require an extensive energy audit with a positive outcome to replace the established conventional technologies.
Microwave Plasma Systems
Microwave irradiation is a facile and efficient means of generating plasmas and has been used for that purpose in several applications. Methane (CH4) utilization, for instance, remains a thoroughly researched area in microwave plasma systems. Hence, understanding the mechanism behind its decomposition can help identify decisive factors.
One of the earliest reports on CH4 decomposition in cold microwave air plasma, Oumghar et al. methodically investigated air plasma interaction with CH4 molecules. The major products were C2 hydrocarbons and carbon monoxide (CO). Figure 9 shows the active species and the products formed in different regions in the plasma reactor. The regions are defined by the distance, d from the plasma discharge along the reactor axis. Region 1 is at the edge of plasma discharge (d = 0–2 cm). Region 2 is the onset of post-discharge region (d = 2–4 cm), and region 3 is the post-discharge region (d > 4 cm). The end products depend on the location at which methane (CH4) is introduced in the plasma reactor. The percentage yield for acetylene (C2H2), ethylene (C2H4), and ethane (C2H6) are highest in region 1. C2H2 diminishes in region 2 while C2H2 and C2H4 are still produced in small amount. Region 3 shows the presence of carbon-monoxide (CO) and hydrogen (H2) only. The product distribution can be explained by the active species present in each region. Free electrons (e−) predominant in region 1 activate CH4 that leads to the formation of activated methyl CHx (x = 1, 2, 3) species. C2 products are a result of radical recombination of CHx species within region 1 and 2. Atomic oxygen (O (\(1_{D}\))) and activated nitrogen species (N*2), predominant in region 2 and 3, are postulated to form CO and H2 by subsequent dehydrogenation of CHxO species (x = 1, 2, 3) [84]. Microwave plasma processes require careful tuning of input power to control product distribution. For example, higher acetylene selectivity over ethane and ethylene could only be achieved by increasing microwave power to 250 W [85]. It implies that dehydrogenation of CHx radicals is favored over their dimerization as the input power increases.
In microwave plasma systems, it is very important to determine if the chemical reaction is driven by high gas temperature or the active plasma species. In another report, Oumghar et al. compared product distribution under a mixture of pure N2 and CH4 plasma. In CH4/N2 system, the plasma temperature was not more than 900 °C, implying acetylene formation solely due to the interaction of active plasma species. The higher acetylene selectivity over ethane and ethylene is only favored when CH4 is introduced at a distance less than 2 cm from the discharge region, highlighting the important role of reactant feed placement in microwave plasma systems. As postulated, the excited CH4 molecules decompose into CHx radicals, C atoms, and H2 gas in a set of simultaneous reactions. CHx radicals recombine to form C2 hydrocarbons. At the same time, free H and NH radicals combine with CHx to form HCN while N2+ and N2H+ species form NH radical leading to the formation of a small amount of NH3 [86]. Heintze et al. showed that in a pulsed microwave plasma system, a high acetylene selectivity could be achieved by creating a mix of CH4 and H2 plasma. Atomic hydrogen played an important role in plasma chemistry by promoting dehydrogenation over dimerization of CHx radicals. The longer duration of the plasma pulses increased H atom in the system promoting acetylene formation over ethane and ethylene [87]. The longer pulsing mode also increases gas temperature and hence, it was unclear whether the selectivity of acetylene was enhanced by the rising plasma temperature or the presence of activated H-species. In the case of ammonia synthesis from N2/H2 plasma powered by microwaves, it is well known that NHx radicals are precursors NH3 formation. The NHx radicals could be produced through the combination of several activated species present in the plasma such as N2+, H, N, and N2H+. NH3 is formed by recombination of NHx radicals with H-atoms [88, 89].
Plasma is a collection of free radicals and ions formed by activation of gas molecules. The recombination of these activated species leads to various product distribution. Microwave activated plasma, in particular, has more products than any other non-thermal plasma sources owing to the generation of a large number of activated species. The product selectivity in a microwave plasma reaction can be controlled either by external factors such as input power or by introducing another plasma species. More control on product selectivity can be achieved by introducing catalysts in microwave plasma systems. The synergistic effects of plasma and catalysis are discussed in detail in the following section.
Microwave Plasma-Enhanced Catalytic Systems
The effects of microwave plasma on catalytic reactions can be approached in two ways. The catalyst can either be placed within the plasma or at a predefined distance from the plasma generation region. The latter scenario becomes a two-stage reaction system. Either way, non-thermal plasma catalytic systems is known to intensify gaseous reactions by enabling the highly reactive species to interact with catalyst surface for better conversion, selectivity, and energy efficiency.
One of the earliest investigations on placing a catalyst in plasma was reported by Sugiyama et al. In a N2/H2 mix plasma, NH3 could be produced by the surface adsorption of N species which combines with H2 either from gas or on the surface to form NHx species. N2 alone cannot adsorb on a catalyst surface at room temperature unless atomized using a high energy source such as microwave [90]. This pathway is similar to microwave plasma systems without catalysts. The additional steps such as adsorption of activated N species accelerate the ammonia formation. This theory was supported by an independent study where N2 was shown to chemisorb on ruthenium black catalyst in a nitrogen plasma discharge. In fact, the introduction of H2 gas into the reaction lead to hydrogenation of the adsorbed N-species to form NHx, a precursor to ammonia formation [63]. In a similar study the yield of ammonia increased by approximately 75% in the presence of a catalyst in a microwave driven N2/H2 plasma [65]. In a plasma-catalyst system, plasma contributes by generating highly reactive species while the catalyst brings in the complicated surface chemistry. The interaction of microwave enhanced plasma species on the heterogeneous catalyst surface can be explained by a several theories including the classical-Langmuir–Hinshelwood mechanism. In addition to the intricacies of surface adsorption and desorption, the free radicals in plasma not only contributes to the activation of gaseous molecules but can also promote electron transfer on catalysts surface leading to enhanced surface heating and reaction acceleration. The dielectric heating also plays a role depending on the catalyst material [91]. In conclusion, introducing catalyst in microwave plasma synthesis of ammonia increase its yield by the combined effects of plasma and catalyst.
As mentioned in Sect. 6.2, microwave plasma produces a greater number of active species. Placing a catalyst in a reaction with a higher product distribution such as C1 to C2 reactions, can offer advantages in product selectivity. Liu et al. reported the highest selectivity for ethylene in oxidative coupling of methane (OCM) in a cold plasma-catalytic reaction. However, on changing the catalyst, ethane had higher selectivity over ethylene. The catalysts acted as a source of charged species enhancing and stabilizing the plasma state and altered the product distribution. Similar to microwave heated catalytic systems, it is essential to distinguish between the homogenous gas phase and the heterogeneous gas–solid reactions in a microwave plasma-enhanced catalytic system [92]. In OCM, the homogeneous gas phase reaction in the plasma state produces an active species of oxygen (O−) which is known to activate methane to form CHx species [93]. Indeed, the methane conversion and product selectivities were highest in O2/CH4 plasmas than CH4 alone [39].
It is apparent that, in the presence of a catalyst, microwave plasma can significantly change reaction pathways and hence the product distribution. The role of catalyst in improving energy efficiency in non-thermal plasma systems needs further investigation. While the intricacies of activated species interaction with catalyst surface can be explained with well-established theories, the practicality of the process can only be assessed by a thorough economic analysis.
Conclusion
In this review, we have compared and critically analyzed microwave enhanced plasma reactions in various chemical technologies. A separate section on reaction mechanism study brings together various concepts that explain catalyst interactions with reactants under microwave irradiation and microwave plasma. A summary on the commercialized microwave plasma technologies is also presented.
Microwave plasma-enhanced catalytic reactions have the potential to transform some of the conventional and widely used processes at a fundamental level. Specifically, microwave plasma catalytic process can achieve high product selectivity and yields which eliminates unnecessary unit operations or reduces the size of process equipment. Meanwhile, energy efficiency can be improved due to lower temperature and lower pressure operation as compared with conventional thermally heated process. These features highlight the process intensification potential. Successful scale-up of this technology could lead to a sustainable, cleaner and energy efficient future in chemical processing.
Microwave plasma is an established technology in some industrial scale processes such as etching, CVD, and food packaging. However, microwave plasma-enhanced catalytic technology is still at a fundamental research stage. At this stage, it has been shown to improve catalytic reactions in terms of conversion, yield and, product distribution. Scaling up these laboratory scale reactions is a research challenge by itself. The first requirement in a large scale chemical production is the high energy input for a continuous plasma generation. A pilot scale gasification unit, for instance, requires two microwave generators of 75 kW power output for a throughput of 1.9 ton syngas/day [94]. Additionally, reaction kinetic models that can encompass the complex interaction of plasma and catalysts are few. A wide scale economic assessment that can prove the profitability of these large scale processes is also required. A combined breakthrough in developing a microwave source with power output greater than 100 kW and predictive reaction models for process optimization and control are the major requirements in large scale chemical processing in microwave plasma.
References
Eliasson B, Kogelschatz U (1991) Nonequilibrium volume plasma chemical processing. IEEE Trans Plasma Sci 19:1063–1077. https://doi.org/10.1109/27.125031
Gomez E, Rani DA, Cheeseman CR et al (2009) Thermal plasma technology for the treatment of wastes: a critical review. J Hazard Mater 161:614–626. https://doi.org/10.1016/j.jhazmat.2008.04.017
Bonizzoni G, Vassallo E (2002) Plasma physics and technology; industrial applications. Vacuum 64:327–336
Hippler R (2001) Low temperature plasma physics: fundamental aspects and applications. Wiley, Hoboken
Nehra V, Kumar A, Dwivedi HK (2008) Atmospheric non-thermal plasma sources. Int J Eng 2:53–68
Lebedev YA (2010) Microwave discharges: generation and diagnostics. In: Journal of physics: conference series
Ferreira CM, Moisan M (1993) Microwave discharges: fundamentals and application. Springer, Berlin
Kaiser M, Baumgärtner KM, Mattheus A (2012) Microwave plasma sources—applications in industry. Contrib Plasma Phys 52:629–635. https://doi.org/10.1002/ctpp.201210059
Tendero C, Tixier C, Tristant P et al (2006) Atmospheric pressure plasmas: a review. Spectrochim Acta Part B 61:2–30. https://doi.org/10.1016/j.sab.2005.10.003
George SM (1995) Introduction: heterogeneous catalysis. Chem Rev 95:475–476. https://doi.org/10.1021/cr00035a001
Hoxie E, Fracasso C (2011) Known knowns… known unknowns… and unknown unknowns: processing the research journey. NeuroQuantology 9:515–517. https://doi.org/10.1088/0022-3727/49/24/243001
Hellund EJ (1961) The plasma state. Reinhold Publishing Corporation, New York
Chen HL, Lee HM, Chen SH et al (2009) Removal of volatile organic compounds by single-stage and two-stage plasma catalyss systems: a review of the performance enhancement mechanisms, current status, and suitable applications. Environ Sci Technol 43:2216–2227. https://doi.org/10.1021/es802679b
Roland U, Holzer F, Kopinke FD (2005) Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: part 2. Ozone decomposition and deactivation. Appl Catal B Environ 58:217–226. https://doi.org/10.1016/j.apcatb.2004.11.024
Van Durme J, Dewulf J, Leys C, Van Langenhove H (2008) Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: a review. Appl Catal B Environ 78:324–333. https://doi.org/10.1016/j.apcatb.2007.09.035
Petitpas G, Rollier JD, Darmon A et al (2007) A comparative study of non-thermal plasma assisted reforming technologies. Int J Hydrogen Energy 32:2848–2867. https://doi.org/10.1016/j.ijhydene.2007.03.026
Laroussi M, Akan T (2007) Arc-free atmospheric pressure cold plasma jets: a review. Plasma Process Polym 4:777–788. https://doi.org/10.1002/ppap.200700066
Pankaj SK, Bueno-Ferrer C, Misra NN et al (2014) Applications of cold plasma technology in food packaging. Trends Food Sci Technol 35:5–17. https://doi.org/10.1016/j.tifs.2013.10.009
Thirumdas R, Sarangapani C, Annapure US (2014) Cold plasma: a novel non-thermal technology for food processing. Food Biophys 10:1–11. https://doi.org/10.1007/s11483-014-9382-z
Cordova-Lopez E, Ortoneda-Pedrola M, Al-Shamma’a AI, Wylie SR (2009) Microwave plasma applications in automotive and food industries. In: IET conference on high power RF technologies
Schneider J, Baumgärtner KM, Feichtinger J et al (2005) Investigation of the practicability of low-pressure microwave plasmas in the sterilisation of food packaging materials at industrial level. Surf Coat Technol 200:962–966. https://doi.org/10.1016/j.surfcoat.2005.01.114
Gutierrez RL, Bourgeous KN, Salveson A et al (2006) Microwave UV: a new wave of tertiary disinfection. Proc Water Environ Found 2006:2853–2864
Al-Shamma’a AI, Pandithas I, Lucas J (2001) Low-pressure microwave plasma ultraviolet lamp for water purification and ozone application. J Phys D Appl Phys 34:2775–2781
Liang Q, Yan CS, Meng Y et al (2009) Recent advances in high-growth rate single-crystal CVD diamond. Diam Relat Mater 18:698–703. https://doi.org/10.1016/j.diamond.2008.12.002
Yan CYC, Ho SHS, Lai J, et al (2006) Development of the microwave plasma CVD technique for rapid growth of large single-crystal diamond. In: The 33rd IEEE international conference on plasma science 2006
Butler JE, Mankelevich YA, Cheesman A et al (2009) Understanding the chemical vapor deposition of diamond: recent progress. J Phys: Condens Matter 21:364201. https://doi.org/10.1088/0953-8984/21/36/364201
Schlemm H, Mai A, Roth S et al (2003) Industrial large scale silicon nitride deposition on photovoltaic cells with linear microwave plasma sources. Surf Coatings Technol 174–175:208–211. https://doi.org/10.1016/S0257-8972(03)00611-X
Suzuki K, Okudaira S, Sakudo N, Kanomata I (1977) Microwave plasma etching. Jpn J Appl Phys 16:1979–1984. https://doi.org/10.1143/JJAP.16.1979
Suzuki K, Ninomiya K, Mishimatsu S (1984) Microwave plasma etching. Vacuum 34:953–957. https://doi.org/10.1016/0042-207X(84)90177-5
De la Fuente JF, Kiss AA, Radoiu MT, Stefanidis GD (2017) Microwave plasma emerging technologies for chemical processes. J Chem Technol Biotechnol 92:2495–2505. https://doi.org/10.1002/jctb.5205
Sanchez AL (2010) Method and apparatus for plasma gasification of carbonic material by means of microwave radiation
White TL, Paulauskas FL, Bigelow TS (2010) System to continuously produce carbon fiber via microwave assisted plasma processing
Zakaria Z, Kamarudin SK (2016) Direct conversion technologies of methane to methanol: an overview. Renew Sustain Energy Rev 65:250–261. https://doi.org/10.1016/j.rser.2016.05.082
Su S, Beath A, Guo H, Mallett C (2005) An assessment of mine methane mitigation and utilisation technologies. Prog Energy Combust Sci 31:123–170. https://doi.org/10.1016/j.pecs.2004.11.001
Reddy VLP, Kim K-H, Song H (2013) Emerging green chemical technologies for the conversion of CH4 to value added products. Renew Sustain Energy Rev 24:578–585. https://doi.org/10.1016/J.RSER.2013.03.035
Holmen A (2009) Direct conversion of methane to fuels and chemicals. Catal Today 142:2–8. https://doi.org/10.1016/j.cattod.2009.01.004
Hu YH, Ruckenstein E (2004) Catalytic conversion of methane to synthesis gas by partial oxidation and CO2 reforming. Adv Catal 48:297–345. https://doi.org/10.1016/S0360-0564(04)48004-3
Suib SL, Zerger RP (1993) A direct, continuous, low-power catalytic conversion of methane to higher hydrocarbons via microwave plasmas. J Catal 139:383–391. https://doi.org/10.1006/jcat.1993.1034
Cho W, Baek Y, Moon SK, Kim YC (2002) Oxidative coupling of methane with microwave and RF plasma catalytic reaction over transitional metals loaded on ZSM-5. Catal Today 74:207–223. https://doi.org/10.1016/S0920-5861(02)00030-5
Nagazoe H, Kobayashi M, Yamaguchi T et al (2006) Characteristics of methane conversion under combined reactions of solid catalyst with microwave plasma. J Chem Eng Japan 39:314–320. https://doi.org/10.1252/jcej.39.314
Scholz WH (1993) Processes for industrial production of hydrogen and associated environmental effects. Gas Sep Purif 7:131–139. https://doi.org/10.1016/0950-4214(93)80001-D
Chen W-H, Hsieh T-C, Jiang TL (2008) An experimental study on carbon monoxide conversion and hydrogen generation from water gas shift reaction. Energy Convers Manag 49:2801–2808. https://doi.org/10.1016/j.enconman.2008.03.020
Wang YF, Tsai CH, Chang WY, Kuo YM (2010) Methane steam reforming for producing hydrogen in an atmospheric-pressure microwave plasma reactor. Int J Hydrogen Energy 35:135–140. https://doi.org/10.1016/j.ijhydene.2009.10.088
Heintze M, Magureanu M (2002) Methane conversion into aromatics in a direct plasma-catalytic process. J Catal 206:91–97. https://doi.org/10.1006/jcat.2001.3467
Ohnishi R, Kojima R, Shu Y et al (2004) Highly stable performance of catalytic methane dehydro-condensation to benzene and naphthalene on Mo/HZSM-5 by addition and a periodic switching treatment of H2. Stud Surf Sci Catal 147:553–558. https://doi.org/10.1016/s0167-2991(04)80110-1
Wang D, Lunsford JH, Rosynek MP (1997) Characterization of a Mo/ZSM-5 catalyst for the conversion of methane to benzene. J Catal 169:347–358. https://doi.org/10.1006/jcat.1997.1712
Edenhofer O, Pichs-Madruga R, Sokona Y et al (2014) Climate change 2014: mitigation of climate change. Fifth assessment report of the intergovernmental panel on climate change. Clim Chang 2014:33–107. https://doi.org/10.1103/PhysRevD.70.106002
Yang H, Xu Z, Fan M et al (2008) Progress in carbon dioxide seperation and capture: a review. J Environ Sci 20:14–27. https://doi.org/10.1016/S1001-0742(08)60002-9
Whipple DT, Kenis PJA (2010) Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J Phys Chem Lett 1:3451–3458. https://doi.org/10.1021/jz1012627
Wang WN, Soulis J, Jeffrey Yang Y, Biswas P (2014) Comparison of CO2 photoreduction systems: a review. Aerosol Air Qual Res 14:533–549. https://doi.org/10.4209/aaqr.203.09.0283
Liu C, Xu G, Wang T (1999) Non-thermal plasma approaches in CO2 utilization. Fuel Process Technol 58:119–134. https://doi.org/10.1016/S0378-3820(98)00091-5
Rusanov VD, Fridman AA, Sholin GV (2011) The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Uspekhi Fiz Nauk 134:447–474. https://doi.org/10.3367/ufnr.0134.198106a.0185
Lebouvier A, Iwarere SA, D’Argenlieu P et al (2013) Assessment of carbon dioxide dissociation as a new route for syngas production: a comparative review and potential of plasma-based technologies. Energy Fuels 27:2712–2722. https://doi.org/10.1021/ef301991d
Spencer LF, Gallimore AD (2013) CO2 dissociation in an atmospheric pressure plasma/catalyst system: a study of efficiency. Plasma Sour Sci Technol 22:9. https://doi.org/10.1088/0963-0252/22/1/015019
Chen G, Georgieva V, Godfroid T et al (2016) Plasma assisted catalytic decomposition of CO2. Appl Catal B Environ 190:115–124. https://doi.org/10.1016/j.apcatb.2016.03.009
Berthelot A, Bogaerts A (2017) Modeling of CO2 splitting in a microwave plasma: how to improve the conversion and energy efficiency. J Phys Chem C 121:8236–8251. https://doi.org/10.1021/acs.jpcc.6b12840
Fridman Alexander (2008) Plasma chemistry. Cambridge University Press, New York
Goede APH, Bongers WA, Graswinckel MF, et al (2014) Production of solar fuels by CO2 plasmolysis. In: EPJ web of conferences
Bongers W, Bouwmeester H, Wolf B et al (2017) Plasma-driven dissociation of CO2 for fuel synthesis. Plasma Process Polym 14:1600126. https://doi.org/10.1002/ppap.201600126
Tsuji M, Tanoue T, Nakano K, Nishimura Y (2001) Decomposition of CO2 into CO and O in a microwave-excited discharge flow of CO2/He or CO2/Ar mixtures. Chem Lett 30:22–23. https://doi.org/10.1246/cl.2001.22
Vesel A, Mozetic M, Drenik A, Balat-Pichelin M (2011) Dissociation of CO2 molecules in microwave plasma. Chem Phys 382:127–131. https://doi.org/10.1016/j.chemphys.2011.03.015
Walzel P (2012) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH
Kunimori K, Osumi M, Kameoka S, Ito S (1992) Plasma-induced nitrogen chemisorption on a ruthenium black catalyst: formation of NH3 by hydrogenation of the chemisorbed nitrogen. Catal Lett 16:443–446. https://doi.org/10.1007/BF00764903
Hong J, Prawer S, Murphy AB (2018) Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain Chem Eng 6:15–31. https://doi.org/10.1021/acssuschemeng.7b02381
Tanaka S, Uyama H, Mastsumoto O (1994) Synergistic effect of catalysts and plasmas on synthesis of ammonia and hydrazine. Plasma Chem Plasma Process 14:491–504
Bai X, Tiwari S, Robinson B et al (2018) Microwave catalytic synthesis of ammonia from methane and nitrogen. Catal Sci Technol. https://doi.org/10.1039/C8CY01355A
Bai M, Zhang Z, Bai M et al (2008) Synthesis of ammonia using CH4/N2plasmas based on micro-gap discharge under environmentally friendly condition. Plasma Chem Plasma Process 28:405–414. https://doi.org/10.1007/s11090-008-9132-4
Gómez-Ramŕez A, Cotrino J, Lambert RM, González-Elipe AR (2015) Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sour Sci Technol. https://doi.org/10.1088/0963-0252/24/6/065011
Peng P, Li Y, Cheng Y et al (2016) Atmospheric pressure ammonia synthesis using non-thermal plasma assisted catalysis. Plasma Chem Plasma Process 36:1201–1210. https://doi.org/10.1007/s11090-016-9713-6
Hong J, Aramesh M, Shimoni O et al (2016) Plasma catalytic synthesis of ammonia using functionalized-carbon coatings in an atmospheric-pressure non-equilibrium discharge. Plasma Chem Plasma Process 36:917–940. https://doi.org/10.1007/s11090-016-9711-8
Debye PJW (1929) Polar molecules. Chemical Catalog Company, Incorporated
Horikoshi S, Serpone N (2016) Microwaves in catalysis: methodology and applications. Wiley, Hoboken
Amini A, Ohno K, Maeda T, Kunitomo K (2018) Effect of the ratio of magnetite particle size to microwave penetration depth on reduction reaction behaviour by H2. Sci Rep 8:1–7. https://doi.org/10.1038/s41598-018-33460-5
Bai X, Robinson B, Killmer C et al (2019) Microwave catalytic reactor for upgrading stranded shale gas to aromatics. Fuel 243:485–492. https://doi.org/10.1016/j.fuel.2019.01.147
Mochizuki D, Sasaki R, Maitani MM et al (2015) Catalytic reactions enhanced under microwave-induced local thermal non-equilibrium in a core–shell, carbon-filled zeolite@zeolite. J Catal 323:1–9. https://doi.org/10.1016/j.jcat.2014.12.003
Amini A, Ohno K, Maeda T, Kunitomo K (2019) A kinetic comparison between microwave heating and conventional heating of FeS-CaO mixture during hydrogen-reduction. Chem Eng J 374:648–657. https://doi.org/10.1016/j.cej.2019.05.226
Ferrari A, Hunt J, Lita A et al (2014) Microwave-specific effects on the equilibrium constants and thermodynamics of the steam-carbon and related reactions. J Phys Chem C 118:9346–9356. https://doi.org/10.1021/jp501206n
Hunt J, Ferrari A, Lita A et al (2013) Microwave-specific enhancement of the carbon-carbon dioxide (Boudouard) reaction. J Phys Chem C 117:26871–26880. https://doi.org/10.1021/jp4076965
Zhou J, Xu W, You Z et al (2016) A new type of power energy for accelerating chemical reactions: the nature of a microwave-driving force for accelerating chemical reactions. Nat Publ Gr. https://doi.org/10.1038/srep25149
Wentao X, Jicheng Z, Zhiming S et al (2016) Microwave catalytic effect: a new exact reason for microwave-driven heterogeneous gas-phase catalytic reactions. Catal Sci Technol 6:698–702. https://doi.org/10.1039/c5cy01802a
Hu J (2018) Direct non-oxidative conversion of shale gas to chemicals: Selective activation, catalyst regeneration, and process intensification. In: ACS Spring 2018, New Orleans
Ramirez A, Hueso JL, Mallada R, Santamaria J (2017) In situ temperature measurements in microwave-heated gas-solid catalytic systems. Detection of hot spots and solid-fluid temperature gradients in the ethylene epoxidation reaction. Chem Eng J 316:50–60. https://doi.org/10.1016/j.cej.2017.01.077
Ramirez A, Hueso JL, Abian M et al (2019) Escaping undesired gas-phase chemistry: microwave-driven selectivity enhancement in heterogeneous catalytic reactors 5:1–7. https://doi.org/10.1126/sciadv.aau9000
Oumghar A, Legrand JC, Diamy AM, Turillon N (1995) Methane conversion by an air microwave plasma. Plasma Chem Plasma Process 15:87–107. https://doi.org/10.1007/BF01596683
Onoe K, Fujie A, Yamaguchi T, Hatano Y (1997) Selective synthesis of acetylene from methane by microwave plasma reactions. Fuel 76:281–282. https://doi.org/10.1016/S0016-2361(96)00228-1
Oumghar A, Legrand JC, Diamy AM et al (1994) A kinetic study of methane conversion by a dinitrogen microwave plasma. Plasma Chem Plasma Process 14:229–249. https://doi.org/10.1007/BF01447080
Heintze M, Magureanu M, Kettlitz M (2002) Mechanism of C2 hydrocarbon formation from methane in a pulsed microwave plasma. J Appl Phys 92:7022–7031. https://doi.org/10.1063/1.1521518
Uyama H, Matsumoto O (1989) Synthesis of ammonia in high-frequency discharges. Plasma Chem Plasma Process 9:13–24. https://doi.org/10.1007/BF01015824
Nakajima J, Sekiguchi H (2008) Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 516:4446–4451. https://doi.org/10.1016/j.tsf.2007.10.053
Sugiyama K, Akazawa K, Oshima M et al (1986) Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem Plasma Process 6:179–193. https://doi.org/10.1007/BF00571275
Neyts EC (2016) Plasma-surface interactions in plasma catalysis. Plasma Chem Plasma Process 36:185–212. https://doi.org/10.1007/s11090-015-9662-5
Liu C, Marafee A, Mallinson R, Lobban L (1997) Methane conversion to higher hydrocarbons in a corona discharge over metal oxide catalysts with OH groups. Appl Catal A Gen 164:21–33
Suzuki T, Hirota E (1993) Vibrational distribution of CH3 produced by the reaction of O(1D2) atom with CH4. J Chem Phys 98:2387–2398. https://doi.org/10.1063/1.464166
Uhm HS, Na YH, Hong YC et al (2014) Production of hydrogen-rich synthetic gas from low-grade coals by microwave steam-plasmas. Int J Hydrogen Energy 39:4351–4355. https://doi.org/10.1016/J.IJHYDENE.2014.01.020
Acknowledgements
The authors acknowledge financial support from the West Virginia Higher Education Policy Commission under Grant HEPC.dsr.18.7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiwari, S., Caiola, A., Bai, X. et al. Microwave Plasma-Enhanced and Microwave Heated Chemical Reactions. Plasma Chem Plasma Process 40, 1–23 (2020). https://doi.org/10.1007/s11090-019-10040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10040-7