Abstract
The human tissue-type plasminogen activator (tPA) is a key kinase of fibrinolysis that plays an important role in dissolving fibrin clots to promote thrombolysis. The recombinant human plasminogen activator (rhPA) has more thrombolytic advantages than the wild type tPA. To increase the half-life and thrombolytic activity of tPA, a mutant containing only the essential K2 fibrin-binding and P activating plasminogen domains of the wild type tPA was cloned. This fragment was then inserted into goat β-casein regulatory sequences. Then, a mammary gland-specific expression vector, PCL25/rhPA, was constructed, and the transgenic rabbits were generated. In this study, 18 live transgenic founders (12♀, 6♂) were generated using pronuclear microinjection. Six transgenic rabbits were obtained, and the expression levels of rhPA in the milk had a range of 15.2–630 µg/ml. A fibrin agarose plate assay of rhPA showed that it had strong thrombolytic bioactivity in vitro, and the highest specific activity was >360 (360 times more than that of alteplase). The results indicated that the rhPA containing only the K2 and P domains is efficiently expressed with higher thrombolytic bioactivity in the milk of transgenic rabbits. Our study also demonstrated a new method for the large-scale production of clinically relevant recombinant pharmaceutical proteins in the mammary glands of transgenic rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human tissue-type plasminogen activator (tPA) is a thrombolytic serine protease synthesized and secreted by vascular endothelial cells that plays a significant role in dissolving fibrin clots through fibrinolysis and transforming plasminogen into plasmin in the blood vessels to promote thrombolysis [1–3]. Mature tPA is a single-chain glycoprotein with a MW of 70,000 that consists of 527 amino acids and 17 disulfide bonds [4] and can be divided into the following five regions: the finger domain (F, residues 1–50) with a fibrin-binding site, the epidermal growth factor domain (E or EGF, residues 51–87) with a liver cell membrane receptor-binding site, the kringle1 domain (K1, residues 87–176) with a glycosylation site (Asn117), the kringle2 domain (K2, residues 177–275) with another fibrin-binding site, and the serine protease domain (P, residues 276–527) with the enzyme catalytic site [5, 6]. Each domain has its own specific function. The F and K2 domains can mediate specific interactions with fibrins, and the F domain is the most important in determining the half-life of tPA in vivo. The E and K1 domains are predominantly associated with the elimination of tPA in vivo [7]. The serine protease P domain converts plasminogen into active plasmin, which also plays an important role in thrombolysis [8]. Therefore, the absence of the F domain can decrease the fibrin-binding specificity, inhibiting systemic bleeding. Previous studies found that because there is no F domain, only the K2 domain, thrombolytics have a low affinity for fibrin (only 20 % of the wild type tPA affinity) and will not bind to the surface of the blood clot during thrombolysis, which results in rapid and efficient thrombolysis. Thus, the thrombolytics can better suppress the hemorrhagic side effects [9]. The absence of the E domain can prevent degradation of tPA by liver cells and thus prolong the half-life of tPA in vivo. The loss of the K1 domain can maintain the stability of tPA [10]. Hence, recombinant human plasminogen activator (rhPA) with a deletion in these three domains (F, E, and K1) is expected to have stronger thrombolytic activity than tPA [11–13]. Previous evidence revealed that the K1 and E domains mediate an interaction between tPA and the surface receptor of a liver cell and then accelerate the plasma clearance of tPA [14, 15]. The high mannose oligosaccharide chains in the K1 structural domain are also correlated with clearance [16]. Thus, the elimination of the K1 and E domains can play an important role in reducing the clearance and lengthening the half-life, which enhances the thrombolytic activity [17]. As described above, the F domain had a higher affinity for fibrin, and thus, the absence of the F domain can decrease the hemorrhagic side effects [14]. Reteplase is a third-generation thrombolytic agent consisting of the K2 and the protease domains, but it lacks the K1, F and E domains of tPA [18]. A previous study demonstrated that one of the most important advantages of reteplase is that it has a prolonged half-life, but the clinical advantage of tPA is that it is most effective when administered in an accelerated regimen of an initial bolus and two timed infusions, and it is the benchmark to which all new thrombolytic agents are compared [19]. Thus, we focused on tPA in our study.
To date, many proteins have been expressed in the mammary glands of transgenic animals [20]. There are many advantages of this system, including higher productivity, lower costs, post-translational modification, and higher bioactivity using mammary gland bioreactors to produce tPA and rhPA compared to other expression systems [21–23]. Currently, many mammary gland bioreactors have been reported in China and other countries [21, 24, 25]. The first studies on transgenic rabbits were conducted in 1985 by Brem and Hammer et al. [26, 27]. Later, transgenic rabbits became one of the most widely used and effective animals for the production of recombinant proteins in milk. Compared to goats, rabbits have a larger litter size (8–12 bunnies), have a shorter gestation period (30–32 days) [28], are polyestrous animals, have a higher rate of ovulation, have a clearly defined genetic background and are easier to manage and feed. Compared to mice, rabbits are phylogenetically closer to humans [29] and have a higher milk yield (100–300 ml/lactation). Due to these advantages and similarities, rabbits are preferentially used in mammary gland bioreactor studies.
In this study, to increase the half-life and improve the thrombolytic effect of tPA, the mammary gland bioreactor of transgenic rabbits was chosen to produce a mutant rhPA with deletions of the F, E and K1 domains. Then, the modified cDNA and the regulatory sequence of goat β-casein were cloned into the mammary gland-specific expression vector PCL25 [30]. The rhPA without the F, E, and K1 domains was successfully expressed in the milk of the transgenic rabbits, and its expression levels and bioactivity in vitro were analyzed. The aim of this study was to evaluate the practicability of producing novel highly efficient thrombolytic drugs using transgenic rabbit mammary gland bioreactors.
Materials and methods
Materials
The PCL25 plasmid [30] was previously generated and cloned by the Engineering Research Center for Transgenic Animal Pharmaceutics, Yangzhou University, Jiangsu Province, China. The structure of the rhPA expression construct is shown in Fig. 1. This PCL25/rhPA vector contains the 5′ and 3′ regions of the goat β-casein regulator gene, cmv, and rhPA (only the coding sequences of the K2 and P domains) (Fig. 1). The experiment was carried out using Chinese albino rabbits (female) weighing 2.5–3.0 kg that were provided by the Yangzhou Medical Laboratory Animal Center. The rabbits were housed with 12 h (7:00–19:00) daylight at 20 °C. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) and China, unless otherwise specified.
This study was approved by the Institutional Animal Care and Use Committee of Yangzhou University. All animals were maintained and processed in accordance with the Developing Guidelines on the Care and Use of Animals [31]. All surgeries were performed under atropine sulfate and zoletil-50 anesthesia, and all efforts were made to minimize any suffering.
Methods
Generation of transgenic rabbits
A 17 kb DNA fragment was obtained by digesting the PCL25/rhPA vector with SalI and NotI, purifying the DNA using agarose gel electrophoresis and diluting it to 5 ng/µl. The purified DNA was microinjected into the fertilized eggs of the superovulated female rabbits [24]. Sexually mature female rabbits (6–12 months old) were superovulated using a standard regimen described in previous studies [28, 32]. The protocols for the surgery and embryo transfer were described earlier [33, 34]. The average pregnancy lasted 30–32 days. Transgenic founder rabbits were identified with PCR analysis of the extracted genomic DNA. The PCR was performed using CMV enhancer-rPA-specific primers designed by Primer 5.0, Oligo (CMV/rhPA set; the primer sequences were as follows: F1: 5′-CGTGGATAGCGGTTT GA-3′; R1: 5′-GAGCCCTCCTTTGATGC-3′).
Enzyme-linked immunosorbent assay
The whey samples from the transgenic rabbits were centrifuged at 10,000×g for 30 min, and the fat in the upper layer with a lower turbidity was dislodged to gather the whey. Gel filtration chromatography was used to separate and purify the whey. Additionally, the sodium dodecyl sulfate (SDS) polyacrylamide gel method and reversed-phase high-performance liquid chromatography were used to test the proteins. Then, the purified rhPA protein was collected and used to coat a microplate to construct a solid-phase antibody. rhPA was successively added to the microplate coated with a monoclonal antibody, and this was then combined with the rhPA labeled with horseradish peroxidase (HRP) to form an antibody-antigen enzyme-labeled antibody complex. After thorough washing, the complex was mixed with the chromogenic substrate tetramethylbenzidine (TMB). Following catalysis of HRP, TMB turned blue, and with the acid, it finally turned yellow. The intensity of the color and the rhPA in samples were positively correlated. Absorbance (optical density, OD) was tested via enzyme-linked immunosorbent assay (ELISA) at 450 nm, and the concentrations of rhPA in the samples were calculated using a standard curve. The alteplase, whey from normal non-transgenic rabbits, and phosphate-buffered saline (PBS)/ddH2O were used as the positive control, negative control, and blank control, respectively. Meanwhile, the saliva and blood of transgenic rabbits were used for specific detection. All samples were added at 100 μl per well in a 1:1 volume of 0.1 M carbonate/bicarbonate buffer (pH 9.6) into 96-well microplates and incubated overnight at 4 °C. Detailed procedures can be found in other studies [28, 32].
Western blot analysis
Skim milk samples obtained from all the transgenic rabbits were diluted for analysis with 100 volumes of Tris-buffered saline (125 mM NaCl, 25 mM Tris, pH 7.4, 5 mM KCl, 2 mM phenylmethylsulfonyl fluoride), and then, these samples were diluted 1:1 with a 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (100 mM tris-Cl, pH 6.8, 200 mM 1,4-dithiothreitol, 4 % (w/v) SDS, 0.2 % bromophenol blue, 20 % glycerol). These samples were denatured in boiling water (100 °C) for 5 min, and 15 µg of each sample was separated with 12 % (w/v) SDS-PAGE using a Bio-Rad 1.5 mM spacers (denaturing PAGE; Mini-Protean II Electrophoresis Cell; Bio-Rad Laboratories, Hercules, CA).
Separated unstained proteins were transferred onto polyvinylidene fluoride membranes (0.45 µM; BioTrace, Pall Co., assembled in Mexico; Lot T215932) using transfer buffer (1.93 g/l tris, 9 g/l glycine) and 1 mA/cm2 for 180 min. The membranes were blocked at 37 °C for 2 h in 20 mM Tris, 137 mM NaCl (pH 7.6), 0.1 % Tween-20, and 10 % fetal bovine serum and then incubated at 4 °C overnight with an anti-rPA primary antibody (1:2000 dilution, against human tPA, mouse monoclonal IgG, sc-59721; Santa Cruz Biotechnology, Santa Cruz, CA). After four washes with the washing buffer (20 mM tris, 137 mM NaCl, 1 % Tween-20, pH 7.6), the membranes were incubated with an HRP-conjugated goat secondary antibody (1:2000 dilution, goat anti-mouse IgG-HRP, sc-2005; Santa Cruz Biotechnology) for 3 h at 37 °C. Finally, the membranes were incubated in the colorimetric solution (100 ml volumes with 50 mg DAB, 0.05 M TB, 30 µl 30 % H2O2, pH 7.6) for 20 min at room temperature after washing thoroughly four times.
Activity analysis (fibrin agarose plate assay, FAPA)
The whey samples were diluted for analysis using 200 volumes of tris-buffered saline. The fibrinogen-thrombin-agarose gel plate was prepared and consisted of 1.0 % (w/v) agarose, 10 mg/ml fibrinogen containing a small amount of plasminogen, and 1 U/ml thrombin in PBS (137 mM NaCl, 10 mM Na2HPO4, 3 mM KCl, 2 mM KH2PO4, pH 7.4) [35].
The agarose gel was boiled and melted completely, and it was then placed in a 15 ml centrifuge tube. When the temperature of the gel dropped to 55–60 °C, 1 ml fibrinogen pre-incubated at 37 °C and 1 ml thrombin pre-incubated at 42 °C were added, mixed rapidly, and then poured onto a 9 cm diameter transparent plexiglass plate. After the cells were equilibrated to room temperature, wells were drilled, and covers were placed on top. Finally, a 20 µl sample was loaded in each well and incubated at 37 °C overnight or longer. To measure fibrinolytic activity of rhPA, the diameters of the thrombolytic transparent circles were observed and measured.
Results and analysis
Generation of transgenic rabbits
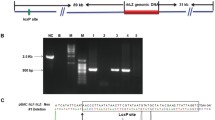
In total, 326 microinjected embryos were transferred to 15 rabbits, and 11 became pregnant. This resulted in 12 female and 6 male transgenic founders from 57 overall offspring, which were analyzed by PCR and sequencing screening. Then, agarose gel electrophoresis was subsequently conducted, and the 566 bp PCR product was identified. The PCR product is shown in Fig. 2. The efficiency of pregnancy, embryo survival, and transgene integration was 73.3 % (11/15), 17.5 % (57/326), and 31.6 % (18/57), respectively.
The agarose gel was stained with ethidium bromide to visualize the rhPA of transgenic rabbits. rhPA, recombinant human plasminogen activator; (a 1–18 F0 represents the rabbit genome to be tested, M DL2000 DNA Marker, B1, B2 double distilled water as the blank control, N1, N2 native rabbit gene as the negative control, P PCR amplification products of microinjection as the positive control); (b 1–19 F0 represents the rabbit genome to be tested, M DL2000 DNA Marker, B1, B2 double distilled water as the blank control, N1, N2 native rabbit gene as the negative control, P PCR amplification products of microinjection as the positive control); (c 1–10 F1 represents the rabbit genome to be tested, M DL2000 DNA Marker, B1, B2 double distilled water as the blank control, N1, N2 native rabbit gene as the negative control, P PCR amplification products of microinjection as the positive control); (d 1–10 F1 represents the rabbit genome to be tested, M DL2000 DNA Marker, B1, B2 double distilled water as the blank control, N1, N2 normal rabbit gene as the negative control, P PCR amplification products of microinjection as the positive control)
Expression of rhPA in the milk of transgenic rabbits
To characterize the expression of rhPA, milk was collected from the 12 female transgenic animals during lactation. The expression level of rhPA in milk was determined using an ELISA; the standard curve was generated with the OD450 as the horizontal axis and the content of rhPA protein in whey as the longitudinal axis (Fig. 3). The results showed that six rabbits from the 12 female founders expressed rhPA, an expression rate of 50 % (6/12). Six corresponding transgenic rabbit lines were generated, and the expression levels of rhPA in the milk were 15.2–630 µg/ml (Table 1). The results in Fig. 3 and Table 1 were obtained after the sample was diluted 500 times.
The concentrations of rhPA in the whey samples. rhPA, recombinant human plasminogen activator; (the standard curve of the concentrations of alteplase: 0.0267, 0.0433, 0.0875, 0.175, 0.350, 0.700 and 1.400 µg/ml; K17, K30, K06, K10, K34 and K29: the detection results of the whey of transgenic rabbits after a 500 times dilution)
To assess possible ectopic expression of rhPA in the transgenic rabbits, rhPA levels in the saliva and blood of lactating rabbits were measured using ELISA. There was no indication of rhPA in the saliva or blood from the transgenic rabbits.
Compared to the non-transgenic wild type rabbits, the six transgenic rabbits expressed a 39 kDa target-specific protein in the milk as shown in the western blot analysis. The detailed results of the western blot analysis are provided in Fig. 4.
Analysis of expression of rhPA in the milk of transgenic rabbits using western blot analysis. Skim milk samples obtained from transgenic rabbits were diluted for analysis using 100 volumes of Tris-buffered saline and 15 µl milk per lane. Lane M molecular weight standards, lane N negative control (whey of normal non-transgenic rabbit), Lane B blank control (triple distilled water), Lanes K06, K10, K17, K29, K30, and K34 whey from the transgenic founder rabbits (arrow indicates rhPA as a 39 kDa band). rhPA recombinant human plasminogen activator
Specific activity assay of rhPA in the milk of transgenic rabbits
tPA is a serine protease that converts plasminogen into active plasmin, which digests fibrin and dissolves fibrin clots. Here, the activity of rhPA (specific activity) in the milk was quantified using FAPA and ELISA. The fibrinolytic activity of rhPA was measured using the size of the thrombolytic transparent circle (Fig. 5a, b).
FAPA of milk from transgenic rabbits. Milk samples were diluted for analysis using 200 volumes of Tris-buffered saline.a Wells 1–10 alteplase standard concentrations were 2880, 1440, 720, 360, 180, 90, 45, 22.5, 11.25, and 0 µg/ml, respectively); wells 11–16 milk samples of transgenic founders K30, K24, K31, K34, K29, and K40, respectively; well 17 milk samples of normal non-transgenic rabbit; well 18 PBS. The diameters of the fibrinolysis transparent circle (12 h, 1–18 wells) were 30.9, 27.5, 24.8, 23.5, 23.0, 22.5, 20.8, 18.6, 14.2, 0, 15.1, 0, 0, 20.5, 27.1, 0, 0 and 0 mM, respectively. b Wells 1–8 alteplase standard concentrations are 288, 144, 72, 36, 18, 9, 4.5, and 0 µg/ml, respectively; wells 9–14 milk samples of transgenic founders K06, K07, K17, K10, K15, and K08, respectively; well 17 milk samples of normal non-transgenic rabbit. The diameters of the fibrinolysis transparent circle (24 h, 1–15 wells) are 25.2, 22.4, 20.6, 18.5, 16.0, 12.8, 9.3, 0, 16.7, 0, 9.2, 20.4, 0, 0 and 0 mm, respectively. c A standard curve was constructed using the logarithm of the standard concentrations (log µg/ml) (3.46, 3.16, 2.86, 2.56, 2.26, 1.95, 1.65, 1.35, 1.05) for the x-axis and the transparent circle diameter (30.9, 27.5, 24.8, 23.5, 23.0, 22.5, 20.8, 18.6, 14.2 mM) for the y-axis. d A standard curve was constructed with the logarithm of the standard concentrations (log µg/ml) (2.46, 2.16, 1.86, 1.56, 1.26, 0.95, 0.65, 0) for the x-axis and the transparent circle diameter (25.2, 22.4, 20.6, 18.5, 16.0, 12.8, 9.3, 0 mM) for the y-axis
The activity analysis (FAPA) showed that the rhPA products expressed by the six transgenic rabbits had fibrinolytic activity in vitro; the highest activity was equivalent to approximately 227,600 µg/ml of the alteplase standard. A standard curve was constructed using the logarithm of the alteplase standard concentrations (log µg/ml) for the x-axis and the transparent circle diameter (mM) for the y-axis (Fig. 5c, d). Based on the standard curve, the calculated activity of the expressed rhPA in the milk of transgenic rabbits was approximately equivalent to the effects of alteplase (Table 1). The FAPA of rhPA showed that the proteins from the six transgenic rabbits had biological activity in vitro and that the specific activity (equivalent of biological activity/quantity expression) was 49–360 times higher than that of alteplase (Table 1). Furthermore, in the milk of normal non-transgenic rabbits and non-expressing transgenic rabbits, the thrombolytic transparent circles were not observed. The results demonstrated that the highest specific activity of rhPA in the transgenic milk was 360 (compared with alteplase). Hence, this method could be used to evaluate tPA and rhPA activity, although the results may depend on individual differences and/or uncontrolled factors.
Discussion
The aim of this study was to explore the feasibility of producing novel highly efficient thrombolytic drugs by constructing a mammary gland-specific expression vector PCL25/rhPA and creating transgenic rabbits. Our results are the first demonstration that transgenic rabbits could efficiently express rhPA, containing only the K2 and P domains, with higher thrombolytic bioactivity in the milk. Moreover, the highest expression level of rhPA in the milk was approximately 630 µg/ml, and the highest specific activity was approximately 360 times greater than that of alteplase.
In recent years, thrombolytic therapies have been shown to be effective treatments that significantly improve the conditions of patients and animals with thrombotic diseases, such as myocardial infarction, cerebral thrombosis, pulmonary embolism, and other vascular emboli. One widely used thrombolytic drug is human tPA. As early as 1947, it was reported that the plasminogen activator could be obtained from animal tissues and was called fibrinokinase [4]. Pennica was the first to complete tPA gene cloning and sequence analysis in 1983 [36]. Since then, the structural-functional relationships of tPA have been studied by many researchers in both academia and industry [1, 4, 37–40]. Kalyan et al. reported in 1988 that the F-E domain deletion mutants showed a significantly increased half-life in the plasma of mice [41]. Rouf et al. reported that deletion of the F, E, or K domains of tPA increased in vitro stability [42]. In addition, a variant with the above unfavorable locus, deleted or mutated, liminated inhibition by increasing the half-life in vivo, reducing systemic bleeding, and enhancing the specific activity of thrombolysis [43, 44]. These studies suggested the potential for increasing the tPA half-life and thrombolytic effects in vivo. Indeed, the recombinant mutant tPA (rhPA) with deletions of the F, E, and K1 domains maintained the K2 domain and the P domain in our study. To demonstrate that this mutant can be normally expressed as the target recombinant protein, we demonstrated expression of the protein, and the highest expression of rhPA in the milk was 630 µg/ml, and the highest biological activity was approximately 360-fold greater than that of alteplase.
Currently, tPA and its recombinant mutants have predominantly been expressed in Escherichia coli [45, 46], the seaweed Laminaria japonica [47], Chinese hamster ovary cells [48], and the insect cell expression systems [6, 49–51]. There are, however, a few reports on the expression of tPA or rhPA in the mammary glands of mammals [38, 39, 52, 53]. Sha et al. [53] and Zhou et al. [39] reported that when the protein was expressed in mouse milk, the milk yield decreased. Another study conducted by Ebert et al. [38] in goats reported a lower level of expression and found that the lactation periods of the transgenic goats with high levels of tPA in the milk were shortened (only by 30–60 days) [52]. However, there has been no study on the expression of the rhPA in the mammary gland of rabbits. Considering the merits and demerits of the previous studies that successfully expressed recombinant human tPA and wild type tPA, there is a gap in the research, and thus, we assessed the practicability of expressing rhPA in the mammary glands of transgenic rabbits. The thrombolytic activity of the rhPA, a mutant protein with the E, F, and K1 domains removed, was evaluated in this study.
Cheng et al. [30] reported that the expression level of recombinant human lactoferrin (rhLF), carried by the mammary gland-specific expression vector of PCL25 containing goat β-casein regulatory sequences and a CMV hybrid promoter/enhancer, showed a mean concentration of 3.25 mg/ml in transgenic mice. Hence, a PCL25/rhPA vector, in which the rhLF coding sequence was replaced with the 1.1 kb rhPA coding sequence, was constructed. The successful expression of rhPA in the milk of six independent transgenic founder rabbits was evaluated in the present study, and the expression levels showed a range of 15.2–630 µg/ml. This variation may be due to the presence of different recombinant proteins, integration sites, or inherent factors in the different species and different rabbits. The highest activity of rhPA obtained from the milk of the six transgenic independent founder rabbits was equivalent to approximately 227.6 mg/ml of the alteplase standard, as determined by the standard curve (K29). The expression of rhPA was confirmed by western blot analysis, which showed a band of approximately 39 kDa. The FIFA showed that the highest biological activity of the six transgenic rabbits expressing rhPA was 360 times more than that of alteplase, which indicates that the thrombolytic activity of the rhPA obtained from the milk of transgenic rabbits greatly exceeded the activity of alteplase. Therefore, we successfully demonstrated that the PCL25/rhPA expression construct was a reasonable and effective method for expressing rhPA in the milk of transgenic rabbits.
In conclusion, our results indicated that the first system expressing rhPA, which contains only the K2 and P domains, from transgenic rabbits showed efficient production of rhPA with higher thrombolytic bioactivity in the milk. Simultaneously, a new method has been developed for the large-scale production of clinically relevant recombinant pharmaceutical proteins in the mammary glands of transgenic rabbits.
References
Barrett AJ (2004) Handbook of proteolytic enzymes. E-STREAMS: electronic reviews of science and technology. Elsevier, Amsterdam, pp 2946–2952
Baumer W, Herrling GM, Feige K (2013) Pharmacokinetics and thrombolytic effects of the recombinant tissue-type plasminogen activator in horses. BMC Vet Res 9:158
DeMers G, Meurer WJ, Shih R, Rosenbaum S, Vilke GM (2012) Tissue plasminogen activator and stroke: review of the literature for the clinician. J Emerg Med 43(6):1149–1154
Collen D, Lijnen HR (2009) The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol 29(8):1151–1155
Khodabakhsh F, Dehghani Z, Zia MF, Rabbani M, Sadeghi HM (2013) Cloning and expression of functional reteplase in Escherichia coli Top10. Avicenna J Med Biotechnol 5(3):168–175
Lin L, Hu K (2014) Tissue plasminogen activator and inflammation: from phenotype to signaling mechanisms. Am J Clin Exp Immunol 3(1):30–36
Hong-Ying SS-GL, Jian-Quan C (2006) Expression of a variant of human tissue-type plasminogen activator in transgenic mouse milk. J Exp Anim Sci 43(3):211–218
Lijnen HR, Collen D (1991) Strategies for the improvement of thrombolytic agents. Thromb Haemost 66(1):88–110
Shafiee F, Moazen F, Rabbani M, Mir Mohammad Sadeghi H (2015) Optimization of the expression of reteplase in Escherichia coli Top10 using arabinose promoter. Jundishapur J Nat Pharm Prod 10(1):e16676
Smalling RW, Bode C, Kalbfleisch J, Sen S, Limbourg P, Forycki F, Habib G, Feldman R, Hohnloser S, Seals A (1995) More rapid, complete, and stable coronary thrombolysis with bolus administration of reteplase compared with alteplase infusion in acute myocardial infarction. Circulation 91(11):2725–2732
Lian LF, Xu F, Tang ZP et al (2014) Intraclot recombinant tissue-type plasminogen activator reduces perihematomal edema and mortality in patients with spontaneous intracerebral hemorrhage. J Huazhong Univ Sci Technol 34:165–171
Dotan A, Kaiserman I, Kremer I, Ehrlich R, Bahar I (2014) Intracameral recombinant tissue plasminogen activator (r-tPA) for refractory toxic anterior segment syndrome. Br J Ophthalmol 98(2):252–255
Meunier JM, Wenker E, Lindsell CJ, Shaw GJ (2013) Individual lytic efficacy of recombinant tissue plasminogen activator in an in vitro human clot model: rate of “nonresponse”. Acad Emerg Med 20(5):449–455
Verheijen JH, Mullaart E, Chang GT, Kluft C, Wijngaards G (1982) A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost 48(3):266–269
Verheijen JH, Caspers MP, Chang GT, de Munk GA, Pouwels PH, Enger-Valk BE (1986) Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J 5(13):3525–3530
Gillis JC, Wagstaff AJ, Goa KL (1995) Alteplase. A reappraisal of its pharmacological properties and therapeutic use in acute myocardial infarction. Drugs 50(1):102–136
Rijken DC, Groeneveld E, Barrett-Bergshoeff MM (1994) In vitro stability of a tissue-type plasminogen activator mutant, BM 06.022, in human plasma. Thromb Haemost 72(6):906–911
Aghaabdollahian S, Rabbani M, Ghaedi K, Sadeghi HM (2014) Molecular cloning of reteplase and its expression in E. coli using tac promoter. Adv Biomed Res 3:190
Ellis K, Brener S (2004) New fibrinolytic agents for MI: as effective as current agents, but easier to administer. Cleve Clin J Med 71(1):29–30
Rudolph NS (1999) Biopharmaceutical production in transgenic livestock. Trends Biotechnol 17(9):367–374
Lubon H (1998) Transgenic animal bioreactors in biotechnology and production of blood proteins. Biotechnol Ann Rev 4:1–54
Houdebine LM (2009) Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis 32(2):107–121
Bosze Z, Baranyi M, Whitelaw CB (2008) Producing recombinant human milk proteins in the milk of livestock species. Adv Exp Med Biol 606:357–393
Serova IA, Dvoryanchikov GA, Andreeva LE, Burkov IA, Dias LP, Battulin NR, Smirnov AV, Serov OL (2012) A 3387 bp 5′-flanking sequence of the goat alpha-S1-casein gene provides correct tissue-specific expression of human granulocyte colony-stimulating factor (hG-CSF) in the mammary gland of transgenic mice. Transgenic Res 21(3):485–498
Wang Y, Tong J, Li S, Zhang R, Chen L, Wang Y, Zheng M, Wang M, Liu G, Dai Y, Zhao Y, Li N (2011) Over-expression of human lipoprotein lipase in mouse mammary glands leads to reduction of milk triglyceride and delayed growth of suckling pups. PLoS One 6(6):e20895
Brem GBB, Goodman HM et al (1985) Production of transgenic mice, rabbits and pigs by microinjection. Theriogenology 20:251–252
Hammer RE, Pursel VG, Rexroad CE Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL (1985) Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315(6021):680–683
Xue F, Ma Y, Chen YE, Zhang J, Lin TA, Chen CH, Lin WW, Roach M, Ju JC, Yang L, Du F, Xu J (2012) Recombinant rabbit leukemia inhibitory factor and rabbit embryonic fibroblasts support the derivation and maintenance of rabbit embryonic stem cells. Cell Reprog 14(4):364–376
Fan J, Watanabe T (2003) Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther 99(3):261–282
Cheng YAL, Yuan YG et al (2012) Hybrid expression cassettes consisting of a milk protein promoter and a cytomegalovirus enhancer significantly increase mammary-specific expression of human lactoferrin in transgenic mice. Mol Reprod Dev 79:573–585
Bayne K (1998) Developing guidelines on the care and use of animals. Ann N Y Acad Sci 862:105–110
Du FXJ, Zhang J et al (2009) Beneficial effect of young oocytes for rabbit somatic cell nuclear transfer. Cloning Stem Cells 11:131–140
Lin TACC, Sung LY et al (2011) Open-pulled straw vitrification differentiates cryotolerance of in vitro cultured rabbit embryos at the eight-cell stage. Theriogenology 75:760–768
Jindal HK, Merchant E, Balschi JA, Zhangand Y, Koren G (2012) Proteomic analyses of transgenic LQT1 and LQT2 rabbit hearts elucidate an increase in expression and activity of energy producing enzymes. J Proteomics 75(17):5254–5265
Granelli-Piperno A, Reich E (1978) A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med 148(1):223–234
Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, Ward CA, Bennett WF, Yelverton E, Seeburg PH, Heyneker HL, Goeddel DV, Collen D (1983) Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 301(5897):214–221
Medved L, Nieuwenhuizen W (2003) Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost 89(3):409–419
Ebert KM, Selgrath JP, DiTullio P, Denman J, Smith TE, Memon MA, Schindler JE, Monastersky GM, Vitale JA, Gordon K (1991) Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: generation of transgenic goats and analysis of expression. Nat Biotechnol 9(9):835–838
Zhou Y, Lin Y, Wu X, Xiong F, Lv Y, Zheng T, Huang P, Chen H (2012) The high-level expression of human tissue plasminogen activator in the milk of transgenic mice with hybrid gene locus strategy. Mol Biotechnol 50(2):137–144
Nielsen VGMR, Ley ML et al (2014) Tissue-type plasminogen activator-induced fibrinolysis is enhanced in patients with breast, lung, pancreas and colon cancer. Blood coagul fibrinolysis 25:248–253
Kalyan NK, Lee SG, Wilhelm J, Fu KP, Hum WT, Rappaport R, Hartzell RW, Urbano C, Hung PP (1988) Structure-function analysis with tissue-type plasminogen activator. Effect of deletion of NH2-terminal domains on its biochemical and biological properties. J Biol Chem 263(8):3971–3978
Rouf SA, Moo-Young M, Chisti Y (1996) Tissue-type plasminogen activator: characteristics, applications and production technology. Biotechnol Adv 14(3):239–266
Zhang L, Chopp M, Teng H, Ding G, Jiang Q, Yang XP, Rhaleb NE, Zhang ZG (2014) Combination treatment with N-acetyl-seryl-aspartyl-lysyl-proline and tissue plasminogen activator provides potent neuroprotection in rats after stroke. Stroke 45(4):1108–1114
Gaberel T, Macrez R, Gauberti M, Montagne A, Hebert M, Petersen KU, Touze E, Agin V, Emery E, Ali C, Vivien D (2013) Immunotherapy blocking the tissue plasminogen activator-dependent activation of N-methyl-d-aspartate glutamate receptors improves hemorrhagic stroke outcome. Neuropharmacology 67:267–271
Mattes R (2001) The production of improved tissue-type plasminogen activator in Escherichia coli. Semin Thromb Hemost 27(4):325–336
Obukowicz MG, Gustafson ME, Junger KD, Leimgruber RM, Wittwer AJ, Wun TC, Warren TG, Bishop BF, Mathis KJ, McPherson DT et al (1990) Secretion of active kringle-2-serine protease in Escherichia coli. Biochemistry 29(41):9737–9745
Zhang Y, Jiang P, Gao J, Liao J, Sun S, Shen Z, Qin S (2008) Recombinant expression of rt-PA gene (encoding Reteplase) in gametophytes of the seaweed Laminaria japonica (Laminariales, Phaeophyta). Sci China C 51(12):1116–1120
Davami F, Sardari S, Majidzadeh AK, Hemayatkar M, Barkhrdari F, Omidi M, Azami M, Adeli A, Davoudi N, Mahboudi F (2010) Expression of a novel chimeric truncated t-PA in CHO cells based on in silico experiments. J Biomed Biotechnol 2010:108159
Gouveia RM, Morais VA, Peixoto C, Sousa M, Regalla M, Alves PM, Costa J (2007) Production and purification of functional truncated soluble forms of human recombinant L1 cell adhesion glycoprotein from Spodoptera frugiperda Sf9 cells. Protein Expr Purif 52(1):182–193
Masroori N, Halabian R, Mohammadipour M, Roushandeh AM, Rouhbakhsh M, Najafabadi AJ, Fathabad ME, Salimi M, Shokrgozar MA, Roudkenar MH (2010) High-level expression of functional recombinant human coagulation factor VII in insect cells. Biotechnol Lett 32(6):803–809
Jahanian-Najafabadi A, Bouzari S, Oloomi M, Roudkenar MH, Mayr LM (2012) Attempts to express the A1-GMCSF immunotoxin in the baculovirus expression vector system. Biosci Biotechnol Biochem 76(4):749–754
Ebert KM, DiTullio P, Barry CA, Schindler JE, Ayres SL, Smith TE, Pellerin LJ, Meade HM, Denman J, Roberts B (1994) Induction of human tissue plasminogen activator in the mammary gland of transgenic goats. Biotechnology 12(7):699–702
Yang QEHY, Zhou GB, Yang ZQ, Zhu SE (2007) Stepwise in-straw dilution and direct transfer using open pulled straws (OPS) in the mouse: a potential model for field manipulation of vitrified embryos. J Reprod Dev 53:211–218
Acknowledgments
This study was supported by the National Major Special Projects on New Cultivation for Transgenic Organisms (2011ZX08008-004), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Jiangsu Province Science and Technology Support Project (BE2013679) and the Graduate Research and Innovation Projects in Yangzhou University (CXLX-1435). We thank our teacher, Professor Yong-Cheng, for providing technical assistance. We would also like to thank the laboratory personnel for their roles in helping with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, S., Ge, X., Cheng, Y. et al. High-level expression of a novel recombinant human plasminogen activator (rhPA) in the milk of transgenic rabbits and its thrombolytic bioactivity in vitro. Mol Biol Rep 43, 775–783 (2016). https://doi.org/10.1007/s11033-016-4020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4020-0