Abstract
Objective
To investigate the feasibility of producing human IgG1 Fc fragment fused factor IX (FIX-Fc) in the milk of transgenic animals, for an alternative possible solution to the unmet need of FIX-Fc products for hemophilia B treatment.
Results
Six founder lines of transgenic mice harboring FIX-Fc cassette designed to be expressed specifically in the mammary gland were generated. FIX-Fc protein was secreted into the milk of transgenic mice with preserved biological activity (with the highest value of 6.2 IU/mL), similar to that of the non-fused FIX transgenic milk. RT-PCR and immunofluorescence analysis confirmed that FIX-Fc was specifically expressed in the mammary gland. The blood FIX clotting activities were unchanged, and no apparent health defects were observed in the transgenic mice. Moreover, the stability of FIX protein in milk was increased by the Fc fusion.
Conclusions
It is feasible to produce biologically functional FIX-Fc in the mammary gland of transgenic mice. Our preliminary results provide a foundation for the potential scale-up production of FIX-Fc in the milk of dairy animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophilia B is an X chromosome-linked bleeding disorder caused by the deficiency in coagulation factor IX (FIX) and affects about 80,000 males worldwide (Nuttall 2007). Patients with hemophilia B might suffer from severe joint damage and life-threatening bleeding complications that are caused by repeated spontaneous or trauma-induced bleeding. Currently, the standard treatment for hemophilia B is protein replacement therapy with plasma-derived or recombinant FIX concentrates. However, due to the short serum half-life of FIX (14–27 h), patients have to receive frequent intravenous injections, making the treatment adherence a difficulty (Björkman 2013). Long-acting FIXs, such as human IgG1 Fc fragment or human serum albumin fused FIX, have been developed to reduce the injection frequency (Powell et al. 2013; Santagostino et al. 2016). Clinical studies showed prolonged pharmacokinetics and pharmacodynamics of these fusion proteins, making them the next generation drugs for hemophilia B treatment (Carcao 2014). At present, commercially available FIX-Fc concentrate is manufactured by mammalian cell cultures. The average annual cost for a moderate or severe hemophilia B patient treated with FIX-Fc is approximately $700,000 in the U.S.A (Tortella et al. 2018). The high cost of this product has limited its clinical use for prophylactic treatments in developed countries and precluded its use in most of the developing countries (von Mackensen et al. 2017). Therefore, seeking other sources of affordable long-acting FIX is vital.
The mammary glands of transgenic dairy animals are promising substitutes for mammalian cell cultures, as they can produce cost-effective therapeutic proteins in large quantities (Houdebine 2009; Maksimenko et al. 2013). There are already two milk-derived product, human C1 inhibitor and human antithrombin, produced from the milk of transgenic rabbits and goats, respectively, have been approved for clinical use by the US-FDA and EMA. These products showed promising safety and efficacy in clinical trials and demonstrated the commercial reality of producing biodrugs by transgenic animals (Paidas et al. 2014; Riedl et al. 2017). Recently, the rapid developments in gene-editing technology, such as CRISPR/Cas system, are revolutionizing this field by circumventing the limitations for the production of transgenic dairy animals (Bertolini et al. 2016). Thus, the commercial application of mammary glands of transgenic animals for biodrugs production could promising in the near future. Previously, we and others have reported producing FIX in the milk of transgenic mice, sheep, goats, and pigs (Clark et al. 1989; Yan et al. 2006; Lisauskas et al. 2008; Amiri Yekta et al. 2013; Lee et al. 2014). These milk FIXs were presented with high antigen and bioactivity levels, suggesting mammary glands are capable of producing complex recombinant proteins, such as coagulation factors. Taken together, it is reasonable to express bioengineered FIXs, for example FIX-Fc, in the transgenic mammary glands to produce affordable long-acting FIXs.
The overall objective of this study was to evaluate the feasibility of producing FIX-Fc in the milk of transgenic animals. FIX-Fc transgenic mice were generated that possess a mammary gland-specific FIX-Fc fusion gene. The FIX-Fc fusion gene expression were determined, and our result showed that FIX-Fc protein could be secreted in the milk of transgenic mice with preserved bioactivity.
Materials and methods
Plasmid construction
The pcDNA3.1-P1A3-FIX plasmid (P1A3-FIX), constructed in our previous research (Yan et al. 2006), was used as a template to generate the FIX-Fc fusion expression vector. The P1A3-FIX construct contains a 6.5 kb goat β-casein promoter (named P1A3, mammary gland-specific) and a FIX minigene, which includes a full-length FIX cDNA and an 800 bp truncated FIX intron I sequence. The FIX minigene was a hyperactive mutant, and the bioactivity was reported to be 2.5–3 times that of the wild-type FIX (Chang et al. 1998). Human IgG1 Fc cDNA (hinge, CH2 and CH3 domains) was introduced into the P1A3-FIX plasmid directly after the FIX minigene by fusion PCR method to construct the FIX-Fc fusion expression plasmid (P1A3-FIXFc). The P1A3FIX and the P1A3-FIXFc plasmid only differ with the Fc part. The schematic structures of the two vectors are shown in Fig. 1.
Transgene constructs schematic for P1A3-FIXFc plasmid. The P1A3-FIX plasmid was used as a template to construct the Fc fusion expression vector. Human IgG1 Fc fragment cDNA was fused directly after FIX minigene, generating the P1A3-FIXFc construct. The P1A3-FIXFc plasmid was linearized before microinjection. The linearized P1A3-FIX fragment contains P1A3 promoter (goat β-casein promoter), FIX minigene (including a full-length human FIX cDNA and a 0.8 kb truncated intron I sequence), and a BGH poly A sequence
Transgenic mice generation
All animal procedures were approved by the Institutional Animal Care and Use Committee at Shanghai Children’s Hospital and were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals. The ICR mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. and housed in a pathogen-free facility at 25 ± 1 °C on a 12-h light/ dark cycle with free access to food and tap water.
Transgenic mice were produced by microinjecting purified, Sal I linearized fragment of plasmid (P1A3-FIXFc) as described previously (Yan et al. 2006). Genomic DNA was isolated from the tail tips for PCR analysis of FIX-Fc transgenic mice. The primers used are listed in Table 1. The founder mice were mated with wild-type (WT) mice to obtain the F1 generation and the same method was used to obtain F2 generations. Milk samples were collected from nursing mice 9–11 days after parturition, as previously described (Yan et al. 2006). For western blot analysis, the milk samples were diluted with two volumes of PBS and defatted by centrifugation at 4 °C for 15 min at 20,000×g. Control milk was obtained from WT mice and treated in the same fashion. Blood samples were collected from mouse tail and anti-coagulated with 0.38% sodium citrate (Sigma; 9:1 v/v). Plasma was obtained by centrifugation at 1500×g for 15 min at room temperature.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol (Invitrogen, China) from the mammary gland, heart, liver, spleen, lung, kidney, and brain of transgenic and WT mice. About 1 μg total RNA was reverse transcribed to obtain cDNA with Reverse Transcriptase M-MLV and Ribonuclease Inhibitor (Takara Biotechnology, China) using oligo-dT primers. RT-PCR was processed with specific primers for the FIX-Fc cDNA and mouse glyceraldehyde-3-phosphate dehydrogenase (mGAPDH) gene, respectively. The sequences of the primers are listed in Table 1.
Quantitative PCR (qPCR)
Genomic DNA collected from the mouse tail was used to determine the copy number of FIX-Fc gene by using qPCR analysis. The sequences of primer pairs specific for human FIX and mTF gene (mouse transferrin, internal control) are listed in Table 1. P1A3-FIX and T-mTF plasmids were used to construct the standard curve (108, 107, 106, 105, 104 copies of the target sequence). Each PCR reaction contained 250 ng DNA, 1 pmol primer, and 12.5 μL SYBR Premix (Takara Biotechnology) in a final reaction volume of 25 μL. The assay was performed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, CA).
Western blot
Western blot analysis was performed according to the standard procedures. Proteins from 10 µL defatted transgenic milk were separated using SDS-PAGE and transferred to PVDF membrane (Bio-Rad, China). For denatured analysis, the samples were boiled at 95 °C for 5 min with reducing loading buffer before SDS-PAGE separation. Mouse anti-human FIX antibody (1:1000, ab97619, Abcam, USA) and HRP-conjugated rabbit anti-mouse IgG (1:2000, DAKO, Denmark) were used to detect FIX-Fc. The bands were visualized by chemiluminescence with an enhanced ECL system (Thermo Fisher, USA). BeneFIX, a commercial, wild-type and mammalian cell-derived FIX, was purchased from Pfizer Inc. (Beijing, China) and used as positive control.
FIX antigen
The FIX concentration was measured using a specific ELISA kit (SEK11503, Sino Biological Inc., China), according to the manufacturer's instructions. Briefly, milk samples were diluted and determined by sandwich ELISA, and the absorbance was measured at 450 nm (Synergy2, BioTek, USA).
FIX activity
The FIX clotting activity levels were determined by aPTT analysis (one-stage activated partial thromboplastin time, 0020006800, Werfen, USA). Samples were diluted and added with the FIX-deficient human plasma (0020011900, Werfen, USA). The standard curve was constructed using the reference standard (pooled human plasma, 0020003110, Werfen) and the FIX activity was calculated by line comparison against the reference standard on ACL Top 700 automatic blood coagulation analyzer (Werfen, USA.).
APTT analysis was also used to determine the milk FIX activity at different storage conditions over time, to examine the effect of Fc fragment for the stability of FIX in the transgenic milk. The FIX-Fc transgenic milk samples were pooled to make a mixture with FIX activity at ~ 1 IU/mL. The non-transgenic milk was added with non-fused FIX to make the control sample, with the same final FIX activity (~ 1 IU/mL). The non-fused FIX added was the same hyperactivity mutant with the FIX part of FIX-Fc protein, and was purified from transgenic bovine milk (purity > 95%, the transgenic cow was constructed with the Sal I linearized fragment of P1A3-FIX plasmid). The two groups of milk samples were incubated at 4 °C (the storage condition) and 37 °C (the physiological condition) for 0, 24, 48, 72, 96, 120 h and 0, 2, 4, 8, 16, 32, 48, 72, 96, 120 h, respectively. Then the samples were tested for the FIX activity using aPTT analysis.
Immunofluorescence analysis
Major tissues (mammary gland, heart, liver, spleen, lung, kidney, brain) of the transgenic and WT mice (negative control) were collected for immunofluorescence analysis. Serial cryosections (5–10 μm) were stained with rabbit-anti-human FIX antibodies (1:200, ab97619, Abcam, USA) and goat-anti-rabbit IgG antibodies conjugated with Cy3 (1:500, A0516, Beyotime Biotechnology, China), and the nuclei were counter-stained with DAPI (5 μg/mL, Sigma). The sections were viewed with a fluorescence microscope (Leica, Germany) and the pictures were merged using microscope software CW4000 (Leica).
Statistics
Data were presented as mean ± SEM and analyzed using GraphPad Prism 7. Statistical comparisons between experimental groups were assessed by student's 2-tailed t-test or two-way RM (Repeated Measure) ANOVA followed by Bonferroni’s multiple comparisons test. Differences were considered significant when P values were less than 0.05.
Results
Generation of the FIX-Fc transgenic mice
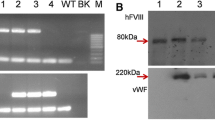
The FIX-Fc transgenic mice were generated using the β-casein promoter-driven FIX-Fc plasmid (Fig. 1). Six transgenic founders, four males (#8, #5, #20, and #44) and two females (#17 and #21) were established and identified by PCR analysis (Fig. 2a). The founders were mated with WT mice, resulting in 17 female transgenic mice litters. The transgene copy number in each line of the transgenic mice was determined by qPCR. As listed in Table 2, the transgene copy number varied in different mouse lines, ranging from 2 to 28, among which, founder #44 presented with the highest value.
Identification of the transgenic mice and expression of FIX-Fc in the milk of transgenic mice. a Genotyping of the P1A3-FIXFc transgenic mice by PCR analysis. P1A3-FIXFc plasmid served as a positive control (PC), and non-transgenic mice DNA served as a negative control (NC). b Western blot analysis of the milk samples from transgenic mice (FIXFc, representative results of founder #17 and its F2 progeny #614) and non-transgenic wild-type mice (WT, negative control). BeneFIX, a commercial, non-fused FIX, was set as positive controls (BF). A non-specific band (* in the figure) was observed under the reducing condition
Expression of FIX-Fc in the milk of transgenic mice
Mid-lactation stage (9–11 days) milk samples were collected from 19 transgenic mice for the detection of FIX-Fc expression. APTT analysis, a classic method for FIX activity determination, was employed to determine the FIX activity in the transgenic milk. The FIX clotting activity was detectable in all the milk samples from the transgenic-positive mice. The average FIX activity in the FIX-Fc transgenic milk was 1.46 ± 0.37 IU/mL, with the highest value of 6.20 IU/mL, in #17 (Table 2). The clotting activity was undetectable in the samples from non-transgenic littermates control. The FIX antigen was determined by a human FIX specific ELISA kit. The average FIX-Fc protein concentration in the transgenic milk was 514.2 ± 141.6 μg/L, and the highest concentration reached was 2500 μg/L in #17 (Table 2). The FIX-Fc antigen levels seemed to correlate well with their activity levels (R = 0.986, P < 0.001).
Western blot analysis was used to detect FIX-Fc protein expression in the transgenic milk. As shown in Fig. 2b, there were strong bands for FIX-Fc proteins under both reducing and non-reducing conditions. Under reducing conditions, the molecular mass (MM) for FIX-Fc (~ 75 kDa) was larger than that of the commercial recombinant FIX control (~ 55 kDa), owing to the fusion of Fc fragment. Under non-reducing conditions, the FIX-Fc band was approximately 150 kDa, which was about twice of that under reducing conditions. These results were consistent with our prediction that the FIX-Fc proteins exist as a dimer form in the milk. In our design, each single Fc chain was linked with a FIX molecular, thus a (FIX-Fc)2 homodimer would be formed when the Fc part dimerized through the disulfide bond in the hinge regain.
Expression of FIX-Fc in various tissues of transgenic mice
RT-PCR and immunofluorescence analyses were used to examine the tissue-specific expression of FIX-Fc in transgenic mice. As shown in Fig. 3a, the FIX-Fc transcripts were found in lactating mammary glands, but not in other tested tissues (heart, liver, spleen, lung, kidney, and brain). Immunofluorescence analysis further confirmed that FIX-Fc protein was specifically expressed in the transgenic mammary glands, with bright intracellular FIX signals in the whole mammary gland surrounded by bright FIX staining in extracellular spaces (Fig. 3b, c). No apparent changes in the structures of the tested organs were noted.
Expression of FIX-Fc in various tissues of transgenic mice. a RT-PCR results of FIX-Fc expression in the various tissues of transgenic mice. P1A3-FIXFc plasmid served as a positive control (PC), and the mammary gland cDNA of WT mice was used as a negative control (NC). Mouse GAPDH was amplified to normalize DNA used in each PCR reaction. b Immunofluorescence analysis of mammary gland tissues from lactating transgenic mice and WT mice. c Various tissues of transgenic mice, including mammary gland, heart, liver, spleen, lung, kidney, and brain from the lactating transgenic mice. The positive stain for FIX was red (stained with specific anti-FIX antibody), and the stain for cell nucleus was blue (stained with DAPI). The scale bar was 100 μm. Original magnifications: × 100. d APTT analysis of the blood FIX clotting activity of the transgenic (n = 12) and WT mice (n = 4). Statistical comparisons between the two groups were assessed by student's 2-tailed t test
The blood FIX clotting activity of lactating mice was determined by aPTT analysis, and no differences were observed between the transgenic and WT mice (Fig. 3d), showing that the blood clotting capability in these transgenic mice remained unchanged.
Fc-fragment effect on stabilization of the FIX-Fc protein in the milk of transgenic mice
FIX activity in the milk was monitored for different storage conditions (4 °C for the storage condition, and 37 °C for the physiological condition) at different time points to assess the stability effect from the Fc-fragment. The non-transgenic milk added with purified non-fused FIX protein, that was same hyperactive mutant form as the FIX part of the FIX-Fc protein, was used as control. As shown in Fig. 4a, under 37 °C condition, the clotting activity of non-fused FIX decreased rapidly over time, with ~ 50% activity lost at 4 h, and subsequently became undetectable at 48 h. While in the FIX-Fc group, the clotting activity began to decline after 48 h at 37 °C, with ~ 50% activity lost at 72 h, demonstrating a postponed degradation rate. Under 4 °C condition, the overall trend of the clotting activity decline was comparable in the two groups, with ~ 50% activity lost at 72 h (Fig. 4b).
Fc fragment enhanced FIX-Fc protein stability in the milk of transgenic mice. Milk samples were collected and pooled for transgenic and non-transgenic mice, respectively. Control samples were prepared by adding corresponding non-fusion FIX into the non-transgenic milk to comparable activity levels (~ 1 IU/mL). The bioactivity of FIX or FIX-Fc was measured by aPTT assay for different temperatures a 37 °C, physiological condition and b 4 °C, short storage condition. The values were determined from three independent experiments, ***P < 0.001, ****P < 0.0001
Discussion and conclusion
In this study, we generated a transgenic mouse model expressing recombinant FIX-Fc in the mammary gland and the FIX-Fc protein was secreted into the milk with preserved coagulation activity. Thus, it is feasible to produce bioactive FIX-Fc in the milk of transgenic animals.
The transgenic mice were generated using the FIX-Fc fusion expression vector which has a human IgG1 Fc cDNA inserted into a FIX minigene construct that we previously reported to be able to expressed in the milk of transgenic mice (Yan et al. 2006). The FIX minigene in these constructs are in a hyperactive mutant form that previously shown to have a 2.5–3 times higher bioactivity than that of the wild-type FIX (Chang et al. 1998).
The average FIX activity obtained from the FIX-Fc milk was slightly higher than that from the non-fused FIX milk in the previous work (1.46 ± 0.37 IU/mL versus 1.03 ± 0.88 IU/mL), suggesting the FIX activity was well-preserved when expressed as an Fc fusion protein. Consistent with previous reports, we observed that the FIX clotting activity levels in the transgenic milk were not strictly correlated with the transgene copy number among different mouse lines. This might be a common phenomenon in transgene animals generated by random integration of foreign gene according to these reports (Kong et al. 2009; Zhang et al. 2012; Qian et al. 2014). Several factors, such as position-effect and promoter methylation, may also play important roles in modulating the transgene expression.
In this study, the FIX-Fc fusion construct displayed the same mammary-specific property as the non-fusion FIX construct from our previous report, when driven by a β-casein promoter. The FIX-Fc transcripts and protein were only detected in the lactating mammary gland, and not in other tissues tested using RT-PCR and immunofluorescence analyses.
Fc fragment is reported to increase the stability of Fc fusion proteins in mammalian cell cultures (Carter 2011; Czajkowsky et al. 2012). In this study, we observed that the clotting activity in FIX-Fc milk declined much slower than in non-fusion FIX milk over time at 37 °C. The amino acid sequences of the FIX-Fc and the non-fused FIX protein were identical except the Fc part. Our results support that Fc fragment might exert a stabilization effect for the FIX-Fc fusion protein in milk. As this phenomenon was observed at 37 °C (the physiological temperature) but not at 4 °C, it is possible that Fc fragment might protect the FIX protein from proteolytic degradation by enzymes such as plasmin that wildly exist in milk (Samis et al. 2000; Zhao et al. 2015; Green et al. 2006; Caroprese et al. 2007; Ismail and Nielsen 2010). Fc fragment might increase the resistance of the FIX protein to the proteolytic enzymes by masking the cleavage sites or by binding to certain milk proteins that might act as stabilizers. Beyond this benefit, Fc fusion proteins can be purified through protein-G/A affinity chromatography, so that it is possible to purify Fc fusion proteins from milk at a more reasonable cost (Carter 2011). Taken together, Fc fusion technology might be helpful in the large-scale production of recombinant proteins in transgenic livestock.
We noticed that the clotting activity levels of FIX-Fc transgenic milk were comparable to its non-fusion counterparts, yet the FIX-Fc antigen levels were lower (514.2 ± 141.6 vs. 4510.9 ± 1709.8 μg/L) (Yan et al. 2006). This result is not likely a result of the ELISA kit used (see “Materials and methods” section), since similar result were observed when repeated using a different ELISA kit (abcam 108,831) for FIX-Fc antigen determination, (data not shown). We suspect that it is possible the Fc fragment might affect the part that the FIX conjugate to its antibody. Furthermore, as the current system was optimized for producing the native FIX gene that included a full FIX cDNA and an 800 bp truncated FIX intron I sequence, specified optimization might be needed to increase the expression efficiency of Fc fusion genes in transgenic mammary glands. Future efforts will be directed towards optimizing FIX-Fc vector by using additional cis-acting elements, intron sequences or signal peptide, etc. Besides, the extended half-life of FIX-Fc in vivo needs to be further defined.
The fact that no changes in the blood FIX activity was detected between the transgenic and WT mice, suggesting that blood coagulation is not affected by the FIX-Fc expression in the transgenic mice. No obvious health side effects were observed in the FIX-Fc transgenic mice.
In conclusion, we demonstrated that FIX-Fc fusion protein could be specifically secreted into the milk of transgenic mice with preserved biological activity. This work provides supporting evidence for the study of expressing Fc fused recombinant coagulation factors and other proteins at an industrial scale from dairy animals, such as cows or goats, which have potential clinical applications.
References
Amiri Yekta A, Dalman A, Eftekhari-Yazdi P et al (2013) Production of transgenic goats expressing human coagulation factor IX in the mammary glands after nuclear transfer using transfected fetal fibroblast cells. Transgenic Res 22:131–142. https://doi.org/10.1007/s11248-012-9634-y
Bertolini LR, Meade H, Lazzarotto CR et al (2016) The transgenic animal platform for biopharmaceutical production. Transgenic Res 25:329–343. https://doi.org/10.1007/s11248-016-9933-9
Björkman S (2013) Population pharmacokinetics of recombinant factor IX: implications for dose tailoring. Haemophilia 19:753–757. https://doi.org/10.1111/hae.12188
Carcao M (2014) Changing paradigm of prophylaxis with longer acting factor concentrates. Haemophilia 20:99–105. https://doi.org/10.1111/hae.12405
Caroprese M, Schena L, Marzano A et al (2007) Contribution of macrophages to plasmin activity in ewe bulk milk. Ital J Anim Sci 6:545–547. https://doi.org/10.3168/jds.2006-691
Carter PJ (2011) Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res 317:1261–1269. https://doi.org/10.1016/j.yexcr.2011.02.013
Chang J, Jin J, Lollar P et al (1998) Changing residue 338 in human factor IX from arginine to alanine causes an increase in catalytic activity. J Biol Chem 273:12089–12094. https://doi.org/10.1074/jbc.273.20.12089
Clark AJ, Bessos H, Bishop JO et al (1989) Expression of human anti-hemophilic factor ix in the milk of transgenic sheep. Bio/Technology 7:487–492. https://doi.org/10.1038/nbt0589-487
Czajkowsky DM, Hu J, Shao Z, Pleass RJ (2012) Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 4:1015–1028. https://doi.org/10.1002/emmm.201201379
Green KA, Nielsen BS, Castellino FJ et al (2006) Lack of plasminogen leads to milk stasis and premature mammary gland involution during lactation. Dev Biol 299:164–175. https://doi.org/10.1016/j.ydbio.2006.07.021
Houdebine L-M (2009) Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis 32:107–121. https://doi.org/10.1016/j.cimid.2007.11.005
Ismail B, Nielsen SS (2010) Invited review: plasmin protease in milk: current knowledge and relevance to dairy industry. J Dairy Sci 93:4999–5009. https://doi.org/10.3168/jds.2010-3122
Kong Q, Wu M, Huan Y et al (2009) Transgene expression is associated with copy number and cytomegalovirus promoter methylation in transgenic pigs. PLoS ONE 4:e6679. https://doi.org/10.1371/journal.pone.0006679
Lee MH, Lin YS, Tu CF, Yen CH (2014) Recombinant human factor IX produced from transgenic porcine milk. Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/315375
Lisauskas SFC, Cunha NB, Vianna GR et al (2008) Expression of functional recombinant human factor IX in milk of mice. Biotechnol Lett 30:2063–2069. https://doi.org/10.1007/s10529-008-9818-y
Maksimenko OG, Deykin AV, Khodarovich YM, Georgiev PG (2013) Use of transgenic animals in biotechnology: prospects and problems. Acta Naturae 5:33–46
Nuttall GA (2007) Hemostasis and thrombosis: basic principles and clinical practice, 5th ed. Lippincott Williams & Wilkins, Philadelphia
Paidas MJ, Forsyth C, Quéré I et al (2014) Perioperative and peripartum prevention of venous thromboembolism in patients with hereditary antithrombin deficiency using recombinant antithrombin therapy. Blood Coagul Fibrinolysis 25:444–450. https://doi.org/10.1097/MBC.0000000000000076
Powell JS, Pasi KJ, Ragni MV et al (2013) Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med 369:2313–2323. https://doi.org/10.1056/NEJMoa1305074
Qian X, Kraft J, Ni Y, Zhao FQ (2014) Production of recombinant human proinsulin in the milk of transgenic mice. Sci Rep 4:1–8. https://doi.org/10.1038/srep06465
Riedl MA, Grivcheva-Panovska V, Moldovan D et al (2017) Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio-oedema: a phase 2, multicentre, randomised, double-blind, placebo-controlled crossover trial. Lancet 390:1595–1602. https://doi.org/10.1016/S0140-6736(17)31963-3
Samis JA, Ramsey GD, Walker JB et al (2000) Proteolytic processing of human coagulation factor IX by plasmin. Blood 95:943–951
Santagostino E, Martinowitz U, Lissitchkov T et al (2016) Long acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood 127:1–33. https://doi.org/10.1182/blood-2015-09-669234
Tortella BJ, Alvir J, McDonald M et al (2018) Real-world analysis of dispensed IUs of coagulation factor IX and resultant expenditures in hemophilia B patients receiving standard half-life versus extended half-life products and those switching from standard half-life to extended half-life products. J Manag Care Spec Pharm 24:643–653. https://doi.org/10.18553/jmcp.2018.17212
von Mackensen S, Kalnins W, Krucker J et al (2017) Haemophilia patients’ unmet needs and their expectations of the new extended half-life factor concentrates. Haemophilia 23:566–574. https://doi.org/10.1111/hae.13221
Yan J-B, Wang S, Huang W-Y et al (2006) Transgenic mice can express mutant human coagulation factor IX with higher level of clotting activity. Biochem Genet 44:347–358. https://doi.org/10.1007/s10528-006-9034-1
Zhang R, Cui D, Wang H et al (2012) Functional recombinant human anti-HBV antibody expressed in milk of transgenic mice. Transgenic Res 21:1085–1091. https://doi.org/10.1007/s11248-012-9589-z
Zhao J, Xu W, Ross JW et al (2015) Engineering protein processing of the mammary gland to produce abundant hemophilia B therapy in milk. Sci Rep 5:1–13. https://doi.org/10.1038/srep14176
Acknowledgements
This work was supported by the National Key Research and Development Program (2016YFC0905102, 2016YFC1000503, 2019YFA0801402), National Natural Science Foundation (81500108), National Basic Research Program (2014CB964703), National Science and Technology Major Project of China (2016ZX08012-002), Clinical Medical Center and Key Disciplines Construction Grogram of Shanghai (2017ZZ02019), Shanghai Science and Technology Support Program (13431901300), and KingMed Diagnostics Project of Academician Workstation (2017B090904030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, H., Gong, X., Xu, M. et al. Production of biologically active human factor IX-Fc fusion protein in the milk of transgenic mice. Biotechnol Lett 42, 717–726 (2020). https://doi.org/10.1007/s10529-020-02808-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02808-1