Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that can cause significant social, communication and behavioral challenges. Environmental contribution to ASD is due in large part to the sensitivity of the developing brain to external exposures such as lead (Pb), and mercury (Hg) as toxic heavy metals or due to a poor detoxification ability as the phenotype of this disorder. Selenium (Se) as an antioxidant element that counteracts the neurotoxicity of Hg, and Pb, presumably through the formation of nontoxic complexes. In the present study, Pb, Hg, and Se were measured in red blood cells (RBCs) of 35 children with ASD and 30 age- and gender-matched healthy control children using atomic absorption spectrometry. Receiver Operating Characteristics (ROC) analysis of the obtained data was performed to measure the predictive value of their absolute and relative concentrations. The obtained data demonstrates a significant elevation of Hg and Pb together with a significant decrease in the Se levels in RBCs of patients with ASD when compared to the healthy controls. The ratios of Se to both Pb and Hg were remarkably altered, being indicative of heavy metal neurotoxicity in patients with ASD. In conclusion, the present study indicates the importance of Se for prevention and/or therapy of heavy metal neurotoxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) as a neurodevelopmental disorder clinically presented as deficits in social interaction, repetitive behavior and intellectual deficits (APA 2013; Christensen et al. 2016). Typically, initial signs and symptom of ASD are often noticeable in the early developmental period such as age of the first smile, response to their names, pointing to objects, and ability to play with peers (Huang et al. 2014). However, behavioral patterns and social deficits might not be recognized as indications of ASD until a child is unable to interact socially, or demonstrate cognitive disability or other significant life-stage demands. This complicates the healthcare practitioner’s ability to diagnose it early enough (Johnson et al. 2016).
The incidence of ASD has remarkably grown during the last decades (Elsabbagh et al. 2012). Up to the DSM-IV diagnostic criteria, the most recent recorded prevalence of ASD reach one per 68 which is more than one hundred-fold higher than that recorded earlier in the 1990s, with a 4:1 male to female prevalence ratio (Rutter 2005). The most recent study demonstrated that the worldwide incidence of ASD is nearly 66 of 10,000 children (Hill et al. 2015). Certain studies propose that the observed increase at least partially occurred due to improved diagnosis of ASD (Rimland 2000). However, it has been observed that improved diagnosis, younger ages at diagnosis, and the inclusion of milder cases do not explain the remarkable increase in the incidence of ASD (Hertz-Picciotto and Delwiche 2009).
There is significant evidence that nutritional, immunological, and environmental factors are greatly contributed in the etiology of ASD (London 2000; Herbert 2010; Bjørklund and Chartrand 2016; Bjørklund et al. 2016; Endreffy et al. 2016). In particular, a significant association between ASD and environmental pollution in general and organic pollutants (Kalkbrenner et al. 2010) and metals, in particular, has been demonstrated (Bjørklund 2013; Roberts et al. 2013; Khaled et al. 2016). Many studies show the sensitivity of the developing brain to environmental exposure to lead (Pb) and mercury (Hg) as two heavy metals (Landrigan 2010).
Mercury has been implicated as an environmental risk factor for ASD (Schultz 2010; Kern et al. 2015; Bjørklund et al. 2017a; Khaled et al. 2016) due to its neurotoxic properties (Castoldi et al. 2001; Kern et al. 2012). In particular, a significant correlation between Hg exposure (Mutter et al. 2005) and the level of Hg in the organism (Kern et al. 2016) and the risk of ASD has been ascertained. Mercury accumulation in autistic children may be due to zinc (Zn) deficiency and the consequence metallothionein (MT) dysfunction (Bjørklund 2013). In fact, research indicates that children with ASD are at risk to Zn deficiency, copper (Cu) toxicity, and disturbed MT system functioning (Bjørklund 2013; Li et al. 2014; Macedoni-Lukšič et al. 2015; Crăciun et al. 2016). At the same time, certain contradictions regarding Hg exist (Ip et al. 2004).
Exposure to Pb is known to disrupt normal physiological processes and neurological development of children (Lanphear et al. 2005; Mostafa et al. 2016a). In some children with ASD, increased levels of blood lead (BPb) may induce the production of serum anti-ribosomal P antibodies (Mostafa et al. 2016a). The mechanism of Pb toxicity is mediated through substitution of calcium (Ca) and Zn by Pb (Mason et al. 2014). In the developing brain, Pb can induce an inappropriate release of neurotransmitters and disrupts brain function at a much lower concentration of Ca (Zawia 2003; Qiu et al. 2010; El-Ansary et al. 2011). Using a log-linear model, Lanphear et al. (2005) through the use of a log-linear model, recorded a negative correlation between IQ and the increase BPb levels (a 6.9 IQ point decrease for an increase of BPb from 2.4 to 30 μg/dL). They conclude that exposure of children who have maximal BPb levels <7.5 μg /dL to environmental Pb is associated with intellectual deficits. A “cognitive reserve” may protect from the effects of Pb on cognitive performance (Bleecker et al. 2007). There is some evidence that the neurobehavioral deficit is reversible with decreasing Pb exposure (Chuang et al. 2005; Winker et al. 2005, 2006). Despite the presence of data on Pb neurotoxicity, its impact on ASD is unclear. Certain studies demonstrated significantly higher levels of Pb in hair (Mohamed Fel et al. 2015), and blood of children with ASD (Macedoni-Lukšič et al. 2015). The research groups did not observe a significant increase in hair (Skalny et al. 2016a), blood (Tian et al. 2011) and teeth (Adams et al. 2007) Pb content in children with ASD. At the same time, erythrocyte (El-Ansary et al. 2011) and blood (Tian et al. 2011) Pb levels significantly correlated with plasma neurotransmitters and gene expression in children with ASD, respectively. Moreover, a growing body of data provided a background for considering ASD as a form of both Pb and Hg toxicity (Yassa 2014).
Selenium (Se) is an essential trace element having a very narrow range between deficient, essential, and toxic doses (Roman 2016). Earlier studies demonstrated that Se has a neuroprotective effect due to its antioxidant and anti-inflammatory properties (Bräuer and Savaskan 2004). In particular, research indicates that Se and selenoproteins play a role in the protection against cognitive decline, including Alzheimer’s disease (Deng et al. 2015; Aaseth et al. 2016). Selenium is an antagonist to Hg and acts as a natural protective agent in Hg neurotoxicity (Bjørklund 2015; Bjørklund et al. 2017b). It is also notable that the increasing body of data demonstrated that Se has not only a neuroprotective but also a neurotoxic effect (Vinceti et al. 2014).
Moreover, it is well known that trace element ratios are more important than their individual or absolute levels being indicative of the antagonistic interactions between the elements. In particular, the review of the existing studies demonstrated that the Hg/Se ratio is significantly associated with neurological outcome (Skalny et al. 2016b). However, the existing data on the level of Hg, Pb, Se, and especially their ratio in ASD are rather contradictory.
Therefore, the objective of the present study was to assess the relative abundance of Pb, Hg, and Se in red blood cells (RBCs) of ASD children in comparison to healthy age- and gender-matched control children.
Material and methods
Compliance with ethical standards
The protocol of the present study was approved by the Ethical Committee of the Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia. The parents or the legal guardians of the investigated participants, according to the Helsinki principles, signed a written consent to participation in this study.
Participants
The present study was conducted on 35 male children with ASD. They were recruited from the Autism Research and Treatment Center, Faculty of Medicine, King Khalid Hospital, Riyadh, Saudi Arabia. The children were attending the Well Baby Clinic at King Khalid University Hospital for routine check-ups of their growth parameters. The patients met the criteria for ASD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) (APA 2000). The range of the children with ASD varied between 3 and 12 years (7.0 ± 2.34 years). Patients who had associated neurological diseases (such as tuberous sclerosis and cerebral palsy), metabolic disorders (e.g., phenylketonuria), concomitant infection, allergic manifestations, or autoimmune disorders were excluded from the study.
The control group comprised 30 age- and sex-matched neurotypical children. Their mean age was 7.2 ± 2.14 years. They were recruited from the Well Baby Clinic at King Khalid University Hospital, Riyadh, Saudi Arabia. The control children were normal healthy children and were unrelated to the ASD children. They demonstrated no clinical findings suggestive of allergic manifestations, immunological disorders, or infections.
Blood sampling
After overnight fast, 10 ml blood samples were collected from both groups in metal-free test tubes containing sodium heparin as anticoagulant. Tubes were centrifuged at 3500 rpm at room temperature for 15 min. Red blood cells were separated by centrifugation and kept frozen at −80 °C for later metal analyses. The tubes used for collection and storage of samples were free of Hg, Pb, and Se elements. Aliquots of the samples (0.5 ml) were transported on dry ice to the central laboratory for metal analyses.
Mercury
To measure total Hg in RBCs, the flameless atomic absorption method of Magos (1971) was used. Red blood cells were diluted with saline to 20 ml, followed by the addition of 1 ml of a 1% cysteine solution, 10 ml of 8MH2 SO4 and 1 ml of SnCl2 (100 mg/ml). After immediate aeration at a constant rate of 2.5 l/min, 20 ml of 45% NaOH was added trough the reaction vessel. The SnCl2 reagent was used to release all of the inorganic Hg from the samples. Aeration was stopped after the recorder pen had settled back to within a few chart divisions (2 or 3) of its original baseline, which was approximately 1 to 1.5 min, depending on the actual aeration rate. The concentration of Hg was measured using a flameless atomic absorption Hg analyzer (model MV-253R, Sugiyamagen Environmental Science Co., Ltd., Japan), and the concentration was calculated using a standard calibration curve prepared using standard Hg concentrations.
Lead
Lead concentrations were determined in RBCs using a modified method of that described by Miller et al. (1987), and Parsons and Slavin (1993). 0.1 of RBCs were digested in 3.9 ml of 0.5 N nitric acid. Lead quantification was based on the measurement of light absorbed at 283.3 nm by the ground-state atoms of Pb from a hollow cathode lamp using atomic absorption spectrophotometry (Hitachi Polarized Zeeman Atomic Absorption Spectrophotometer 180–80, Japan).
Selenium
A Perkin-Elmer model Il00B (Perkin-Elmer, USA), atomic absorption spectrophotometer, equipped with a deuterium arc background corrector, a model HGA 700 graphite furnace, AS-70 autosampler, and a model FX-800 Epson printer were used.
Statistical analysis
The obtained data were treated with Statistica 10.0 (Statsoft, Tulsa, OK, USA). Distribution normality was assessed using Shapiro-Wilk test. Data were expressed as median and the respective 25 and 75 percentile boundaries as well as mean and the respective standard deviations (SD). As the distribution was not Gaussian, Mann-Whitney U-test was used for paired group comparisons. Correlation analysis was performed using Spearman’s rank correlation coefficient.
SPSS computer software was also used for Receiver Operating Characteristic (ROC) analysis. ROC analysis was performed as a comprehensive tool to assess the accuracy of the measured biomarkers. The area under the curve (AUC) provided a useful measure to compare different biomarkers. An AUC value close to one indicated an excellent diagnostic and predictive marker; while, a curve that was close to the diagonal (AUC = 0.5) had no diagnostic value. An AUC close to one is always accompanied by satisfactory values of specificity and sensitivity of the biomarker.
Results
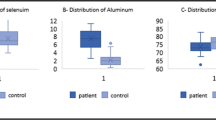
The obtained data demonstrate that ASD significantly affected the level of the studied elements (Table 1). In particular, mean erythrocyte Se levels in children with ASD were significantly lower than the control values by 42%. It was noticed that the mean values of Pb and Hg in RBC were significantly elevated by 55 and 35% as compared to the controls, respectively. The remarkable alteration of the absolute concentration of the three measured elements was reflected in their ratios (Se/Pb, Se/Hg, and Pb/Hg). While Se/Pb and Se/Hg ratios were significantly decreased by 63 and 65% in comparison to the respective control values, Pb/Hg was not significantly changed (p > 0.05).
Correlation analysis demonstrates a significant negative association between Se and toxic metal levels in erythrocytes (Table 2). At the same time, the levels of Hg and Pb were positively interrelated. Being in agreement with the group mean values, the RBC levels of Pb and Hg were significantly associated with the Se/Hg and Se/Pb ratios, respectively. It is notable that the estimated ratios of erythrocyte element concentrations (Se/Pb, Se/Hg, and Pb/Hg).
Table 3 shows the AUC, Cutoff values, specificity, and sensitivity of the measured parameters. It can be easily noticed that absolute concentration of Se and Pb together with relative levels of Se with both toxic elements (Se/Pb and Se/Hg) recorded high AUC values and satisfactory sensitivity and specificity, while Hg and Pb/Hg recorded much lower concentrations.
Discussion
The results of the present study demonstrated a significant increase in erythrocyte heavy metal (Pb and Hg) and decreased Se content. The observed elevation of erythrocyte Pb and Hg content is in agreement with the earlier data. The existing data demonstrate that environmental exposure to Pb and Hg has a significant impact on the incidence of ASD. Increased erythrocyte Hg and Pb levels were observed in children with ASD. In particular, Adams et al. (2013) found a significant 41% increase in RBC lead levels in children with ASD. In turn, Geier and the colleagues detected a significant 1.9-fold elevation of erythrocyte Hg levels in ASD (Geier et al. 2010). Also, blood Hg levels were found to be significantly associated with antineuronal antibodies (Mostafa and Refai 2007) and expression of 189 genes (Stamova et al. 2011). Correspondingly, the elevated levels of Hg and Pb were found to be increased in the hair of children suffering from ASD (Fido and Al-Saad 2005; Lakshmi Lakshmi Priya and Geetha 2011; Blaurock-Busch et al. 2011). Moreover, it has been noted that hair Pb and Hg levels are associated with ASD functioning (Lakshmi Priya and Geetha 2011).
Despite the presence of multiple studies demonstrating increased total body burden of heavy metals in ASD, the earlier study by Holmes showed a significant decrease of hair Hg levels in first baby haircuts in ASD as compared to the control values (Holmes et al. 2003). Similarly, Kern et al. (2007) revealed a significant decrease in hair toxic trace elements in ASD children. These observations allowed proposing the fact of altered heavy metal handling in children with ASD. In particular, it is hypothesized that heavy metals are sequestered in the brain of children, where they exert neurotoxic properties (Kern et al. 2007). This hypothesis is also supported by a previous work of Yorbik et al. (2010), in which urinary levels of cadmium (Cd), Pb, and chromium (Cr) of 30 children with ASD reported decreased levels when compared to control. Taken together, these data demonstrate a significant increase in the total body burden of heavy metals may be a consequence of their increased exposure, sequestration, and impaired detoxification and excretion in children with ASD.
Multiple studies have demonstrated Hg neurotoxicity (Aschner et al. 2013) including that in ASD (Geier et al. 2014). The primary mechanisms of Hg neurotoxicity may be mediated through proinflammatory and prooxidant effects of the metal (Gauba et al. 2015). Mostafa and Al-Ayadhi (2015) reported a significant positive association between the elevated levels of blood mercury (BHg) and anti-myelin binding protein autoantibodies in children with ASD compared to control healthy subjects. More recently, a potential relationship between levels of serum neurokinin A, as a pro-inflammatory neuropeptide and BHg in children with ASD was reported (Mostafa et al. 2016b).
Selenium is known to be neuroprotective due to its antioxidant and anti-inflammatory effect that is mediated through the catalytic role of Se in certain selenoproteins (Schweizer et al. 2004). Taking into account the role of oxidative stress in ASD pathogenesis (Chauhan and Chauhan 2006), one could consider Se as a protective agent in ASD due to its antioxidant effect. This supposition is supported by the observation of a significant association between genetic variants of glutathione peroxidase 1, an antioxidant selenoprotein, and susceptibility to ASD (Ming et al. 2010). However, data on Se metabolism in ASD are inconsistent. In particular, the existing studies simultaneously demonstrate a significant decrease (Jory and McGinnis 2008; Lakshmi Priya and Geetha 2011; Blaurock-Busch et al. 2012; Skalny et al. 2016c), increase (Lubkowska and Sobieraj 2009; Yasuda and Tsutsui 2013; Skalny et al. 2016a) or a lack of changes in Se levels in different human samples in ASD.
The remarkably lower Se/Hg ratio in ASD patients compared to healthy controls is in good agreement with the previous report of Sajdel-Sulkowska et al. (2008) and Alabdali et al. (2014) in which significantly higher mean blood and cerebellar levels of 3- nitrotyrosine (3-NT) as marker of oxidative stress, Hg, and the ratio of Hg/Se were recorded in patients diagnosed with ASD compared to controls.
The significantly lower Se/Pb ratio presented in the present study can be related to oxidative stress as pathological mechanism related to ASD. It is well documented that Pb and Se have antagonistic effects. Reduced Se uptake that may affect glutathione peroxidase (GPx) activity as Se- dependent enzyme may increase the susceptibility of the cell to oxidative damage (Schrauzer 1987). The protective effect of Se against Pb toxicity can be through one or more of three mechanisms previously proposed by Othman and El Missiry (1998). These mechanisms are (i) formation of an inactive Se-Pb complex; (ii) stimulation of radical scavenging through the activation of superoxide dismutase (SOD), thereby increasing the removal of the superoxide radical; and (iii) increasing the antioxidant capacity of cells indirectly by increasing the activity of glutathione reductase, which has an important role in maintaining a sufficient level of GSH in the reduced form. Based on this information, the significant lower Se/Pb reported in the present study can easily be related to all the oxidative stress related markers previously reported in patients with ASD (Al-Yafee et al. 2011). Based on the fact that Pb exposure may probably increase the susceptibility of membranes to oxidative stress by altering their integrity through deteriorating their fatty acids components, the significant increase of Pb together with the decrease of Se/Pb ratio can be related to the abnormal abundance of polyunsaturated fatty acids previously reported in ASD patients or animal models of ASD (Yiin and Lin 1995; El-Ansary and Al-Ayadhi 2014).
Also, the observed statistical interaction between Hg and Pb may be indicative of their synergistic effects (Rose et al. 2008).
In conclusion, a significant negative interaction between Se and toxic heavy metals, Pb and Hg, in children with ASD was revealed in the present study. The observed interactions may be indicative of biochemical antagonism between the studied elements, especially regarding neurotoxicity. It is hypothesized, that Se deficiency in ASD, as assessed by RBC levels, increases susceptibility to neurotoxicity of increased Hg and Pb levels. Finally, it is proposed that the relative concentrations of Se, Pb, and Hg can be used for the early diagnosis of ASD as multifactorial disorder related to environmental pollution with heavy metals. However, further detailed studies are required to assess the intimate mechanisms of the observed associations.
References
Aaseth J, Alexander J, Bjørklund G, Hestad K, Dusek P, Roos PM, Alehagen U (2016) Treatment strategies in Alzheimer's disease: a review with focus on selenium supplementation. Biometals 29:827–839
Adams JB, Romdalvik J, Ramanujam VS, Legator MS (2007) Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A 70:1046–1051
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S, Barnhouse S, Lee W (2013) Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol Trace Elem Res 151:171–180
Alabdali A, Al-Ayadhi L, El-Ansary A (2014) Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J Neuroinflammation 11:4. doi:10.1186/1742-2094-11-4
Al-Yafee YA, Al-Ayadhi LY, Haq SH, El-Ansary AK (2011) Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurol 11:139. doi:10.1186/1471-2377-11-139
APA - American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC
APA - American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association Publishing, Arlington
Aschner M, Farina M, Rocha JB (2013) Mercury neurotoxicity. In: Kretsinger RH, Uversky VN, Permyakov EA (eds) Encyclopedia of metalloproteins. Springer, New York, pp 1362–1367
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73:225–236
Bjørklund G (2015) Selenium as an antidote in the treatment of mercury intoxication. Biometals 28:605–614
Bjørklund G, Chartrand M (2016) Nutritional and environmental influences on autism spectrum disorder. J Nutr Disorders The 6:e123. doi:10.4172/2161-0509.1000e123
Bjørklund G, Saad K, Chirumbolo S, Kern JK, Geier DA, Geier MR, Urbina MA (2016) Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol Exp (Wars) 76:257–268
Bjørklund G, Bengtsson U, Chirumbolo S, Kern JK (2017a) Concerns about environmental mercury toxicity: do we forget something else? Environ Res 152:514-516
Bjørklund G, Aaseth J, Ajsuvakova OP, Nikonorov AA, Skalny AV, Skalnaya MG, Tinkov AA (2017b) Molecular interaction between mercury and selenium in neurotoxicity. Coord Chem Rev 332:30–37
Blaurock-Busch E, Amin OR, Rabah T (2011) Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica (Buchar) 6:247–257
Blaurock-Busch E, Amin OR, Dessoki HH, Rabah T (2012) Toxic metals and essential elements in hair and severity of symptoms among children with autism. Maedica (Buchar) 7:38–48
Bleecker ML, Ford DP, Celio MA, Vaughan CG, Lindgren KN (2007) Impact of cognitive reserve on the relationship of lead exposure and neurobehavioral performance. Neurology 69:470–476
Bräuer AU, Savaskan NE (2004) Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev Neurosci 15:19–32
Castoldi AF, Coccini T, Ceccatelli S, Manzo L (2001) Neurotoxicity and molecular effects of methylmercury. Brain Res Bull 55:197–203
Chauhan A, Chauhan V (2006) Oxidative stress in autism. Pathophysiology 13:171–181
Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee LC, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ 65(3):1–23. doi:10.15585/mmwr.ss6503a1
Chuang HY, Chao KY, Tsai SY (2005) Reversible neurobehavioral performance with reductions in blood lead levels–a prospective study on lead workers. Neurotoxicol Teratol 27:497–504
Crăciun EC, Bjørklund G, Tinkov AA, Urbina MA, Skalny AV, Rad F, Dronca E (2016) Evaluation of whole blood zinc and copper levels in children with autism spectrum disorder. Metab Brain Dis 31:887–890
Deng Z, Fu H, Xiao Y, Zhang B, Sun G, Wei Q, Ai B, Hu Q (2015) Effects of selenium on lead-induced alterations in Aβ production and Bcl-2 family proteins. Environ Toxicol Pharmacol 39:221–228
El-Ansary A, Al-Ayadhi L (2014) Relative abundance of short chain and polyunsaturated fatty acids in propionic acid-induced autistic features in rat pups as potential markers in autism. Lipids Health Dis 13:140. doi:10.1186/1476-511X-13-140
El-Ansary A, Al-Daihan SK, El-Gezeery AR (2011) On the protective effect of omega-3 against propionic acid-induced neurotoxicity in rat pups. Lipids Health Dis 10:142. doi:10.1186/1476-511X-10-142
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5:160–179
Endreffy I, Bjørklund G, Dicső F, Urbina MA, Endreffy E (2016) Acid glycosaminoglycan (aGAG) excretion is increased in children with autism spectrum disorder, and it can be controlled by diet. Metab Brain Dis 31:273–278
Fido A, Al-Saad S (2005) Toxic trace elements in the hair of children with autism. Autism 9:290–298
Gauba P, Shakeel M, Gaur S (2015) Mercury neurotoxicity: a review of case studies. Asian J Multidiscip Stud 3:9–16
Geier DA, Audhya T, Kern JK, Geier MR (2010) Blood mercury levels in autism spectrum disorder: is there a threshold level? Acta Neurobiol Exp (Wars) 70:177–186
Geier M, Kern JK, King PG, Sykes L, Geier DA (2014) Mercury induced autism. In: Patel VB, Preedy VR, Martin CR (eds) Comprehensive guide to autism. Springer, New York, pp 1411–1432
Herbert MR (2010) Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol 23:103–110
Hertz-Picciotto I, Delwiche L (2009) The rise in autism and the role of age at diagnosis. Epidemiology 20:84–90
Hill AP, Zuckerman K, Fombonne E (2015) Epidemiology of autism spectrum disorders. In: Robinson-Agramonte MA (ed) Translational approaches to autism spectrum disorder. Springer, Cham, pp 13–38
Holmes AS, Blaxill MF, Haley BE (2003) Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicol 22:277–285
Huang JP, Cui SS, Han Y, Irva HP, Qi LH, Zhang X (2014) Prevalence and early signs of autism spectrum disorder (ASD) among 18–36 month old children in Tianjin of China. Biomed Environ Sci 27:453–461
Ip P, Wong V, Ho M, Lee J, Wong W (2004) Mercury exposure in children with autistic spectrum disorder: case-control study. J Child Neurol 19:431–434
Johnson NL, Burkett K, Reinhold J, Bultas MW (2016) Translating research to practice for children with autism spectrum disorder. Part I: definition, associated behaviors, prevalence, diagnostic process, and interventions. J Pediatr Health Care 30:15–26
Jory J, McGinnis W (2008) Red-cell trace minerals in children with autism. Am J Biochem Biotechnol 4:101–104
Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J (2010) Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology 21:631–641
Kern JK, Grannemann BD, Trivedi MH, Adams JB (2007) Sulfhydryl-reactive metals in autism. J Toxicol Environ Health A 70:715–721
Kern JK, Geier DA, Audhya T, King PG, Sykes LK, Geier MR (2012) Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol Exp (Wars) 72:113–153
Kern JK, Geier DA, Deth RC, Sykes LK, Hooker BS, Love JM, Bjørklund G, Chaigneau CG, Haley BE, Geier MR (2015) Systematic assessment of research on autism spectrum disorder and mercury reveals conflicts of interest and the need for transparency in autism research. Sci Eng Ethics. doi:10.1007/s11948-015-9713-6
Kern JK, Geier DA, Sykes LK, Haley BE, Geier MR (2016) The relationship between mercury and autism: a comprehensive review and discussion. J Trace Elem Med Biol 37:8–24
Khaled EM, Meguid NA, Bjørklund G, Gouda A, Bahary MH, Hashish A, Sallam NM, Chirumbolo S, El-Bana MA (2016) Altered urinary porphyrins and mercury exposure as biomarkers for autism severity in Egyptian children with autism spectrum disorder. Metab Brain Dis 31:1419-1426
Lakshmi Priya MD, Geetha A (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 142:148–158
Landrigan PJ (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 22:219–225
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 113:894–899
Li SO, Wang JL, Bjørklund G, Zhao WN, Yin CH (2014) Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 25:1216–1220
London EA (2000) The environment as an etiologic factor in autism: a new direction for research. Environ Health Perspect 108(Suppl 3):401–404
Lubkowska A, Sobieraj W (2009) Concentrations of magnesium, calcium, iron, selenium, zinc and copper in the hair of autistic children. Trace Elem Electrolytes 26:72–77
Macedoni-Lukšič M, Gosar D, Bjørklund G, Oražem J, Kodrič J, Lešnik-Musek P, Zupančič M, France-Štiglic A, Sešek-Briški A, Neubauer D, Osredkar J (2015) Levels of metals in the blood and specific porphyrins in the urine in children with autism spectrum disorders. Biol Trace Elem Res 163:2–10
Magos L (1971) Selective atomic-absorption determination of inorganic mercury and methylmercury in undigested biological samples. Analyst 96:847–853
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:840547. doi:10.1155/2014/840547
Miller DT, Paschal DC, Gunter EW, Stroud PE, D'Angelo J (1987) Determination of blood lead with electrothermal atomic absorption using a L'vov platform and matrix modifier. Analyst 112:1701–1704
Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S (2010) Genetic variant of glutathione peroxidase 1 in autism. Brain Dev 32:105–109
Mohamed Fel B, Zaky EA, El-Sayed AB, Elhossieny RM, Zahra SS, Salah Eldin W, Youssef WY, Khaled RA, Youssef AM (2015) Assessment of hair aluminum, lead, and mercury in a sample of autistic Egyptian children: environmental risk factors of heavy metals in autism. Behav Neurol 2015:545674. doi:10.1155/2015/545674
Mostafa GA, Refai TMK (2007) Antineuronal antibodies in autistic children: relation to blood mercury. Egypt J Pediatr Allergy Immunol 5:21–30
Mostafa GA, Al-Ayadhi LY (2015) Reduced levels of plasma polyunsaturated fatty acids and serum carnitine in autistic children: relation to gastrointestinal manifestations. Behav Brain Funct 11:4. doi:10.1186/s12993-014-0048-2
Mostafa GA, Bjørklund G, Urbina MA, Al-Ayadhi LY (2016a) The positive association between elevated blood lead levels and brain-specific autoantibodies in autistic children from low lead-polluted areas. Metab Brain Dis 31:1047–1054
Mostafa GA, Bjørklund G, Urbina MA, Al-Ayadhi LY (2016b) The levels of blood mercury and inflammatory-related neuropeptides in the serum are correlated in children with autism spectrum disorder. Metab Brain Dis 31:593–599
Mutter J, Naumann J, Schneider R, Walach H, Haley B (2005) Mercury and autism: accelerating evidence. Neuroendocrinol Lett 26:439–446
Othman AI, El Missiry MA (1998) Role of selenium against lead toxicity in male rats. J Biochem Mol Toxicol 12:345–349
Parsons PJ, Slavin W (1993) A rapid Zeeman graphite furnace atomic absorption spectrometric method for the determination of lead in blood. Spectrochim Acta 48:925–939
Qiu LB, Ding GR, Li KC, Wang XW, Zhou Y, Zhou YC, Li YR, Guo GZ (2010) The role of protein kinase C in the opening of blood–brain barrier induced by electromagnetic pulse. Toxicology 273:29–34
Rimland B (2000) The autism epidemic, vaccinations, and mercury. J Nutr Environ Med 10:261–266
Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG (2013) Perinatal air pollutant exposures and autism spectrum disorder in the children of nurses’ health study II participants. Environ Health Perspect 121:978–984
Roman M (2016) Selenium: properties and determination. In: Caballero B, Finglas P, Toldrá F (eds) Encyclopedia of food and health. Academic, Oxford, pp 734–743
Rose S, Melnyk S, Savenka A, Hubanks A, Jernigan S, Mario Cleves M, James SJ (2008) The frequency of polymorphisms affecting lead and mercury toxicity among children with autism. Am J Biochem Biotechnol 4:85–94
Rutter M (2005) Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 94:2–15
Sajdel-Sulkowska EM, Lipinski B, Windom H, Audhya T, McGinnis W (2008) Oxidative stress in autism: elevated cerebellar 3-nitrotyrosine levels. Am J Biochem Biotechnol 4:73–84. doi:10.3844/ajbbsp.2008.73.84
Schrauzer GN (1987) Effects of selenium antagonists on cancer susceptibility: new aspects of chronic heavy metal toxicity. J UOEH 9(Suppl):208–215
Schultz ST (2010) Does thimerosal or other mercury exposure increase the risk for autism. Acta Neurobiol Exp (Wars) 70:187–195
Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE (2004) Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev 45:164–178
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjørklund G, Skalnaya MG, Nikonorov AA, Tinkov AA (2016a) Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis. doi:10.1007/s11011-016-9899-6
Skalny AV, Skalnaya MG, Nikonorov AA, Tinkov AA (2016b) Selenium antagonism with mercury and arsenic: from chemistry to population health and demography. In: Hatfield DL, Schweizer U, Tsuji PA, Gladyshev VN (eds) Selenium. Springer, Cham, pp 401–412
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG, Nikonorov AA, Tinkov AA (2016c) Assessment of serum trace elements and electrolytes in children with childhood and atypical autism. J Trace Elem Med Biol. doi:10.1016/j.jtemb.2016.09.009
Stamova B, Green PG, Tian Y, Hertz-Picciotto I, Pessah IN, Hansen R, Yang X, Teng J, Gregg JP, Ashwood P, Van de Water J, Sharp FR (2011) Correlations between gene expression and mercury levels in blood of boys with and without autism. Neurotox Res 19:31–48
Tian Y, Green PG, Stamova B, Hertz-Picciotto I, Pessah IN, Hansen R, Yang X, Gregg JP, Ashwood P, Jickling G, Van de Water J, Sharp FR (2011) Correlations of gene expression with blood lead levels in children with autism compared to typically developing controls. Neurotox Res 19:1–13
Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y (2014) Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol Lett 230:295–303
Winker R, Barth A, Ponocny-Seliger E, Pilger A, Osterode W, Rüdiger HW (2005) No cognitive deficits in men formerly exposed to lead. Wien Klin Wschr 117:755–760
Winker R, Ponocny-Seliger E, Rüdiger HW, Barth A (2006) Lead exposure levels and duration of exposure absence predict neurobehavioral performance. Int Arch Occup Environ Health 79:123–127
Yassa HA (2014) Autism: a form of lead and mercury toxicity. Environ Toxicol Pharmacol 38:1016–1024
Yasuda H, Tsutsui T (2013) Assessment of infantile mineral imbalances in autism spectrum disorders (ASDs). Int J Environ Res Public Health 10:6027–6043
Yiin SJ, Lin TH (1995) Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res 50:167–172
Yorbik O, Kurt I, Haşimi A, Oztürk O (2010) Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol Trace Elem Res 135:10–15
Zawia NH (2003) Transcriptional involvement in neurotoxicity. Toxicol Appl Pharmacol 190:177–188
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No (RGP-VPP-005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
El-Ansary, A., Bjørklund, G., Tinkov, A.A. et al. Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis 32, 1073–1080 (2017). https://doi.org/10.1007/s11011-017-9996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-9996-1