Abstract
Tachykinins (substance P, neurokinin A, and neurokinin B) are pro-inflammatory neuropeptides that may play an important role in some autoimmune neuroinflammatory diseases, including autism spectrum disorder (ASD). Mercury (Hg) is a neurotoxicant, and potentially one of the main environmental triggers for ASD as it induces neuroinflammation with a subsequent release of neuropeptides. This is the first study to explore the potentially causal relationship between levels of serum neurokinin A and blood mercury (BHg) in children with ASD. Levels of serum neurokinin A and BHg were measured in 84 children with ASD, aged between 3 and 10 years, and 84 healthy-matched children. There was a positive linear relationship between the Childhood Autism Rating Scale (CARS) and both serum neurokinin A and BHg. ASD children had significantly higher levels of serum neurokinin A than healthy controls (P < 0.001). Increased levels of serum neurokinin A and BHg were respectively found in 54.8 % and 42.9 % of the two groups. There was significant and positive linear relationship between levels of serum neurokinin A and BHg in children with moderate and severe ASD, but not in healthy control children. It was found that 78.3 % of the ASD patients with increased serum levels of neurokinin A had elevated BHg levels (P < 0.001). Neuroinflammation, with increased levels of neurokinin A, is seen in some children with ASD, and may be caused by elevated BHg levels. Further research is recommended to determine the pathogenic role of increased levels of serum neurokinin A and BHg in ASD. The therapeutic role of tachykinin receptor antagonists, a potential new class of anti-inflammatory medications, and Hg chelators, should also be studied in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurogenic inflammation is a neurally mediated immune inflammation that is orchestrated by a large number of neuropeptides, mainly tachykinins. Tachykinins (substance P, neurokinin A, and neurokinin B) have been considered as a group of neuropeptides which are released from the excitatory part of the nonadrenergic, noncholinergic excitatory nervous system nerves after exposure to allergens. The biological activity of tachykinins depends on their interaction with three specific tachykinin receptors, neurokinin (NK)1 (specific for substance P), NK2 (specific for neurokinin A) and NK3 (specific for neurokinin B) receptors (Maggi 2000; Richardson and Vasko 2002; Geppetti et al. 2008; Ramalho et al. 2011; Almeida et al. 2004).
Current estimates in the US are 1 child in 45 has a diagnosis of autism spectrum disorder (ASD) (Zablotsky et al. 2015). ASD has a multifactorial etiology that involves interactions between genes and environment, including diet (Endreffy et al. 2015). Exposure to neurotoxicant heavy metals such as mercury (Hg) and lead (Pb) have been suggested as a potential cause in presence of genetic predisposition in some children with ASD (Cohly and Panja 2005; Alabdali et al. 2014; Hodgson et al. 2014; Yassa 2014; Macedoni-Lukšič et al. 2015). It has been shown that Hg binds to lymphocyte receptors and/or tissue enzymes, inducing the proliferation and cytokine production from T lymphocytes (Jiang and Moller 1995). This resulted in neuroinflammation and autoimmune reaction (Dastych et al. 1999; Singh and Hanson 2006); suggesting Hg may be one of the main environmental triggers of neuroinflammation and autoimmunity in ASD. Early life exposure to Hg may result in the neurological injury that may lead to developmental defects, including ASD (Monroe and Halvorsen 2009). Several sources and exposure routes of toxic Hg in children have been reported in the literature. Mercurials can be found in various drugs, bleaching creams, antiseptics, disinfectants, as preservatives in cosmetics, toothpastes, lens solutions, vaccines, contraceptives and immunotherapy solutions, fungicides, herbicides and in dental amalgam fillings, as well as in long-lived and migratory fish such as tuna, due to water pollution. Mercury can cause immune, sensory, neurological, motor, and behavioral dysfunction similar to those associated with ASD (Geier et al. 2008). Elevated environmental Hg exposures have been shown to lead to a significant increase in the rates of ASD and special education students. A model prediction show on average that for each 453.6Kg increase in environmental Hg, there was a 61 % increase in the rate of ASD and 43 % increase in the rate of special education students (Palmer et al. 2006). In fact, it has been previously recommended to identify the source of Hg exposure in the population and consider prevention and control measures of environmental pollution (Palmer et al. 2006).
Evidence for an interaction between chronic inflammation in autoimmune diseases and neural dysfunction points to a mechanism linking both nervous and immune systems. In this context, neuropeptides, including tachykinins and neurotrophins have been recognized as key mediators of neuro-immune interactions in some autoimmune diseases, including ASD (Veres et al. 2009). Since the potential role of Hg as an external trigger in ASD has been suggested, the present study aims to 1) characterize the levels of serum neurokinin A and blood mercury (BHg) in children with ASD, and 2) explore a potential relationship between them in ASD.
Methods
Study population
The present study was conducted on 84 children with ASD. They were recruited from the Pediatric Neuropsychiatric Clinic, Faculty of Medicine of Ain Shams University, Cairo, Egypt, during their follow-up visits. All patients fulfilled the criteria for the diagnosis of ASD according to the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (APA 1994). The ASD group comprised 62 males and 22 females. Their ages ranged between 3 and 10 years (mean ± SD; 6.8 ± 1.5 years). Patients who had associated neurological diseases (such as cerebral palsy and tuberous sclerosis), metabolic disorders (e.g., phenylketonuria), allergic manifestations or concomitant infection were excluded from the study.
The control group comprised 84 age- (mean ± SD; 7 ± 1.8 years) and sex- matched healthy children. They included 60 males and 24 females. They were recruited from the Outpatients Clinic, Children’s Hospital, Faculty of Medicine, Ain Shams University. The control children were not related to the ASD children and demonstrated no clinical evidence of infections, allergies, and immunological or neuropsychiatric disorders.
The present study was approved by the ethical committee of the Faculty of Medicine at Ain Shams University in Cairo, Egypt. The parents or the legal guardians of the examined children gave their written consent for participation and publication of the study.
Study measurements
The clinical evaluation of the ASD patients in the present study was based on the clinical history from caregivers, clinical examination, and neuropsychiatric assessment. In addition, the degree of the disease severity was assessed by using the Childhood Autism Rating Scale (CARS) (Schopler et al. 1986), which rates the child on a scale from one to four in each of the 15 areas included (relationship to people; emotional response; imitation; body; object use; listening response; fear or nervousness; verbal communication; non-verbal communication; activity level; adaptation to changes; visual response; taste–smell-touch response and use; level and consistency of intellectual response; and general impressions). According to the scale, children who scored 30–36 were considered to have mild to moderate ASD (n = 43; CARS = 32.5 ± 1.7), while those with scores above 37 (n = 41; CARS = 51.9 ± 6.5) were considered to have a severe degree of ASD.
Blood sampling
Venous blood (2 ml) was collected from each child both in the patient and the control groups. The blood was transferred into a dry clean tube and left to clot at room temperature. The blood samples were, then, spun down at 3000 rpm for 5 min at room temperature. Prompt separation of serum was done and stored at −20 °C until analysis of serum neurokinin A levels. Another 1 ml of blood was collected in a heparinized syringe for immediate assay of BHg.
Assessment of serum neurokinin A
Serum levels of neurokinin A were evaluated with an enzyme-linked immunoassay (ELISA) kit which is highly sensitive to neurokinin A. Neurokinin A-like immunoreactivity, was measured using an antibody isolated from porcine spinal cord. It shows 100 % reactivity to neurokinin A with little cross-reactivity to other tachykinins (Peninsula laboratories, 611 Talorwat, Belmont, CA, USA). All samples were analyzed twice in two independent assays, to assess the inter-assay variation and to ensure reproducibility of the observed results (P > 0.05). No significant cross-reactivity or interference was observed.
Assessment of blood mercury levels
The evaluation of BHg levels was done by a flameless atomic absorption spectrophotometer (Perkin Elmer, FIMS-400) using the cold vapor technique (Mahaffey et al. 2004). To increase accuracy, all samples were analyzed twice in two separate runs to assess inter-assay variations and to ensure reproducibility of the observed results (P > 0.05).
Statistical analysis
The results were analyzed by using the commercially available software package (Statview, Abacus concepts, inc., Berkley, CA, USA). The data were non-parametric. Thus, they were presented as a median and interquartile range (IQR), which is between the 25th and 75th percentiles. Mann–Whitney test was used for comparison between these data. Chi-square test was used for comparison between qualitative variables of the studied groups. Spearman’s rho correlation coefficient “r” was used to determine the relationship between different variables. For all tests, a probability (P) of less than 0.05 was considered significant. In addition, patients were considered to have elevated serum neurokinin A if their levels were above the highest cut-off value which was 120 pg/ml (the 95th percentile of the control values).
Results
Serum levels of neurokinin A
Serum levels of neurokinin A were significantly higher in ASD children than healthy control children, P < 0.001 (Table 1). Forty-six (46) children with ASD (54.8 %) had increased serum levels of neurokinin A.
Patients with severe ASD had significantly higher serum levels of neurokinin A than patients with mild to moderate ASD P < 0.001 (Table 1). In addition, there were significant and positive linear relationship between serum levels of neurokinin A and values of CARS in ASD children (r2 = 0.84; p < 0.0001; Fig. 1; Table 2).
Blood levels of mercury
Blood Hg levels were significantly higher in ASD children than in healthy control children, P < 0.001 (Table 1). Thirty-six (36) children with ASD (42.9 %) had increased BHg levels.
Patients with severe ASD had significantly higher BHg levels than patients with mild to moderate ASD P < 0.001 (Table 1). In addition, there were significant and positive linear relationship between blood levels of Hg and values of CARS in ASD children (r2 = 0.89; p < 0.0001; Fig. 1, Table 2).
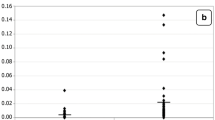
Increased levels of serum neurokinin A and blood mercury
There was a significant and positive linear relationship between levels of serum neurokinin A and BHg in children with moderate ASD (r2 = 0.92; p < 0.001; Fig. 2; Table 2) and severe ASD (r2 = 0.95; p < 0.0001; Fig. 2; Table 2), but not in healthy children (r2 = 0.004; p = 0.0.548; Fig. 2; Table 2).
Discussion
To date, there is only one previous study that investigated serum neurokinin A levels in a group of Saudi patients with ASD (Mostafa and Al-Ayadhi 2011). They found elevated levels of serum neurokinin A in 57.1 % of the patients with ASD (Mostafa and Al-Ayadhi 2011). A previous study investigated serum levels of some neuropeptides in children with ASD, reporting increased levels of serum neurotensin, but no increase in the other studied neuropeptides (β-endorphin and substance P) (Angelidou et al. 2010).
Tachykinin 1 gene is located in the candidate region responsible for ASD and produces substance P and neurokinins. These products modulate glutamatergic excitatory synaptic transmission and are also involved in brain inflammation of some children with ASD. Therefore, tachykinin 1 gene may have some functions associated with the presumably pathophysiology of ASD. To elucidate the genetic background of ASD, one study analyzed the relationship between three single nucleotide polymorphisms of the tachykinin 1 gene and ASD in the Japanese population, but no significant difference was observed between ASD children and healthy controls (Marui et al. 2007). Neuroendocrine hormones, including tachykinins, triggered by stress may lead to immune dysregulation, caused by altered cytokine production. This, in turn, could result in autoimmune or atopic diseases. In several autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and diabetes mellitus, the immune dysregulation may be attributable to an imbalance in the neuroendocrine-immune network, through the overproduction of neuropeptides and cytokines (Frieri 2003).
In the present study, patients with severe ASD had significantly higher serum levels of neurokinin A than patients with mild to moderate ASD. Another study also reported a positive association between serum levels of neurokinin A and the severity of ASD (Mostafa and Al-Ayadhi 2011). This suggests that the extent of the elevations in serum neurokinin A is closely linked to the degree and/or severity of ASD. However, it is not easy to determine whether the increase in serum neurokinin A levels is a mere consequence of ASD or has a pathogenic role in the disease.
While glial cells are recognized for their roles in maintaining neuronal function, there is a growing evidence of the ability of resident glial cells to initiate and/or augment inflammation following exposure to allergens, trauma or infection in the central nervous system (CNS). The tachykinins are found throughout the CNS, with evidence for both neuronal and glial cells as being sources of them. Tachykinins are well known to augment inflammatory responses at peripheral sites, such as the gastrointestinal tract and skin, which raises the possibility that they might have a similar function within the brain. Tachykinins may have a role in augmenting the immune functions of CNS glial cells, contributing to the progression of the detrimental inflammation within the CNS (Marriott 2004). Brain mast cells in some autoimmune neuroinflammatory diseases, such as multiple sclerosis, are activated by neural factors, including tachykinins. Mast cells can stimulate the activated T cells coming in contact with them at the blood–brain barrier. In addition, brain mast cells secrete numerous proinflammatory and vasoactive molecules that can disrupt the blood–brain barrier, a finding that precedes clinical or pathologic signs of some autoimmune neuroinflammatory diseases of CNS (Theoharides et al. 2008).
In this study, BHg levels were significantly higher in ASD children than healthy control children, P < 0.001. In addition, 42.9 % of the children with ASD had increased BHg levels. Previous studies also reported elevated Hg levels in blood (Palmer et al. 2006; Geier et al. 2008; Alabdali et al. 2014; Yassa 2014; Macedoni-Lukšič et al. 2015; Mostafa and Al-Ayadhi 2015) and hair of some children with ASD (Hodgson et al. 2014). In addition, elevated urinary coproporphyrin excretion, which is an indicator of Hg toxicity, was reported in 83 % of ASD children (Geier and Geier 2006).
The main reason for the elevated BHg in children with ASD may be a decreased detoxification capacity due to a dysfunction in metallothionein (MT) function, resulting from genetic polymorphism. MT is a family of cysteine-rich, low-molecular weight, intracellular proteins. These proteins have an extraordinary metal-binding capability and are essential to heavy metal detoxification (Aschner 1996; Bjørklund 2013). Children with ASD cannot adequately up-regulate MT biosynthesis following Hg exposure. Subsequently, children with ASD have significantly decreased level of reduced glutathione (Fido and Al-Saad 2005; Mutter et al. 2005). In addition, ASD patients were described as poor detoxifiers with remarkably less active glutathione-transferase which is important for Hg detoxification (Alabdali et al. 2014).
Autoimmunity to CNS may have a pathogenic role in ASD (Cohly and Panja 2005). Allergic autoimmune reaction after exposure to heavy metals such as Hg may play a causal role in ASD (Singh and Hanson 2006). Mercury and infectious agents are the two main environmental triggers of autoimmunity in ASD (Vojdani et al. 2003). Mercury has been shown to induce proliferation and cytokine production from T lymphocytes (Jiang and Moller 1995). Mercury may be one of the main environmental candidates that trigger autoimmunity in ASD, as it binds to lymphocyte receptors and/or tissue enzymes resulting in autoimmune reaction (Vojdani et al. 2003; Singh and Hanson 2006). Besides its possible role in the induction of autoimmunity to CNS, Hg could induce brain damage by other mechanisms which include the inhibition of glutathione and other antioxidant enzymes, damage of mitochondria with subsequent depletion of the cellular energy and disruption of important neurotransmitters such as serotonin, acetylcholine, glutamate and dopamine. All of these abnormalities have been found in ASD (Fenichel 2000; Bernard et al. 2001; James et al. 2005).
Promising treatments of ASD may involve detoxification of Hg, and supplementation of deficient metabolites. Some have proclaimed that chelation therapy for suspected Hg poisoning may have a role in the treatment of children with ASD with high Hg levels (Mutter et al. 2005). A significant decline in the blood levels of Pb and Hg with the use of meso-2,3-dimercapto-succinic acid (DMSA, succimer) as a chelating agent has been reported. In addition, a reduction in the ASD symptoms has been reported to follow a decrease in blood Pb and Hg levels after the use of DMSA (Yassa 2014).
There was in the present study a significant and positive association between the elevated levels of serum neurokinin A and increased BHg in children with ASD. This is the first study that investigated the relationship between levels of serum neurokinin A and BHg in ASD children.
Many studies reported that tachykinins may also be produced by non-neuronal cells, such as immune cells after exposure to inflammatory stimuli and they exert a profound influence on the inflammatory responses by affecting multiple aspects of immune cell functioning (Zhang et al. 2006). In one study, the elevated sputum neurokinin A levels showed a significant and positive correlation with eosinophil counts in both blood and sputum of asthmatic children during acute asthma exacerbation (Mostafa et al. 2008). Thus, the reason behind the increased serum neurokinin A levels in ASD children may be through the stimulation of the immune cells, after exposure to some environmental antigens, such as exposure to heavy metals, food allergens, and infectious agents, with a subsequent increase and release of tachykinin from these cells.
Mercury is known to be neurotoxic, but its effects on the immune system are less well understood. Mercury stimulates vascular endothelial growth factor and IL-6 release from human mast cells, potentially disrupting the blood–brain-barrier and permit brain inflammation. As a result, moderate doses of Hg may contribute to ASD pathogenesis (Kempuraj et al. 2010). Brain mast cells are also activated by neural factors, including tachykinins, in some autoimmune neuroinflammatory diseases (Theoharides et al. 2008). Mast cells are involved in allergic reactions, but also in innate and acquired immunity, as well as in inflammation. Intriguingly, many patients with ASD have allergic symptoms (Kempuraj et al. 2010). In one study, for example, serum neurokinin A levels were significantly correlated with serum levels of anti-ribosomal P protein antibodies in a group of ASD children (Mostafa and Al-Ayadhi 2011). In a more recent study, BHg levels were significantly associated with the production of serum anti-myelin basic protein autoantibodies in another group of children with ASD (Mostafa and Al-Ayadhi 2015). Thus, both Hg and neurokinin A may have effects on the immune system by stimulating mast cells in ASD children.
The present study revealed that the increase of BHg levels may promote the induction of neuroinflammation and autoimmunity through stimulation of the production of neurokinin A. Based on the findings of this first study investigating the relationship between BHg levels and serum neurokinin A in children with ASD, future studies exploring potential relationships between elevated levels of BHg and other heavy metals and the production of neuropeptides in children with ASD are needed.
Conclusions
Neuroinflammation, with subsequently increased levels of neurokinin A, in some children with ASD, may be induced by elevated BHg levels. Further research is recommended to determine the pathogenic role of increased levels of serum neurokinin A and BHg in children with ASD. The therapeutic role of tachykinin receptor antagonists, a potential new class of anti-inflammatory medications, and Hg chelators, should also be studied in ASD.
References
Alabdali A, Al-Ayadhi L, El-Ansary A (2014) A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav Brain Funct 10:14. doi:10.1186/1744-9081-10-14
Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML (2004) Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11:2045–2081
Angelidou A, Francis K, Vasiadi M, Alysandratos KD, Zhang B, Theoharides A, Lykouras L, Sideri K, Kalogeromitros D, Theoharides TC (2010) Neurotensin is increased in serum of young children with autistic disorder. J Neuroinflammation 7:48. doi:10.1186/1742-2094-7-48
APA - American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. American Psychiatric Association, Washington
Aschner M (1996) The functional significance of brain metallothioneins. FASEB J 10:1129–1136
Bernard S, Enayati A, Redwood L, Roger H, Binstock T (2001) Autism: a novel form of mercury poisoning. Med Hypotheses 56:462–471
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73:225–236
Cohly HH, Panja A (2005) Immunological findings in autism. Int Rev Neurobiol 71:317–341
Dastych J, Walczak-Drzewiecka A, Wyczolkowska J, Metcalfe DD (1999) Murine mast cells exposed to mercuric chloride release granule-associated N-acetyl-beta-D-hexosaminidase and secrete IL-4 and TNF-alpha. J Allergy Clin Immunol 103:1108–1114
Endreffy I, Bjørklund G, Dicső F, Urbina MA, Endreffy E (2015) Acid glycosaminoglycan (aGAG) excretion is increased in children with autism spectrum disorder, and it can be controlled by diet. Metab Brain Dis. doi:10.1007/s11011-015-9745-2
Fenichel GM (2000) Disorders of cranial volume and shape. In: Fenichel GM (ed) Clinical pediatric neurology: a signs and symptoms approach, 5th edn. WB Saunders, Philadelphia, pp 353–373
Fido A, Al-Saad S (2005) Toxic trace elements in the hair of children with autism. Autism 9:290–298
Frieri M (2003) Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allergy Asthma Immunol 90(6 Suppl 3):34–40
Geier DA, Geier MR (2006) A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotox Res 10:57–64
Geier DA, King PG, Sykes LK, Geier MR (2008) A comprehensive review of mercury provoked autism. Indian J Med Res 128:383–411
Geppetti P, Nassini R, Materazzi S, Benemei S (2008) The concept of neurogenic inflammation. BJU Int 101(Suppl 3):2–6
Hodgson NW, Waly MI, Al-Farsi YM, Al-Sharbati MM, Al-Farsi O, Ali A, Ouhtit A, Zang T, Zhou ZS, Deth RC (2014) Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Exp Biol Med (Maywood) 239:697–706
James SJ, Slikker W, Melnyk S, New E, Pogribna M, Jernigan S (2005) Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. Neurotoxicology 26:1–8
Jiang Y, Moller G (1995) In vitro effects of HgCl2 on murine lymphocytes. I. Preferable activation of CD4+ T cells in a responder strain. J Immunol 154:3138–3146
Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, Theoharides TC (2010) Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation 7:20. doi:10.1186/1742-2094-7-20
Macedoni-Lukšič M, Gosar D, Bjørklund G, Oražem J, Kodrič J, Lešnik-Musek P, Zupančič M, France-Štiglic A, Sešek-Briški A, Neubauer D, Osredkar J (2015) Levels of metals in the blood and specific porphyrins in the urine in children with autism spectrum disorders. Biol Trace Elem Res 163:2–10. doi:10.1007/s12011-014-0121-6
Maggi CA (2000) The troubled story of tachykinins and neurokinins. Trends Pharmacol Sci 21:173–175
Mahaffey KR, Clickner RP, Bodurow CC (2004) Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect 112:562–570
Marriott I (2004) The role of tachykinins in central nervous system inflammatory responses. Front Biosci 9:2153–2165
Marui T, Funatogawa I, Koishi S, Yamamoto K, Matsumoto H, Hashimoto O, Nanba E, Nishida H, Sugiyama T, Kasai K, Watanabe K, Kano Y, Kato N, Sasaki T (2007) Tachykinin 1 (TAC1) gene SNPs and haplotypes with autism: a case–control study. Brain Dev 29:510–513
Monroe RK, Halvorsen SW (2009) Environmental toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial disruption. NeuroToxicology 30:589–598
Mostafa GA, Al-Ayadhi LY (2011) The possible link between the elevated serum levels of neurokinin A and anti-ribosomal P protein antibodies in children with autism. J Neuroinflammation 8:180. doi:10.1186/1742-2094-8-180
Mostafa GA, Al-Ayadhi LY (2015) The possible association between elevated levels of blood mercury and the increased frequency of serum anti-myelin basic protein auto-antibodies in autistic children. J Clin Cell Immunol 6:2. doi:10.4172/2155-9899.1000310
Mostafa GA, Reda SM, Abd El-Aziz MM, Ahmed SA (2008) Sputum neurokinin A in Egyptian asthmatic children and adolescents: relation to exacerbation severity. Allergy 63:1244–1247
Mutter J, Naumann J, Schneider R, Walach H, Haley B (2005) Mercury and autism: accelerating evidence? Neuro Endocrinol Lett 26:439–446
Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C (2006) Environmental mercury release, special education rates, and autism disorder: an ecological study of Texas. Health Place 12:203–209
Ramalho R, Raquel Soares R, Nuno Couto N, Moreira A (2011) Tachykinin receptors antagonism for asthma: a systematic review. BMC Pulm Med 11:41. doi:10.1186/1471-2466-11-41
Richardson JD, Vasko MR (2002) Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302:839–845
Schopler E, Reichler RJ, Renner BR (1986) The childhood autism rating scale (CARS): for diagnostic screening and classification of autism. Irvington, New York
Singh VK, Hanson J (2006) Assessment of metallothionein and antibodies to metallothionein in normal and autistic children having exposure to vaccine-derived thimerosal. Pediatr Allergy Immunol 17:291–296
Theoharides TC, Kempuraj D, Kourelis T, Manola A (2008) Human mast cells stimulate activated T cells: implications for multiple sclerosis. Ann N Y Acad Sci 1144:74–82
Veres TZ, Rochlitzer S, Braun A (2009) The role of neuro-immune cross-talk in the regulation of inflammation and remodelling in asthma. Pharmacol Ther 122:203–214
Vojdani A, Pangborn JB, Vojdani E, Cooper EL (2003) Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol 16:189–199
Yassa HA (2014) Autism: a form of lead and mercury toxicity. Environ Toxicol Pharmacol 38:1016–1024
Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ (2015) Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. National health statistics reports; no. 87. National Center for Health Statistics, Hyattsville. http://www.cdc.gov/nchs/data/nhsr/nhsr087.pdf. Accessed 21 December 2015
Zhang Y, Berger A, Milne CD, Paige CJ (2006) Tachykinins in the immune system. Curr Drug Targets 7:1011–1020
Acknowledgments
This research project was supported by a grant from the Research Center of the Center for Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Mostafa, G.A., Bjørklund, G., Urbina, M.A. et al. The levels of blood mercury and inflammatory-related neuropeptides in the serum are correlated in children with autism spectrum disorder. Metab Brain Dis 31, 593–599 (2016). https://doi.org/10.1007/s11011-015-9784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9784-8