Abstract
This study investigates both the level of toxic metals in children with autism and the possible association of those toxic metals with autism severity. This study involved 55 children with autism ages 5–16 years compared to 44 controls with similar age and gender. The study included measurements of toxic metals in whole blood, red blood cells (RBC), and urine. The autism group had higher levels of lead in RBC (+41 %, p = 0.002) and higher urinary levels of lead (+74 %, p = 0.02), thallium (+77 %, p = 0.0001), tin (+115 %, p = 0.01), and tungsten (+44 %, p = 0.00005). However, the autism group had slightly lower levels of cadmium in whole blood (−19 %, p = 0.003). A stepwise, multiple linear regression analysis found a strong association of levels of toxic metals with variation in the degree of severity of autism for all the severity scales (adjusted R 2 of 0.38–0.47, p < 0.0003). Cadmium (whole blood) and mercury (whole blood and RBC) were the most consistently significant variables. Overall, children with autism have higher average levels of several toxic metals, and levels of several toxic metals are strongly associated with variations in the severity of autism for all three of the autism severity scales investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background and Significance
Determination of toxic metal exposure in classic lead poisoning, such as due to ingestion of lead paint, is relatively easy and involves measuring blood levels of lead. However, in autism, the problem appears to usually not be high exposure, but rather decreased excretion. The half-life of lead, mercury, and other toxic metals in the blood is weeks to months, so those metals rapidly leave the blood and accumulate in tissue and/or bone. Since biopsies of those tissues are invasive, this makes assessment of toxic metal exposure in autism more complex.
Many studies suggest that children with autism have a decreased ability to excrete toxic metals, leading to a higher body burden. The decreased ability to excrete toxic metals is partly due to low glutathione [1–4] since glutathione conjugation (and subsequent excretion in the feces) is the primary pathway for removal of some toxic metals. Another factor that also decreases ability to excrete toxic metals in feces is increased use of oral antibiotics [5–8] since oral antibiotics have been shown (in rats) to almost completely inhibit excretion of mercury [9, 10] due to their effect on altering gut flora. This is consistent with two studies which found lower levels of mercury in the baby hair of children with autism, [8, 11], one study which found decreased lead, arsenic, and cadmium in hair of young children with autism [12], and one study [13] reanalyzed correctly [14] which found abnormal ratios of mercury in blood to mercury in hair consistent with an excretion problem.
One study in Kuwait [15] found children with autism had dramatically higher levels of mercury (15 times higher, p < 0.001) and somewhat higher levels of lead (2.1 times higher, p < 0.001) and uranium (3 times higher, p < 0.001) compared to neurotypical children. The levels of mercury in the Kuwait study (median of 4.5 ppm in the children with ASD) are far higher than in most other studies and clearly suggest a toxic exposure to mercury in those Kuwaiti children. In one study in the USA [8], there was a small subset (10 %) of children with ASD who had similar high levels, whereas most of the ASD children had lower levels than the neurotypical group. So, the Kuwaiti study provides strong evidence of exposure to toxic levels of mercury in the Kuwaiti children with autism, whereas the other studies suggest a problem with excretion of mercury.
Evidence of increased body burden of toxic metals in children with autism includes a small study [7] which found children with autism had twice the level of mercury in whole baby teeth as did neurotypical children, but similar levels of lead. In contrast, a recent small study of enamel of baby teeth did not find differences in levels of lead or mercury in children with autism vs. neurotypical children [16]. Enamel (exterior of tooth) is completely formed by 3–12 months of age [17], whereas dentin (interior of tooth) is living tissue until the tooth is lost, so it reflects exposure throughout childhood [18]. If both studies are valid, then this may suggest that increased mercury deposition occurred primarily during childhood after the age of 3–12 months, but further research is needed to validate that possible interpretation of these small studies.
One recent large study of mercury levels in red blood cells (RBC) found that children with autism had significantly higher levels of mercury in their RBC (1.9× higher, p < 0.0001) than did typical children [19]. However, a recent similar very large study of mercury levels in whole blood did not find a significant difference in mercury levels between children with autism and controls, although they did find that mercury levels correlated with seafood consumption and dental amalgams [20].
One study of urinary excretion of chromium, lead and cadmium in Turkey [21] found that children with ASD had increased excretion of chromium, but decreased levels of excretion of cadmium and lead compared to neurotypical controls. However, the age and gender of the controls were unspecified and may not have matched those of the autism group.
Another study investigated the administration of dimercaptosuccinic acid (DMSA), a medication which binds to some toxic metals and excretes them in the urine; that study found that children with autism excreted mercury at three to six times the level of neurotypical children after both groups received the DMSA [22]. One group [23] attempted a similar study, but their study was seriously flawed due to very small sample size (15 autism and 4 neurotypicals) and very insensitive measurement techniques such that the toxic metals were measureable in less than 15 % of the autism cases and none of the control cases, so they were lacked the necessary sensitivity to observe differences between the groups.
Measurements of urinary porphyrins are an indirect method to assess exposure to certain toxic metals, especially lead and mercury, due to their effect on inhibiting the porphyrin pathway. Three studies [24–26] of urinary porphyrins found that children with autism had higher levels of porphyrins which may be associated with increased mercury body burden, and one study [27] found a significant correlation of autism severity with mercury-related porphyrins. One study [28] did not find differences in pre-coprophyrin (associated with mercury), but did find differences in several other porphyrins including significantly elevated levels of coproporphyrin (associated with lead and mercury). That study also investigated levels of mercury in urine and did not find significant differences in urinary excretion of mercury in children with autism vs. controls; however, mercury is primarily excreted in the bile, not the urine, unless there is use of a chelator such as DMSA that is excreted in the urine.
Three environmental studies [29–31] have linked autism incidence with increased level of airborne mercury and (in one study) other airborne toxins.
One study [32] investigated the incidence of autism in the grandchildren of survivors of acrodynia, a disorder involving unusual susceptibility to mercury, and found an incidence of 1 in 22 compared to 1 in 160 in the general population. So, it appears that the grandchildren of individuals known to be unusually vulnerable to mercury were themselves at much greater risk of developing autism, presumably due to a genetic vulnerability to mercury.
One study investigated the effect of DMSA on children with autism. One paper [33] found that 22–45 % (p < 0.005) of the variation in autism severity was associated with urinary levels of toxic metals pre and post administration of DMSA, based on a regression analysis. The open-label treatment study found that DMSA therapy was effective in removing toxic metals, improving glutathione levels, and partially improving platelet status (a marker of inflammation) [34]. DMSA therapy also resulted in improvements in autism severity, with the degree of improvement being associated with the baseline level of toxic metals and glutathione [35].
Overall, many studies suggest that children with autism have an impaired ability to excrete mercury and other toxic metals due to low glutathione (which binds to toxic metals and excretes them in the bile) and excessive oral antibiotic use (which greatly inhibits fecal excretion). Many but not all studies suggest that children with autism have a higher body burden of mercury and other toxic metals, and that airborne mercury is a risk factor for autism.
The purpose of this paper is to further investigate the toxicological status of children with autism compared to age- and gender-matched neurotypical children. Specifically, we hypothesize that:
-
1.
Children with autism will have higher levels of some toxic metals in their blood and urine, presumably due to a combination of increased exposure, increased absorption, and an impairment in the fecal excretion of toxic metals.
-
2.
The severity of autism will be associated with level of toxic metals in blood and urine.
The data collected for this study were part of a larger study which also evaluated the nutritional and metabolic status of children with autism compared to neurotypical controls of similar age and gender [4].
Methodology
This paper reports on the toxicological status of children with autism compared to neurotypical children. This study was conducted with the approval of the Human Subjects Institutional Review Board of Arizona State University.
Participants
Participants were recruited from Arizona with the help of the Autism Society of America—Greater Phoenix Chapter (by email and mailing of the IRB-approved invitation letter) and the Arizona Division of Developmental Disabilities (by mailing of the IRB-approved invitation letter). Also, neurotypical controls were recruited by asking participants to share the IRB-approved invitation letter for neurotypical families with their friends and neighbors. All parents and (where possible) children signed parent consent/child assent forms.
Enrollment Criteria
-
1.
Age 5–16 years old
-
2.
No usage of a vitamin/mineral supplement in the last 2 months
-
3.
No current use of any chelation treatment
-
4.
Autism group: prior diagnosis of autism, PDD/NOS, or Asperger’s by a psychiatrist or similar professional, with written verification (no additional assessment was done in this study)
-
5.
Control group: in good mental and physical health and no siblings with autism spectrum disorders, and no evidence of attention deficit disorder by parent report (no additional assessment was done in this study)
Participants
The characteristics of the study participants are listed in Table 1.
Study Protocol
-
1.
Participant parents contacted the study coordinator, and the study was explained by telephone. Consent/assent forms were sent to the parents for review, and then signed copies were brought to the study coordinator. The principal investigator (J.B. Adams) also discussed the study personally with each participant.
-
2.
Parents of children with autism completed three questionnaires relating to the severity and symptoms of autism (see below).
-
3.
The study physician conducted a physical exam to determine that the children were in adequate health for participating in the study.
-
4.
Morning blood samples were collected after an overnight fast (8–12 h). Morning urine samples were collected, and in almost all cases, these were first-morning (overnight) urines.
-
5.
All study data (questionnaires and laboratory samples) were assigned a coordinating subject code. All laboratory analyses were done blinded to subject group (autism or control).
Lab Measurements
Levels of many toxic metals were measured in whole blood, red blood cells (RBC), and urine (see Tables 2 and 3), based on the testing available from Doctor’s Data (St. Charles, IL, USA—www.doctorsdata.com). Doctor’s Data are certified by CLIA, the Clinical Laboratory Improvement Amendments program operated by the US Department of Health and Human Services which oversees approximately 200,000 laboratories in the USA.
Urine is a measure of recent exposure (several days), RBC is a measure of longer term exposure (several months), and whole blood is intermediate between urine and RBC since it includes a mixture of short-term (serum) and long-term (cellular) components.
Whole blood and packed red blood cells were collected in a potassium EDTA trace metal free (royal blue top; BD Vacutainer, Franklin Lakes, NJ). Packed red blood cells were spun for 15 min in a centrifuge at 1,500×g (g force). The plasma and buffy coat were removed and the remaining packed red blood cells were submitted for testing. Elemental analysis was performed after digesting an aliquot of sample using a temperature controlled microwave digestion system (Mars5; CEM Corp; Matthews, SC) following the same procedure for nitric acid microwave digestion and sample procedure as used previously for hair [36]. The digested sample was analyzed by inductively coupled plasma–mass spectrometry (ICP-MS; Elan DRCII; Perkin Elmer Corp; Shelton, CT). Results were verified for precision and accuracy using controls from Doctor’s Data and Seronorm whole blood controls (Sero; Billingstad, Norway).
For some of the elements, a fraction of the samples had undetectable levels and that is reported in Table 2. For cases where over half of the samples had undetectable levels, the results should be interpreted with great caution.
Assessing Autistic Symptoms and Severity
Three tools were used to assess the severity and symptoms of autism, namely the pervasive developmental disorder behavior inventory (PDD-BI) [37], autism treatment evaluation checklist (ATEC) [38], and severity of autism scale (SAS) [33]. For the PDD-BI, we used a slightly modified autism composite, in which the semantic/pragmatic problems (SemPP) subscale is ignored. The reason is that the SemPP is difficult to interpret, since children with no spoken language inappropriately score as less severe than those with limited language. Therefore, following the example of our previous study [33] we exclude the SemPP subscale in calculating the autism composite score, resulting in a modified autism composite score consisting of the sum of the sensory/perceptual approach, ritualisms/resistance to change, social pragmatic problems, social approach behaviors, and phonological and semantic pragmatic subscales.
Statistical Analysis
Several types of statistical analyses were used, depending on the research question being addressed. In comparing variables between groups (such as children with autism vs. neurotypical children), two-sided unpaired t tests were used. The unpaired t tests were either done assuming equal variance or unequal variance, based on the results of a test for equal variance. For individual comparisons, a p value of 0.05 or lower was assumed significant. However, in order to maintain an overall significance of 5 % when multiple comparisons were considered, a smaller per test p value was considered significant based on a Bonferroni analysis—this is defined at the beginning of the results section. Pearson correlation coefficients were obtained to determine the strengths of linear relationships among the variables involved in the analyses. Some of the data for toxic metals in urine and blood were not normally distributed, so in those cases a non-parametric Wilcoxon test was used instead of a t test.
Note that for a few measurements there was some data below our detection limit. In those cases, we substituted the value of the detection limit for the data point; so for cases where some samples were below detection limit, our reported measured values are an upper bound to the true value.
Regression analysis was employed to examine the relationship between the severity of autism (assessed by the ATEC, PDD-BI, and SAS) and the levels of toxic metals in whole blood, RBC, and urine. For the selected dependent and independent variables, step-wise linear regression analyses were conducted: initially, all independent variables were included in the regression; then at each step, the variable with the highest p value was eliminated, and this process was continued until the adjusted R 2 value began declining. Thus, the goal was to determine the best fit to the sample data for the selected model, taking into account the correlation among the independent variables.
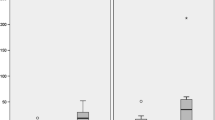
Average levels of toxic metals in whole blood, RBC, and urine which were significantly different between the autism and neurotypical groups. The average values are shown in columns, rescaled to the average neurotypical value. The vertical bars display the 25th and 75thpercentiles. The number of asterisks indicates the p value (*p < 0.05, **p < 0.01, ***p < 0.001). In all the cases but one, the autism group had higher levels of toxic metals. In most cases, the variation between the 25th and 75th percentiles is greater for the autism group
Results
Detection Limits
Different metals were measurable in different compartments, with urinary levels generally being the most sensitive. For whole blood, detectable levels of arsenic, barium, cadmium, cobalt, lead, and mercury were measured; levels of nickel, platinum, silver, thallium, and uranium were also measured, but they were below the laboratory’s detection limits of 3, 0.2, 0.1, 0.1, and 0.1 mcg/L, respectively, for those elements.
For RBC, detectable levels of arsenic, lead, and mercury were measured. Levels of cadmium and thallium were measured, but were below the laboratory detection limits of 0.001 and 0.0001 mcg/g, respectively.
For urine, detectable levels of aluminum, antimony, arsenic, bismuth, cadmium, lead, mercury, nickel, thallium, tin, tungsten, and uranium were measured. Levels of beryllium, platinum, and thorium were below the laboratory detection limit in almost all cases—only one child (with autism) had detectable beryllium (0.2 mcg/g-creatinine) and only one child (with autism) had detectable platinum (0.05 mcg/g-creatinine).
Levels of Toxic Elements
Toxic elements were measured in the whole blood (WB), RBC, and urine, and their values were given in Table 2 and Fig. 1. The distribution of toxic elements was not normally distributed; rather, it was skewed toward higher levels such that the mean was sometimes much higher than the median. So, Table 2 reports both the mean and the median, and Wilcoxon test instead of a t test was used for comparing the autism and neurotypical groups. Applying the Bonferroni analysis to the 21 comparisons, p values are defined as: “significant” = p < 0.002, “marginally significant” = p < 0.005, and “possibly significant” = p < 0.05.
Compared to the controls, the children with autism had significantly higher levels of lead in RBC and thallium and tungsten in urine. There were also possibly significant higher levels of lead and tin in urine. There was a possibly significant slightly lower level of cadmium in the whole blood (but not in urine).
For tungsten in urine, one of the typical children had an unusually high level (2.7 mcg/g creatinine), which was 16 times higher than the average of the other children, and 4.5 times higher than the next highest value. So, the mean value is reported with and without this data.
Table 3 lists the levels of toxic metals in which more than half of the samples had levels below the detectable limits. There were no significant differences between the autism and neurotypical group, but this should be interpreted with caution due to the detection limits.
We also investigated the correlations of levels of some toxic metals measured in WB, RBC, and urine—see Table 4. For lead, there are very strong correlations among values in WB, RBC, and urine. For arsenic, there are weak correlations between WB and RBC, and between WB and urine, but none between RBC and urine. Arsenic levels in urine are very susceptible to consumption of arsenic-rich foods such as shellfish, so lower correlation with WB and RBC is expected. (Speciation of arsenic could distinguish between organic and inorganic forms, and might yield stronger correlations). For cadmium, there was no correlation of levels in WB and urine, presumably because cadmium levels were low and cadmium is primarily excreted in the bile, not the urine. (Cadmium was not measured in RBC because the concentration in serum is much higher than in RBC). For other toxic metals either, no data was available, or most of the data were below the detectable limit, preventing any direct comparison.
Correlations with Autism Severity
We calculated the correlations of each biomarker with each of the three autism severity scales. Table 5 lists the biomarkers which had the highest correlation with autism severity (R > 0.34 in absolute magnitude, corresponding to a p value of 0.01 or lower). Note that because we are simultaneously investigating correlations with many biomarkers, the cut-off for significance is not p = 0.05, but below 0.001. So none of the results are significant, but some are possibly significant. Cadmium (WB) and mercury (WB and RBC) had correlations of R > 0.34 in absolute magnitude, but those correlations are only possibly significant.
Regression Analysis
The regression analysis (see Table 6) yielded highly significant results for each autism severity scale, with all three severity scales having high adjusted R 2 (0.38–0.47). Cadmium (WB) and mercury (RBC and WB) were the most consistently significant variables.
Discussion
Levels of toxic metals in blood and urine reflect a combination of exposure, absorption, and excretion of toxic metals. Higher levels of toxic metals in blood or urine suggest a combination of increased exposure, increased absorption, and/or decreased fecal excretion (most toxic metals are conjugated to glutathione, excreted in the bile into the intestines, and then expelled in the feces). The major differences between the autism group and the neurotypical group were a much higher level of lead in RBC, and much higher urinary levels of several toxic metals, including primarily thallium and tungsten, and possibly lead and tin. The higher amount of lead in urine correlated with higher amounts in the RBC and WB; however, this correlation did not hold for arsenic or cadmium, and could not be determined for thallium, tin, or tungsten. Higher amounts of toxic metals in the blood and urine are suggestive of higher body burden, so the results of this study suggest that a subset of children with autism have higher body burdens of lead, thallium, and tungsten.
The reason for the higher body burden of toxic metals may relate to increased exposure to toxic metals, increased absorption due to intestinal permeability [39] and/or decreased ability to excrete toxic metals (due to low glutathione and abnormal gut bacteria due to increased oral antibiotic use). There are likely also genetic factors which result in increased vulnerability to mercury [32].
The higher levels of toxic metals in the autism group are consistent with measurements of decreased plasma reduced glutathione (−21 %, p < 0.0001) in this same group [4] and in other studies [1–3]. Glutathione binds to toxic metals (especially lead, mercury, and cadmium) and is excreted with them in the bile, so decreased amounts of glutathione would tend to result in a higher body burden of toxic metals, as found here.
The higher level of toxic metals may be partially due to higher levels of oral antibiotic use in children with autism. Antibiotic use results in a near-total loss of the ability to excrete mercury [9, 10]. The reason appears to be that normal gut anaerobes are able to convert methylmercury (which is rapidly absorbed) into inorganic mercury (which is poorly absorbed and hence mostly excreted). In contrast, most strains of yeast and Escherichia coli carry out the reverse reaction, namely the methylation of inorganic mercury to methylmercury [40]. Thus, high oral antibiotic use would result in a loss of normal gut flora and an increase in yeast and E. coli, resulting in a loss of ability to demethylate methylmercury and enhanced methylation of inorganic mercury, resulting in decreased fecal excretion and increased uptake of mercury. The effects of oral antibiotics on gut flora may be long-lasting unless treated.
The higher level of toxic metals in the autism group is also consistent with the higher level of oxidative stress in this same group [4], including higher levels of the ratio of oxidized glutathione (GSSG) to reduced glutathione (GSH; +49 %, p < 0.0001) and higher levels of plasma nitro-tyrosine (+125 %, p < 0.0001). Higher levels of toxic metals would tend to increase oxidative stress and deplete glutathione.
The slightly lower (marginally significant) level of cadmium in WB was inconsistent with slightly higher (not significant) levels in the urine. Since both are reasonable measures of body burden, this suggests that overall cadmium body burden might not be very different in children with autism compared to typical children.
The median levels of urinary toxic metals reported in this paper are similar to those reported at baseline (unprovoked) in another study of children with autism [33], despite some differences in age (5–16 years in the present study vs. 3–8 years in the other study [33]; Fig 2). Levels of the metals which were found to be elevated in this study (lead, tin, thallium, and tungsten) were at similar levels (tin, thallium, tungsten) or higher levels (lead) in that study. Overall, the reasonable agreement of the present results with those of the previous study lends confidence to the present results.
Comparison of the median levels of urinary excretion of toxic metals in children with autism in this study compared to baseline urines (unprovoked) in children with autism in a previous study [33, 34]. Levels are normalized to the average level of the controls (value of one). The bars indicate the 25th and 75th percentiles for each measurement
A similar study of children with autism in Turkey [21] found somewhat different results, namely significantly lower levels of urinary excretion of lead and cadmium in children with autism vs. neurotypical children. However, the age and gender of the controls were unspecified and may not have matched those of the autism group, which may have affected the results. The differences with this study may be due to differences in geographic exposure to toxic metals (Turkey vs. Arizona). The lower level of urinary cadmium in the Turkish study is intriguing since the present study found lower levels of cadmium in whole blood (but no difference in levels in urine).
Correlation Analysis
High amounts of mercury (both WB and RBC) correlated with more severe autism (see Table 5), which is consistent with the known neurotoxicity of mercury. However, since most of the measurements were below our detection limit, this finding should be interpreted with caution.
The modest inverse correlation of WB cadmium with autism severity is surprising, as a direct (positive) correlation would be expected. It is possible that this is just a statistical artifact, since many correlations were investigated, but it is worth further investigation.
Regression Analysis
The regression analysis found very significant associations with the variation in autism severity, especially for the PDD-BI, followed by the SAS and then the ATEC. Mercury (WB and RBC) followed by cadmium (WB) were the most consistently significant variables, although other biomarkers were also significant. Since the levels of toxic metals are often strongly correlated [33], it is important not to over-interpret the results as being specific to a particular toxic metal. Also, different geographic regions will have different levels of toxic metals, so different toxic metals may be more or less important in different parts of the country.
The finding of a strong association of the level of toxic metals with variation in autism severity is generally consistent with another study by our group [33]. That study involved measuring level of toxic metals in urine both before and after administration of dimercaptosuccinic acid (DMSA), an FDA-approved medication for treating lead toxicity. Together, these studies provide compelling evidence that the levels of toxic metals are strongly associated with variations in autism severity.
These significant associations may offer clues to the etiology of autism. For example, there has been extensive speculation that toxic metals may contribute to the severity of autism, in part because of low glutathione [1–4] which is needed to remove some toxic metals. We hypothesize that reducing early exposure to toxic metals may help prevent or ameliorate autism, and treatment to remove toxic metals may reduce symptoms of autism; this hypothesis needs further exploration, as there is a growing body of research to support it.
Limitations of This Study
-
1.
The diagnosis of an autism spectrum disorder by a qualified medical professional was verified in writing, but there no additional verification. Similarly, for the neurotypical children, no additional verification was made beyond the parental report.
-
2.
The sample size of 55 children with ASD and 44 neurotypical children was large enough to observe many significant differences between the two groups, but some differences were only marginally or possibly significant—another study, preferably with larger number of participants—is needed to verify some of the observations.
-
3.
Some of the measurements of toxic metals were at levels near or below the detection limit. This was especially true of mercury, and measurements should be repeated with more sensitive methods.
-
4.
Medication effects: 45 % of the children with autism was taking one or more medications (see Table 1), and it is possible that some of those medications might affect detoxification ability.
-
5.
All the study participants were from Arizona, so the results for this region may be somewhat different from other parts of the USA or the world due to different environmental exposures.
Conclusions
This study found that children with autism, on average, had higher levels of lead in RBC (+41 %, p = 0.002), and higher urinary levels of lead (+74 %, p = 0.02), thallium (+77 %, p = 0.0001), tin (+115 %, p = 0.01), and tungsten (+44 %, p = 0.00005). The reason for the higher body burden of toxic metals may relate to increased exposure to toxic metals, increased absorption due to intestinal permeability, and/or decreased ability to excrete toxic metals (due to low glutathione and abnormal gut bacteria due to increased oral antibiotic use).
Regression analysis revealed that some toxic metals are strongly associated with variations in the severity of autism for each of the three autism severity scales investigated. Since toxic metals can significantly impair neurological development and function, it seems likely that the higher levels of toxic metals in the autism group may account for some of their symptoms.
We hypothesize that reducing early exposure to toxic metals may help to prevent or ameliorate autism, and treatment to remove toxic metals may help reduce some of the symptoms of autism, although much more research is needed to investigate these hypotheses.
References
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617
James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW (2006) Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 141:947–956
James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, Hubanks A, Gaylor DW (2009) Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr 89((1):425–430, Epub 2008 Dec 3
Adams JB, Audhya T, Mcdonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S, Barnhouse S, Lee W (2011) Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond) 8(1):34, Jun 8
Konstantareas MM, Homatidis S (1987) Ear infections in autistic and normal children. J Autism Dev Disord 17:585–594
Adams JB, Holloway CE, Margolis M, George F (2003) Heavy metal exposures, developmental milestones, and physical symptoms in children with autism. Conference Proceedings of the Fall 2003 Defeat Autism Now! Conference on Oct 3–5, 2003 in Portland, Oregon, p. 71–75.
Adams JB, Romdalvik J, Ramanujam VMS, Legator MS (2007) Mercury, lead, and zinc in baby teeth of children with autism vs. controls. J Toxicology Environ Health A 70:1046–1051
Adams JB, Romdalvik J, Levine KE, Hu L-W (2008) Mercury in first-cut baby hair of children with autism vs. typically-developing children. Toxicol Environ Chem 90(4):739–753
Rowland IR, Davies M, Evans J (1980) Tissue content of mercury in rats given methylmercury chloride orally: influence of intestinal flora. Arch Environ Health 35:155–160
Rowland IR, Robinson RD, Doherty RA (1984) Effect of diet on mercury metabolism and excretion in mice given methylmercury: role of gut flora. Arch Env Health 39:401–408
Holmes AS, Blaxill MF, Haley BE (2003) Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicology 22(4):277–285
Kern JK, Grannemann BD, Trivedi MH (2007) Adams J.B. sulfhydryl-reactive metals in autism. J Toxicol Environ Health A 70:1–7
Ip P, Wong V, Ho M, Lee J, Wong W (2004) Mercury exposure in children with autistic spectrum disorder: case–control study. J Child Neurol 19:431–434
DeSoto MC, Hitlan RT (2007) Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurology 22:1308–1311
Fido A, Al-Saad S (2005) Toxic trace elements in the hair of children with autism. Autism 9(3):290–298
Abdullah MM, Ly AR, Goldberg WA, Clarke-Stewart KA, Dudgeon JV, Mull CG, Chan TJ, Kent EE, Mason AZ, Ericson JE (2012) Heavy Metal in Children’s Tooth Enamel: Related to Autism and Disruptive Behaviors? J Autism Dev Disord 42:929–936
Ash MM, Nelson SJ (2003) Wheeler’s dental anatomy, physiology and occlusion, 8th edn. Elsevier, St Louis
Farmer JG, MacKenzie AB, Moody GH (2006) Human teeth as historical biomonitors of environmental and dietary lead: some lessons from isotopic studies of 19th and 20th century archival material. Environ Geochem Heal 28:421–430
Geier DA, Audhya T, Kern JK, Geier MR (2010) Blood mercury levels in autism spectrum disorder: is there a threshold level? Acta Neurobiol Exp (Wars) 70(2):177–186
Hertz-Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah IN (2010) Blood mercury concentrations in CHARGE Study children with and without autism. Environ Health Perspect 118(1):161–166
Yorbik O, Kurt I, Haşimi A, Oztürk O (2010) Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol Trace Elem Res 135(1–3):10–15, Epub 2009 Aug 18
Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR (2003) A case–control study of mercury burden in children with autistic spectrum disorders. J Am Phys Surg 8(3):76–79
Soden SE, Lowry JA, Garrison CB, Wasserman GS (2007) 24-Hour provoked urine excretion test for heavy metals in children with autism and typically developing controls, a pilot study. Clin Toxicol (Phila) 45(5):476–481, Jun–Aug
Nataf R, Skorupka C, Amet L, Lam A, Springbett A (2006) Lathe R Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicol Appl Pharmacol 214:99–108
Geier DA, Geier MR (2006) A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotox Res 10:57–64
Geier DA, Geier MR (2007) A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. J Toxicology and Environmental Health A70:1723–1730
Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Nataf R, Geier MR (2009) Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci 280:101–108, Epub - 2008 Sep 24
Woods JS, Armel SE, Fulton DI, Allen J, Wessels K, Simmonds PL, Granpeesheh D, Mumper E, Bradstreet JJ, Echeverria D, Heyer NJ, Rooney JP (2010) Urinary porphyrin excretion in neurotypical and autistic children. Environ Health Perspect 118(10)):1450–1457, Epub 2010 Jun 24
Windham GC, Zhang L, Gunier R, Croen LA, Grether JK (2006) Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect 114(9):1438–1444
Palmer RF, Blanchard S, Wood R (2009) Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place 15(1):18–24, Epub 2008 Feb 12
Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C (2006) Environmental mercury release, special education rates, and autism disorder: an ecological study of Texas. Health Place 12(2):203–209
Shandley K, Austin DW (2011) Ancestry of pink disease (infantile acrodynia) identified as a risk factor for autism spectrum disorders. J Toxicol Environ Health A 74(18):1185–1194
Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, Zappia I, Newmark S, Gehn E, Rubin RA, Mitchell K, Bradstreet J, El-Dahr JM (2009) The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J Toxicol 2009(532640):7. doi:10.1155/2009/532640
Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, Zappia I, Newmark S, Gehn E, Rubin RA, Mitchell K, Bradstreet J, El-Dahr J (2009) Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part A—medical results. BMC Clin Pharmacol 9:16
Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, Zappia I, Newmark S, Gehn E, Rubin RA, Mitchell K, Bradstreet J, El-Dahr J (2009) Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part B—behavioral results. BMC Clin Pharmacol 9:17
Puchyr RF, Bass DA, Gajewski R, Calvin M, Marquardt W, Urek K, Druyan ME, Quig D (1998) Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol Trace Elem Res 62(3):167–182
Cohen IL, Schmidt-Lackner S, Romanczyk R, Sudhalter V (2003) The PDD behavior inventory: a rating scale for assessing response to intervention in children with pervasive developmental disorder. J Autism Dev Disord 33(1):31–45
Rimland B and Edelson S (2000) Autism treatment evaluation checklist: statistical analyses, Autism Research Institute
de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51(4):418–424
Rowland IR, Grasso P, Davies MJ (1975) The methylation of mercuric chloride by human intestinal bacteria. Experientia 31:1064–1065
Acknowledgments
First and foremost, we thank the many autism families and their friends who volunteered as participants in this research study. We thank the Autism Society of Greater Phoenix and the Arizona Division of Developmental Disabilities for assistance with advertising this study. We thank the staff of the Southwest College of Naturopathic Medicine (N. Foster, M. Harland, B. Peterson, N. Tkacenko) for the help with phlebotomy, and we thank ICDRC for providing use of their offices for participant visits. We thank Vitamin Diagnostics and Doctor’s Data for providing testing for this study. We thank Jon Pangborn for commenting on the manuscript.
Declaration of Competing Interests
The authors declare that they have no competing interests.
Funding Acknowledgements
This work was supported by the Autism Research Institute and the Legacy Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adams, J.B., Audhya, T., McDonough-Means, S. et al. Toxicological Status of Children with Autism vs. Neurotypical Children and the Association with Autism Severity. Biol Trace Elem Res 151, 171–180 (2013). https://doi.org/10.1007/s12011-012-9551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9551-1