Abstract
ASD is a complex condition defined by many causes, one of them being excessive concentrations of necessary and harmful chemicals in children. The serum, hair, and nails of children with ASD have lower levels of critical trace elements, according to studies. It is quite obvious that bio elements are involved in physiology and pathophysiology. Thus, this study examined trace element contents in serum samples from children with autism spectrum disorder (ASD), specifically zinc (Zn), aluminum (Al), and selenium (Se). The study also looked for links between trace element levels and autistic severity. The study included 47 children with autism spectrum disorder, and the Gilliam’s Scale was used for severity. The study also included 53 healthy kids with age and gender-matched with those of ASD. For serum trace element analysis, graphite furnace atomic absorption spectrophotometry was used. The study found significant decreases in selenium and zinc concentration (OR, 5.25; CI, 1.96 ~ 14.08; p < 0.001) and increases in aluminum level (OR, 39.34; CI, 8.20 ~ 89.45; p < 0.001) in children with ASD compared to the control group. The area under the curve (AUC) values of 0.85 for Se, 0.98 for Al, and 0.7 for Zn showed high sensitivity and specificity for all parameters. Results indicate a strong positive connection between ASD and their levels of selenium (Se) and zinc (Zn) (β, 0.48; CI, 0.280 ~ 0.679; p < 0.001 and β, 0.31; CI, 0.10 ~ 0.52; p = 0.005). There is a negative correlation between ASD and aluminum (Al) (β 0.83; CI, 0.71 ~ 0.95; p < 0.001). This element may be a biomarker for autism in youngsters. High odds ratio (OR) values indicate trace element risk in autistic children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ASD is a disability that is identified based on observable behaviors, which of difficulties in social communication, social interaction, and impairment in language. Individuals with autism also exhibit a limited range of interests and frequently engage in repetitive behaviors and mannerisms [1]. Autism spectrum disorder (ASD) was initially documented in 1943, and afterward, there has been a notable surge in the prevalence of ASD on a global scale [2]. Despite extensive research and analysis spanning several decades, the precise etiology of autism remains elusive. This is mostly due to the complex and varied nature of the condition, which manifests with variable degrees of severity [3]. The incidence of ASD has seen a notable rise in recent years. According to recent data, the prevalence of ASD in the USA was found to be one in 54 children aged 8 years across 11 different sites. Additionally, the incidence of ASD was seen to be 4.3 times higher in males compared to girls. In China, the prevalence of ASD among children aged 6 to 12 is estimated to be 0.7%. These findings are based on studies conducted in the respective regions [4, 5]. The escalating incidence of ASD has engendered apprehensions within the realm of public health. The precise etiology of ASD remains uncertain; nonetheless, it is widely acknowledged that the development of ASD is influenced by a combination of genetic and environmental variables [6]. Research has shown that an imbalance of trace elements can lead to impairments in the nervous system and biological dysfunctions linked with (ASD) [7]. Zinc (Zn), copper (Cu), and selenium (Se) are essential trace elements that play crucial roles in multiple physiological functions and are needed for various enzymatic processes. Therefore, the biochemical regulation of these elements is of utmost importance [8]. Previous studies have noted that the antagonistic interactions between zinc (Zn) and copper (Cu) can have a significant impact on the pathophysiology of ASD by influencing the metallothionein system, which in turn leads to the induction of cellular excitotoxicity [9]. Zinc can detoxify heavy metals if interacts with them increasing the synthesis of metallothionein. The heavy metals compete with copper and zinc for binding with metallothionein because of having a high affinity for binding with it [10]. Selenium is a crucial component of numerous antioxidant enzymes, such as thioredoxin reductase, iodothyronine deiodinase, and glutathione peroxidase. Selenium plays a vital antioxidant role in the regular activities of humans and shields cells from the damaging effects of free radicals as a component of enzymes that catalyze oxidation and reduction reactions [11]. Selenium can exert its detoxification effect on mycotoxin poisoning by reducing reactive oxygen species production and mitochondrial dysfunction, enhancing cell viability and function, and inhibiting the immune response [12]. Also, Se can further counteract the immunotoxin effects of T-2 toxins on T lymphocytes [13]. Se can detoxify heavy metals such as cadmium, inorganic mercury, methylmercury, thallium, and to a limited extent silver, depending on the selenium/mineral interaction. If the selenide combines with the heavy metal, ions will be given a metal selenide, which is metabolically inert [14]. Additional trace metals arsenic (As), lead (Pb), aluminum (Al), and cadmium (Cd) are known to have a significant impact on autism spectrum disorder (ASD) by triggering neuroinflammation, oxidative stress, excitotoxicity, and disrupting the balance of critical ions within the body. Furthermore, the elements manganese (Mn) and iron (Fe) have been found to potentially contribute to neurotoxicity, which may have a significant impact on the development of autism spectrum disorder (ASD) [15]. Numerous studies have been conducted to investigate the presence of essential and hazardous elements in children diagnosed with ASD. However, the predominant focus of these investigations has been on the analysis of trace element concentrations in blood or hair [16,17,18]. Previous studies have observed a correlation between trace element concentrations and the presence of social communication difficulties and repetitive behaviors in children with ASD as compared to typically developing children [19]. In addition, individuals who have received a diagnosis of ASD may manifest a range of characteristic behaviors associated with the condition, such as challenges in communication, repetitive actions, self-harming tendencies, disturbances in sleep patterns, and difficulties in adhering to dietary norms [20]. Furthermore, it is important to note that the manifestation of these autistic symptoms might exhibit significant variability across individuals [21]. Numerous studies have been conducted to evaluate the association between ASD and the levels of trace elements [22]. Therefore, it is essential to acknowledge the significance of evaluating the association between ASD and blood trace elements as a viable approach for the early detection of the condition, because is cost-effective.
Study Design
The current study comprised two cohorts. The first cohort consisted of children diagnosed with autism spectrum disorder (ASD) at the Hamaem Al-Salam Center in Iraq/Najaf, totaling 47 participants. The second cohort served as the control group and included 53 individuals. Furthermore, the study exclusively focused on children diagnosed with autism spectrum disorder (ASD), while children with other mental disorders were deliberately excluded from the research. It is noteworthy to remark that the control samples were obtained from youngsters residing in the Karbala province of Iraq. The Gillim scale was employed to assess the severity of ASD in the individuals. Ultimately, the age range of participants in both groups spanned from 3 to 12 years of age (mean ± SD, 6.35 ± 2.37).
Results
Characteristic Features
The questionnaire is completed by the parents of the children or psychological trainers employed at the autism center who oversee the care of children diagnosed with autism. From the same children, the blood samples were collected in order to assess their clinical characteristics. The aforementioned questions have been incorporated into the compilation of questions as presented in Table 1.
Distribution of Samples
After performing the distribution test (Kolmogorov-Smirnov and Shapiro-Wilk test) for the samples, it was found that they had an abnormal distribution (p-value for all elements less than 0.05), as illustrated in Figure 1. In light of the results of this test, the most effective strategy for carrying out statistical procedures was selected.
Blood Parameters Under Study
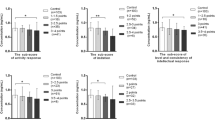
The concentrations of three trace elements, namely selenium, aluminum, and zinc, were measured in the serum specimens of all participants. A comparison was then conducted between the two groups involved in the study, and the results are displayed in Table 2. Interestingly, a significant decrease in the levels of selenium and zinc elements and a significant increase in the levels of aluminum were observed in children with autism spectrum disorder (ASD). Specifically, the odds ratios (OR) and confidence intervals (CI) for selenium were found to be 5.25 (CI, 1.96~14.08; p < 0.001), for aluminum 39.34 (CI, 8.20~89.45; p < 0.001), and for zinc 3.75 (CI, 1.44~9.76; p = 0.02).
Spearman’s correlation coefficient (r) test was used to examine the correlation between elements measured. Through results presented in Table 3, noted a strong negative only between selenium and aluminum (p < 0.001).
The ROC analysis test is the basic measure of accuracy: sensitivity (the probability the diagnostic test is positive for disease for a patient who truly has the disease) and specificity (the probability the diagnostic test is negative for disease for a patient who truly does not have the disease) [23]. It was utilized to calculate the area under the curve for all parameters (Figure 2) and considered an excellent value (AUC of Se, 0.85; AUC of Al, 0.98; and AUC of Zn, 0.70). The optimal cutoff values, sensitivity, and specificity for serum trace elements are presented in Table 4. The percentage sensitivity and specificity for all trace elements under this study are considered excellent pedigree.
Method
Blood Specimen Collection
Venous blood samples, measuring 5 mL each, were obtained from both cohorts, consisting of individuals with (ASD) and a control group. Subsequently, the specimens were transferred into gel tubes and allowed to clot at ambient temperature. Following clotting, the samples were subjected to centrifugation with a force of 3000 times the acceleration due to gravity (xg) for 10 min. The serum was isolated and afterward stored in an Eppendorf tube at a temperature of −20 °C ready for the next step [24].
Determination of Trace Elements
The furnace atomic absorption spectrometry (AAS) (SHIMADZU AA-6300\Japan) technique with low detection limits for 0.2 ppb located in the laboratories of the College of Medicine, University of Karbala was used to determine trace elements in environmental and biological samples by conversion of an element into a free atomic state by means other than flame, such as using electronic furnaces and then measuring the absorbed light of a certain wavelength in the ground state.
The maxim wavelength for all elements is determined by plotting absorbance vs. wavelength in the graph. Moreover, the absorbance maximum is used instead of some other point on the absorption curve because the maximum is the most reliable position to measure. The wavelength for selenium was 196 nm for aluminum (309.3nm) and 213.9 nm for zinc.
Ethical Approval Declarations
The study involved the collection of blood samples from children with autism, following the necessary administrative procedures, including obtaining consent from parents. This process was conducted under the supervision of the Dean of the College of Science and the Head of the Chemistry Department at the University of Kerbala, with oversight provided by the professor overseeing the research. The specimens were obtained from the participants at the central site situated in Najaf, Iraq. Furthermore, the Karbala University Ethics Committee process was followed in a current project under the form number 6633257/KUEC.
Statistical Analysis
The data results were tested using version 26 of the Statistical Package for the Social Sciences (SPSS), developed by IBM. The Shapiro test, which is a normality test, was employed to assess the distribution of the samples. The Shapiro-Wilk test revealed a violation of the assumption of normality, thus necessitating the utilization of the Mann-Whitney U test to analyze the data gathered in this study. The receiver operating characteristic (ROC) analysis test was employed to compute the area under the curve, which is widely regarded as a reliable measure of sensitivity and specificity in detecting and identifying. On the other hand, a linear regression model was employed to examine the association between serum selenium (Se), aluminum (Al), and zinc (Zn) levels and autism spectrum disorder (ASD). To examine the relationship between the variables in this study, a correlation analysis was conducted. The statistical analysis in this study utilized Spearman’s rho correlation coefficient (r). A p-value of 0.05 or below was deemed to be statistically significant in all conducted statistical tests.
Discussion
The results of this study suggest a notable correlation between reduced selenium levels and the occurrence of autistic spectrum disorder. Selenium, along with its related selenoproteins, plays a crucial role in various biological processes, including the manufacture of thyroid hormones, DNA replication, reproductive capacity, and antioxidant defense [25]. Selenium (Se) plays a role in immune function by modulating the activity of activated T lymphocytes. Multiple studies have demonstrated that the administration of selenium (Se) supplements results in an accelerated and enhanced immune response [26]. The vital role of Se in brain function continues to be apparent. Selenoprotein P (SEPP1) is accountable for the transportation of selenium (Se) to the brain and serves a neuroprotective function in mitigating oxidative stress. Mice lacking the selenoprotein P1 (SEPP1) gene display evident impairments in brain function. The mice in this study had brain damage and eventual mortality as a result of insufficient dietary selenium [27]. The majority of selenoproteins have a role in the cellular response to oxidative stress [28], and the ability to counteract oxidative stress is crucial for proper neurodevelopment. The occurrence of heightened oxidative stress has been commonly documented in children diagnosed with ASD [29]. This oxidative stress is mostly attributed to the presence of reactive oxygen species (ROS) [30], elevated levels of lipid peroxidation [31], and diminished concentrations of antioxidants [32]. It is noteworthy that a particular antioxidant, glutathione peroxidase-1 (GPx1), which is a selenoprotein and recognized as a gene associated with ASD, regularly exhibits lower levels in children diagnosed with ASD [33]. In recent studies, it has been demonstrated that Se can inhibit a specific cellular mechanism known as ferroptosis, which is triggered by oxidative stress [34]. While the investigation of ferroptosis has predominantly focused on its occurrence in adult individuals with stroke, traumatic brain injury (TBI), and Parkinson’s disease, there is a possibility that this mechanism also plays a role in nervous development. Therefore, Se deficiency may lead to heightened neuronal susceptibility to oxidative stress in children diagnosed with ASD. Even though Se is essential for healthy brain function, Se neurotoxicity has received a lot of attention lately [35]. Numerous studies have been conducted, especially in the past few years, that indicate different inorganic and organic selenium compounds may have neurotoxic consequences if they are present at spur nutritional levels [36]. All things considered, everyone agrees that inorganic Se species selenite in particular are more neurotoxic than organic Se compounds [37].
The potential association between aluminum (Al) and ASD has been examined by multiple researchers [38, 39]. Despite the prevalence of aluminum in the natural environment, it lacks any discernible biological role within the human body. Numerous studies have demonstrated that aluminum has the potential to induce developmental and immune impairments, disturb hormonal balance, exhibit neurotoxic properties, and impact cognitive functions and behavioral patterns. Aluminum (Al) is a neurotoxin that has been empirically proven to have detrimental effects on the human nervous system for several decades [40]. Previous studies have provided evidence indicating that individuals diagnosed with ASD exhibit a notable presence of aluminum (Al) deposition inside the brain [41]. A study conducted by Melendez et al. demonstrated a correlation between elevated body aluminum levels and the presence of behavioral abnormalities in individuals with ASD [42]. Furthermore, empirical investigations have shown evidence of the capacity of Al compounds to negatively impact social behavior [43]. While there is currently no available evidence suggesting a direct link between aluminum (Al) exposure and catatonia, several researchers have presented findings that support the detrimental impact of Al on motor function [44, 45].
The findings of this study revealed a statistically significant reduction in zinc levels in children diagnosed with ASD. The presence of zinc deficiency can have various impacts on the immunological and nervous systems of the human body. In both the developmental and adult stages of neurogenesis, Zn2+ is an essential component for several processes such as proliferation, migration, differentiation, and survival. Neurons that are deficient in zinc exhibit reduced proliferation, differentiation, and apoptotic pathway activation [46]. In fact, data suggests that the hippocampal region is most likely the most vulnerable to a zinc deficit [47], which reduces progenitor cell number and neuronal differentiation, thus causing irreversible impairment of learning and memory capacity during early development [48]. There is a suggestion that chronic zinc shortage may lead to reduced efficiency of the adaptive immune system and increased reliance on the innate immune system, even though the innate immune system also experiences damage in the presence of zinc deficiency [49]. According to a study conducted by Bonaventura et al. in 2015, it has been documented that zinc possesses anti-inflammatory properties. Additionally, a zinc deficiency has been found to elevate the levels of pro-inflammatory cytokines and enhance the activity of the central nervous system inflammasome [50]. Zinc has been found to protect against maternal insult induced by lipopolysaccharide in animal models. This protection has been observed in the context of preventing aberrant behavior in object recognition tasks and abnormal sickness behavior following immune challenges. Furthermore, recent studies have shown that zinc can also prevent communication deficits in a mouse model of autism [51]. A recent review has also examined the relationship between zinc and the nervous system [52]. Considering the various functions of zinc, it seems advantageous to examine its classification into three overarching categories: structural roles, cellular signaling, and enzymatic co-factors. Zinc serves as an essential co-factor for many enzymes involved in DNA and RNA polymerization, histone modification, and DNA repair through the action of DNA ligase. Zinc plays a crucial role in various facets of protein synthesis inside the central nervous system (CNS) and functions as an autonomous factor in regulating gene expression [53]. In recent times, zinc has been recognized as a crucial constituent in structural proteins, particularly in the context of zinc-finger motifs [43]. Proteins with widespread presence are frequently responsible for the composition of receptors found in the brain, such as estrogen, thyroid hormone, and glucocorticoid receptors [54]. Zinc plays a crucial role in facilitating the folding process and the subsequent development of the functional structure of these receptors [55]. Zinc has been associated with the development of olfactory, cerebellar, and hippocampus regions, and even a slight shortage of zinc has been demonstrated to impact memory and learning [56]. Previous studies have provided evidence indicating that temporary zinc deficit during gestation can have long-lasting effects on memory and learning abilities that extend into adulthood [57].
Conclusion
The findings of this study indicate a correlation between the levels of selenium (Se), aluminum (Al), and zinc (Zn) in serum and autism spectrum disorder (ASD) among patients in Iraq. Additionally, the high percentage of sensitivity and specificity observed for all trace elements examined in this study suggests their potential as reliable biomarkers for diagnosing autism in children. Elevated odds ratio values are indicative of an increased risk of trace element exposure in children diagnosed with autism.
Data Availability
No datasets were generated or analysed during the current study.
References
Hodges H, Fealko C, Soares N (2020) Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 9(1):S55-65. https://doi.org/10.21037/tp.2019.09.09
Wolf JM, Barton M, Jou R (2018) Autism spectrum disorder. Neuropsychol Cond Across Lifespan 45–60. https://doi.org/10.1007/978-3-319-95720-3_16
Waterhouse LH, Waterhouse L (2013) Waterhouse Rethinking autism: Variation and complexity, 1st edn. Elsevier, New York, pp 73–75
Shaw KA (2022) progress and disparities in early identification of autism spectrum disorder: autism and developmental disabilities monitoring network. J Am Acad Child Adolesc Psych 61(7):4–8. https://doi.org/10.1016/j.jaac.2021.11.019
Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X et al (2020) Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. 36(9):961–71. https://doi.org/10.1007/s12264-020-00530-6
Rodrigues TS (2017) Settings Announcement Format _ Quote Question _ Answer Thumb _ Up Textsms. https://doi.org/10.3390/w11050921
Saldanha Tschinkel PF, Bjørklund G, Conón LZZ, Chirumbolo S, Nascimento VA (2018) Plasma concentrations of the trace elements copper, zinc, and selenium in Brazilian children with autism spectrum disorder. Biomed Pharmacother. 106:605–9. https://doi.org/10.1016/j.biopha.2018.06.174
Zhang Y (2017) Trace elements and healthcare: a bioinformatics perspective. Adv Exp Med Biol 1005:63–98. https://doi.org/10.1007/978-981-10-5717-5_4
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73(2):225–236
Zalewska M, Trefon J, Milnerowicz H (2014) The role of metallothionein interactions with other proteins. Proteomics 14(11):1343–1356. https://doi.org/10.1002/pmic.201300496
Kielczykowska M, Kocot J, Pazdzior M, Musik I (2018) Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med. 27(2):245–55. https://doi.org/10.17219/acem/67222.
Wang X, Zuo Z, Deng J, Zhang Z, Chen C, Fan Y et al (2018) Protective role of selenium in immune-relevant cytokine and immunoglobulin production by piglet splenic lymphocytes exposed to deoxynivalenol. Biol Trace Elem Res. 184(1):83–91. https://doi.org/10.1007/s12011-017-1160-6
He Y, Fang J, Peng X, Cui H, Zuo Z, Deng J et al (2014) Effects of sodium selenite on aflatoxin B1-induced decrease of ileal IgA+ cell numbers and immunoglobulin contents in broilers. Biol Trace Elem Res 160(1):49–55. https://doi.org/10.1007/s12011-014-0035-3
Liu X, Lin J, Zhang H, Khan NU, Zhang J, Tang X et al (2022) Oxidative stress in autism spectrum disorder—current progress of mechanisms and biomarkers. Front Psychiatry 13(1):1–35. https://doi.org/10.3389/fpsyt.2022.813304
Leong YK, Chang JS (2020) Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour Technol. 303(May):5–9. https://doi.org/10.1016/j.biortech.2020.122886
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG et al (2017) Assessment of serum trace elements and electrolytes in children with childhood and atypical autism. J Trace Elem Med Biol. 43:9–14. https://doi.org/10.1016/j.jtemb.2016.09.009
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG et al (2017) Analysis of hair trace elements in children with autism spectrum disorders and communication disorders. Biol Trace Elem Res 177(2):215–223. https://doi.org/10.1007/s12011-016-0878-x
Rashaid AHB, Nusair SD, Alqhazo MT, Adams JB, Abu-Dalo MA, Bashtawi MA (2021) Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: a case-control study on Jordanian children. J Trace Elem Med Biol 67(September):1–6. https://doi.org/10.1016/j.jtemb.2021.126790
Fiore M, Barone R, Copat C, Grasso A, Cristaldi A, Rizzo R et al (2020) Metal and essential element levels in hair and association with autism severity. J Trace Elem Med Biol 57:31630927. https://doi.org/10.1016/j.jtemb.2019.126409
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Restrepo B, Angkustsiri K, Taylor SL, Rogers SJ, Cabral J, Heath B et al (2020) Developmental–behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Res 13(10):1778–1789. https://doi.org/10.1002/aur.2354
Zhang J, Li X, Shen L, Khan NU, Zhang X, Chen L et al (2021) Trace elements in children with autism spectrum disorder: a meta-analysis based on case-control studies. J Trace Elem Med Biol 67:34049201. https://doi.org/10.1016/j.jtemb.2021.126782
El-Ansary A, Bjørklund G, Tinkov AA, Skalny AV, Al Dera H (2017) Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis. 32(4):1073–80 (http://springerlink.bibliotecabuap.elogim.com/referenceworkentry/10.1007/978-1-4899-7502-7_739-1)
The World Health Organization (2007) Manual for the laboratory diagnosis of measles and rubella virus infection, 2nd edn. WHO, pp 1–109
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18(3):3292–3311. https://doi.org/10.3390/molecules18033292
Rayman MP (2012) Selenium and human health. Lancet. 379(9822):1256–68. https://doi.org/10.1016/S0140-6736(11)61452-9
Zhang ZH, Song GL (2021) Roles of selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Front Neurosci. 15:1–24. https://doi.org/10.3389/fnins.2021.646518
Raymond LJ, Deth RC, Ralston NVC (2014) Potential role of selenoenzymes and antioxidant metabolism in relation to autism etiology and pathology. Autism Res Treat 2014:1–15. https://doi.org/10.1155/2014/164938
Bjørklund G, Meguid NA, El-Bana MA, Tinkov AA, Saad K, Dadar M et al (2020) Oxidative stress in autism spectrum disorder. Mol Neurobiol. 57(5):2314–32. https://doi.org/10.1007/s12035-019-01742-2
Yui K, Kawasaki Y, Yamada H, Ogawa S (2016) Oxidative stress and nitric oxide in autism spectrum disorder and other neuropsychiatric disorders. CNS Neurol Disord - Drug Targets 15(5):587–596. https://doi.org/10.2174/1871527315666160413121751
Angelova PR, Myers I, Abramov AY (2023) Carbon monoxide neurotoxicity is triggered by oxidative stress induced by ROS production from three distinct cellular sources. Redox Biol 60(April):1–16. https://doi.org/10.1007/s00204-023-03562-9
Manivasagam T, Arunadevi S, Essa MM, SaravanaBabu C, Borah A, Thenmozhi AJ et al (2020) Role of oxidative stress and antioxidants in autism. Adv Neurobiol 24:193–206. https://doi.org/10.1007/978-3-030-30402-7_7
Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S (2010) Genetic variant of glutathione peroxidase 1 in autism. Brain Dev 32(2):105–109. https://doi.org/10.1016/j.braindev.2008.12.017
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262-1279.e25. https://doi.org/10.1016/j.cell.2019.03.032
Zhang ZH, Song GL (2021) Roles of selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Front Neurosci 15:1–24. https://doi.org/10.1016/j.toxlet.2013.11.016
Lopes de Andrade V, Marreilha dos Santos AP, Aschner M (2021) Neurotoxicity of metal mixtures. Adv Neurotoxicol 5:329–364. https://doi.org/10.1007/978-3-319-60189-2_12
Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M et al (2018) Selenium for preventing cancer. Cochrane Database Syst Rev. 2018(1). https://doi.org/10.1002/14651858.CD005195.pub4
Blaylock R, Maroon J (2011) Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-a unifying hypothesis. Surg Neurol Int 2(1):107. https://doi.org/10.4103/2152-7806.83391
Bjørklund G, Skalny AV, Rahman MM, Dadar M, Yassa HA, Aaseth J et al (2018) Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ Res. 166:234–50. https://doi.org/10.1016/j.envres.2018.05.020
Strunecka A, Blaylock RL, Strunecky O (2016) Fluoride, aluminum, and aluminofluoride complexes in the pathogenesis of the autism spectrum disorders: a possible role of immunoexcitotoxicity. J Appl Biomed. 14(3):171–6. https://doi.org/10.4103/sni.sni_407_17
Mold M, Umar D, King A, Exley C (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol. 46:76–82. https://doi.org/10.1016/j.jtemb.2017.11.012
Melendez L (2013) Aluminum and other metals may pose a risk to children with autism spectrum disorder: biochemical and behavioural impairments. Clin Exp Pharmacol 03(01):1–9. https://doi.org/10.4172/2161-1459.1000120
Sheth SKS, Li Y, Shaw CA (2018) Is exposure to aluminum adjuvants associated with social impairments in mice? A pilot study. J Inorg Biochem. 181:96–103. https://doi.org/10.1016/j.jinorgbio.2017.11.012
Wise JP, Young JL, Cai J, Cai L (2022) Current understanding of hexavalent chromium [Cr (VI)] neurotoxicity and new perspectives. 158. Environ Int 158:1–44. https://doi.org/10.1016/j.envint.2021.106877
Ashmawi NS, Hammoda MA (2022) Early prediction and evaluation of risk of autism spectrum disorders. Cureus 14(3):1–10. https://doi.org/10.7759/cureus.23465
Neurobiology PM, Journal E (2021) Neurobiology of zinc a nd its role in neurogenesis Background bbreviations. 1–28. https://doi.org/10.1007/s00394-020-02454-3
Szewczyk B (2013) Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 5(JUL):1–43. https://doi.org/10.3389/fnagi.2013.00033
Choi S, Hong DK, Choi BY, Suh SW (2020) Zinc in the brain: friend or foe? Int J Mol Sci 21(23):1–24. https://doi.org/10.3390/ijms21238941
Summersgill H, England H, Lopez-Castejon G, Lawrence CB, Luheshi NM, Pahle J et al (2014) Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis 5:3–4. https://doi.org/10.1038/cddis.2013.547
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14(4):277–285. https://doi.org/10.1016/j.autrev.2014.11.0.08
Kirsten TB, Queiroz-Hazarbassanov N, Bernardi MM, Felicio LF (2015) Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci 130:12–7. https://doi.org/10.1016/j.lfs.2015.02.027
Gower-Winter SD, Levenson CW (2012) Zinc in the central nervous system: from molecules to behavior. BioFactors 38(3):186–193. https://doi.org/10.1002/biof.1012
Prakash A, Bharti K, Majeed ABA (2015) Zinc: indications in brain disorders. Fundam Clin Pharmacol. 29(2):131–49. https://doi.org/10.1111/fcp.12110
Physiological PM, Journal T (2017) Physiological roles of zinc transporters: molecular and genetic import a nce in zinc homeostasis. https://doi.org/10.1007/s12576-017-0521-4
Ahmed S, Venkatesan G, Prapul DS, Md AR (2023) Zebrafish as a comprehensive model of neurological diseases. Int J Sci Res Arch. 10(1):018–052. https://doi.org/10.30574/ijsra.2023.10.1.0696
Xie Z, Ma X, Ji W, Zhou G, Lu Y, Xiang Z et al (2010) Zbtb20 is essential for the specification of CA1 field identity in the developing hippocampus. Proc Natl Acad Sci U S A 107(14):6510–6515. https://doi.org/10.1073/pnas.0912315107
De Souza AS, Fernandes FS, das Graças Tavares do Carmo M (2011) Effects of maternal malnutrition and postnatal nutritional rehabilitation on brain fatty acids, learning, and memory. Nutr Rev 69(3):132–144. https://doi.org/10.1111/j.1753-4887.2011.00374.x
Author information
Authors and Affiliations
Contributions
Study design and conception, proofreading, and statistical analysis by Narjis Hadi Al-Saadi. Material preparation, data collection, analysis, and writing—first draft by Ali Fadheel Hamoud. Interpretation of the results by Narjis Hadi Al-Saadi and Ali Fadheel Hamoud.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamoud, A.F., Al-Saadi, N.H. The Assessment of Selenium, Aluminum, and Zinc in Children with Autism Spectrum Disorder. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04283-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04283-5