Abstract
The aim of the present study was to determine the levels of metals in blood (zinc (Zn), copper (Cu), aluminium (Al), lead (Pb) and mercury (Hg)), as well as the specific porphyrin levels in the urine of patients with autism spectrum disorder (ASD) compared with patients with other neurological disorders. The study was performed in a group of children with ASD (N = 52, average age = 6.2 years) and a control group of children with other neurological disorders (N = 22, average age = 6.6 years), matched in terms of intellectual abilities (Mann-Whitney U = 565.0, p = 0.595). Measurement of metals in blood was performed by atomic absorption spectrometry, while the HPLC method via a fluorescence detector was used to test urinary porphyrin levels. Results were compared across groups using a multivariate analysis of covariance (MANCOVA). In addition, a generalized linear model was used to establish the impact of group membership on the blood Cu/Zn ratio. In terms of blood levels of metals, no significant difference between the groups was found. However, compared to the control group, ASD group had significantly elevated blood Cu/Zn ratio (Wald χ 2 = 6.6, df = 1, p = 0.010). Additionally, no significant difference between the groups was found in terms of uroporphyrin I, heptacarboxyporphyrin I, hexacarboxyporphyrin and pentacarboxyporphyrin I. However, the levels of coproporphyrin I and coproporphyrin III were lower in the ASD group compared to the controls. Due to observed higher Cu/Zn ratio, it is suggested to test blood levels of Zn and Cu in all autistic children and give them a Zn supplement if needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a developmental disorder that affects essential human behaviours such as social interaction, the ability to communicate ideas and feelings, imagination and the establishment of relationships with others. The previous version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) listed three distinct subgroups for ASD: autistic disorder (AD), Asperger syndrome and pervasive development disorder-not otherwise specified (PDD-NOS) [1]. In 2013, a new edition of this manual was published (DSM-5), which eliminated these subgroups and replaced them with one broad diagnosis of ASD [2]. Autistic individuals are now placed on a continuum depending on the severity of their symptoms. The diagnosis is made on the basis of characteristic clinical signs because there are no known biological markers so far. ASD is a developmental disorder with a complex and heterogeneous aetiology, including both genetic and environmental factors.
Autism was once considered as a rare disorder; however, the reported incidence and prevalence have drastically increased during the last decades. Today, at least 1 in 88 children in the USA is diagnosed with ASD [3]. It is not known to what extent the exceptional growth in the prevalence of all forms of ASD combined is real or not, but there can be no doubt that the registration for the sum of all kinds of ASD must have been affected by a change in the concept in terms of dimensional perspective, by substitution and “addition” of diagnoses, as well as greater awareness, new therapeutic options and specific approaches to the education of these children [4–7].
The aetiopathogenesis remains unexplained in the majority of cases. It still holds true that genetic influences are the most important in ASD, although in recent studies, researchers have found less concordance for monozygotic (0.58) and dizygotic (0.21) twins in comparison with older studies, which indirectly points to the greater importance of environmental factors [8]. With new strategies in the field of genetic research (analysis of genetic linkage and association studies), more and more genes are being discovered that are associated with an increased risk of autism, but despite this, in the majority of autism in clinical practice, no genetic disorder is detected that would clarify the specific clinical picture [9, 10]. The newer genomic screening methods, especially array comparative genomic hybridization, contribute to a more precise clinical genetic diagnostic work-up compared to classical karyotyping. Rare de novo or congenital structural variation in the genome, especially copy-number variations (CNVs), should be found in 5–10 % of children with “idiopathic” autism; however, in a study, which comprised mostly children with ASD and associated mental retardation, this was even higher, at 16.6 % [11].
In the last 10 years, there has been a marked increase in studies on possible environmental risk factors. One of the largest American population studies—CHARGE (an epidemiologic investigation of genetic and environmental factors contributing to autism)—dating from 2006, which links environmental and genetic factors, was however focused on environmental factors that could influence the development of ASD during pregnancy and in the first year of life [12]. Other newer larger population studies are the Study to Explore Early Development (SEED) and especially the Autism Birth Cohort (ABC), which in addition to the interaction between genetic and environmental factors also include a time dimension [13, 14].
Among prenatal infections, rubella holds first place as a risk factor. Excessive alcohol consumption, consumption of certain medicines—valproate, thalidomide and misoprostol—older parental age and maternal metabolic disturbances during pregnancy increase the risk of developing ASD [15–17]. Concerning harmful factors during pregnancy that have been shown to be associated with enhanced risk of autism in the offspring, some of them are extremely well-documented mutagens. This is the case for folate deficiency [18–22] and alcohol [23–26]. Several pesticides are well-documented chemical mutagens. They act by different mechanisms, but some of them are aryl hydrocarbon (Ah) receptor agonists, like the dioxins [27–29], PCBs [29–31] and brominated flame retardants [32].
Within the scope of the CHARGE study, in addition to the previously mentioned factors, they also identified air pollution, febrile illnesses during pregnancy and exposure to pesticides as possible risk factors, while the consumption of folic acid at the beginning and in the first trimester of pregnancy decreased the risk of developing ASD [33–37]. The amount of information concerning immune factors associated with ASD is also on the increase [38–40]. It is still very uncertain, if immune disturbances cause ASD or opposite or if the epidemiological association due to something else is a common aetiological factor (or factors) both in immune disturbances and ASD.
Many studies have studied the possible influence of metals, especially mercury (Hg), zinc (Zn), copper (Cu), aluminium (Al) and lead (Pb) on the development of ASD [41–46]. Mercury is among the most toxic heavy metals for the developing brain. It can cause a number of neurological abnormalities, including an autism-like behavioural picture [47]. A small portion of the population may be particularly sensitive to Hg [48, 49].
Porphyrins, especially specific porphyrins in the urine, can be an indicator of heavy metal poisoning [50, 51]. In autistic children, certain derivatives—coproporphyrin and precoproporphyrin—have been found in a significantly increased amount than in the control groups of children with non-neurological diseases and healthy children [52]. The remaining porphyrins—uroporphyrin, heptacarboxyporphyrin and pentacarboxyporphyrin—show a less consistent pattern of association with ASD [53].
However, porphyria is very commonly a consequence of enhanced oxidative stress in important heme-synthesizing cells and organs [54–56]. Since it is common that ASD patients have other abnormalities telling about enhanced oxidative stress or impaired antioxidant defence [57], it must be a reasonable working hypothesis that porphyria in ASD patients may most commonly be a result of oxidative stress caused by the disease itself rather than being a symptom of poisoning by some toxic metal.
Excessive excretion of porphyrins in the urine is the result of the blockade of key enzymes in porphyrin metabolism, e.g. uroporphyrinogen decarboxylase and coproporphyrinogen oxidase [58–60]. In the case of the inhibition of these two enzymes, the concentration of coproporphyrin and pentacarboxyporphyrin increases in the urine. Precoproporphyrin is produced from pentacarboxyporphyrinogen under the influence of heavy metals, especially Hg. Those children with ASD who have the so-called pica (eating inedible substances) are also at risk of Pb poisoning [61].
Among the trace elements, which are vital for the functioning of living organisms, Zn has a special place. It is important for the activity of at least 300 enzymes [62]. Zinc and Cu are both needed as cofactors in two of the important antioxidant enzymes, viz the intracellular cytosolic and extracellular isozymes of Cu/Zn-dependent superoxide dismutase [63, 64]. These trace elements have an antagonistic relationship to one another in their metabolism, though not in their actual biochemical functions as enzyme cofactors. There are at least two different mechanisms explaining this. Zinc and Cu have a competition for at least one of the systems for active membrane transport, which is important for their absorption from the intestinal lumen into enterocytes [65]. Both Zn and Cu bind to metallothionein at the same time as they serve as positive regulators of the expression of apometallothionein [66]. Disruption of the balance between Zn and Cu, chiefly in the direction of reduced Zn and/or elevated Cu (Cu/Zn), can lead to many health problems [66, 67]. The main source for Zn in the body is almost always the extracellular fluid except for actively phagocytosing cells, which may also get some Zn from “food particles” that they have ingested. For most cells in the body, almost all Zn they need has been transported through the blood plasma. Metallothionein (MT) is important as an intracellular storage form not only for Zn but also for Cu [68, 69]. Zinc is one of the positive regulators of apometallothionein expression, and more apometallothionein expression means more binding capacity for binding of toxic metals by apometallothionein, i.e. binding to MT, and Zn is involved in the detoxification of heavy metals [70]. Metallothionein contains much cysteine, and cysteine is quite essential to understanding MT function besides being needed as a precursor to make MT. The clinical chemical observation in ASD patients is to postulate that the elevated Cu/Zn concentration ratio in blood plasma might be mainly due to the same mechanisms as during the acute phase response in patients with infectious diseases. The most important Cu protein in blood plasma is ceruloplasmin [71, 72], which is one of the important acute phase proteins [73]. Researchers report on the significantly reduced levels of serum Zn and/or the elevated Cu/Zn ratio in people with ASD [45, 46, 74]. Disturbances in Zn and Cu metabolism are almost universal in infectious diseases [75] and several non-infectious chronic inflammatory diseases [76, 77], in addition to being common in patients with ASD [44].
With the purpose of contributing to a better understanding of the possible influence of metals (Pb, Hg, Al, Cu and Zn), the present study was carried out on a group of children with ASD and a control group of children with other neurological disorders to determine the level of previously mentioned metal in blood, as well as specific porphyrins in urine. This study represents the first research on the biochemical indicators in children with ASD in Slovenia.
Methods
Participants
Randomly included in the present study were children who were treated in the Clinical Department for Child, Adolescent and Developmental Neurology, Children’s Hospital, Ljubljana. Prior to inclusion, the parents and children were acquainted with the purpose of the research and asked to give written consent. A group of children with autism spectrum disorders (ASD) (N = 52) on the basis of the diagnostic criteria of DSM-IV-TR [1] and results on the Childhood Autism Rating Scale (CARS) and a control group of children with other neurological disorders and diseases (N = 22) were included in the present study.
Psychological Assessment
Cognitive abilities were, depending on the age of the participants, assessed using the Bayley Scales of Infant Development (BSID-II)—Mental Scale, Wechsler Intelligence Scale for Children (WISC-III) and Raven’s Colour Progressive Matrices (CPM). In order to compare the results of the CPM test with the results of the BSID-II and WISC-III tests, in the present study, the results of the CPM test expressed in percentile ranks and set in a standard value on the normally distributed scale with an average of 100 and a standard deviation of 15 were normalized. The results thus obtained were then combined with the normally distributed results on the scale of general cognitive abilities of WISC-III and the Mental Scales test of BSID-II and divided into five categories (<50, 51–60, 61–70, 71–80 and >81). Although the results of the three instruments used do not measure exactly identical cognitive abilities, their convergent validity is considerable, especially in children older than 2 years [78]. Studies have shown that the predictive validity of the Mental Scale of BSID-II for achievements on the tests of general cognitive abilities such as WISC-III in childhood is high (r = 0.73–0.79) [79] and there is likewise a moderate to high correlation of the results on the CPM and WISC-III tests (average r = 0.67) [80].
Analysis of Metals
Analysis of metals in the blood was carried out at the Clinical Institute for Clinical Chemistry and Biochemistry, University Clinical Centre in Ljubljana, using the following methods: (1) analysis of Zn and Cu by flame atomic absorption spectrometry, (2) analysis of Al and Pb by the electrothermal atomic absorption spectrometry method and (3) analysis of Hg by the cold vapour atomic absorption spectrometry method.
Measurements of Hg were carried out on the Varian SpectrAA-250 plus atomic absorption spectrometer (Varian Australia Pty Ltd Mulgrave, Victoria, Australia) with the additional VGA 77 unit for analysis with cold vapours after acid degradation in the microwave oven (MARSXpress, CEM, USA). Measurements were satisfactory if the absorbance was greater than 0.010 and relative standard deviation less than 5 %.
Aluminium in the serum and Pb in the blood were determined using the electrothermal atomic absorption spectrometry method according to established routine procedures at the Clinical Institute for Clinical Chemistry and Biochemistry. For this method of Al determination, the limit of detection was 1.64 μg/L, and the inaccuracy in the series at a concentration of 63.4 μg/L was 3.67 %. For this method of Pb determination, the limit of detection was 0.015 μg/L, and the inaccuracy in the series at a concentration of 384 μg/L was 4 %.
Copper and Zn in the serum were determined using the flame atomic absorption spectrometry method according to established routine procedures at the Clinical Institute for Clinical Chemistry and Biochemistry. For this method of Cu determination, the limit of detection was 1.2 μmol/L, and the inaccuracy in the series at a concentration of 17.2 μmol/L was 2.4 %. For this method of Zn determination, the limit of detection was 1.6 μmol/L, and the inaccuracy in the series at a concentration of 12.96 μmol/L was 3.7 %.
Analysis of Porphyrins
Analysis of specific porphyrins in the urine was performed by the KOBIS Company. The urine sample was immediately wrapped in Al foil and frozen until analysis at −30 °C. Analysis was carried out using the HPLC technique with a fluorescence detector. The procedure was carried out using the RECIPE reagent kit of the ClinRep Company as follows: After centrifuging (3,000g, 5 min), 1 mL of supernatant was acidified with HCl, the centrifuge was repeated and 50-μL solution was applied on a reverse phase column (C18, 5 μm). The porphyrin fractions were eluted with the mobile phase with a gradient; the porphyrin fractions in the eluate were detected by a fluorescence detector.
The analysis of specific porphyrins was carried out separately for the two groups of fractions: (a) uroporphyrin I, heptacarboxyporphyrin I, hexacarboxyporphyrin I and pentacarboxyporphyrin I; and (b) coproporphyrin I and coproporphyrin III. They were divided into two groups on the evidence of previous research, where it was shown that coproporphyrin I and III are, compared to the other porphyrin fractions (uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin), most reliably associated with Hg poisoning in humans and animals [53].
Statistical Analysis
The two groups were compared according to average age by a t test and according to gender and co-morbidity using appropriate non-parametric tests (χ 2 test, Fisher’s exact test, Mann-Whitney U test). For statistical assessment of the differences between the two groups according to levels of metals in the blood and porphyrins in the urine, a multivariate analysis of covariance (MANCOVA) was carried out in advance, followed by calculation of the five generalized linear models using the SPSS 19.0 software package (IBM SPSS Statistics, 2010). Spearman’s non-parametric correlation coefficient was used for assessment of the correlation between biochemical parameters and the level of general cognitive abilities. The level of acceptable α error for all performed statistical analyses was 0.05.
Results
Participant Characteristics
The two groups of children were comparable with regard to age and most of the key variables, although they differed according to gender, since there were more females in the control group (Table 1). The children’s age range was 1–16 years. The diagnoses in the control group of children are shown in Table 2.
Cognitive abilities were assessed by the Bayley Scales of Infant Development (BSID-II)—Mental Scale in 29 children with ASD (54.7 %) and in 13 children in the control group (54.7 %), the Wechsler Intelligence Scale for Children (WISC-III) in 19 children with ASD (35.8 %) and 10 children in the control group (43.5 %) and Raven’s Colour Progressive Matrices (CPM) in 5 children with ASD (9.5 %). The level of general cognitive abilities was comparable in both groups (Mann-Whitney U = 565.0, p = 0.595), as seen in Table 3.
Metals in the Blood
The average values of metals in blood of children in the ASD group and the control group are shown in Table 4. MANCOVA did not confirm that membership of a group (Wilks’ Λ = 0.914, df 1 = 5, df 2 = 40, p = 0.591, η 2 = 0.086), gender (Wilks’ Λ = ., df 1 = 5, df 2 = 40, p = 0.546, η 2 = 0.093) or age (Wilks’ Λ = ., df 1 = 5, df 2 = 40, p = 0.296, η 2 = 0.137) correlated with blood levels of metals. Due to the absence of statistically important effects, additional analyses by generalized linear models were not performed.
Using generalized linear models, the differences between the two groups with regard to the ratio between Cu and Zn were analysed. Calculation of regression coefficients (b) (Table 5) showed that the older children, regardless of group, had reduced ratios between Cu and Zn and that children with ASD had significantly elevated ratios between Cu and Zn compared to children from the control group (95 % confidence interval for children with ASD = 1.86–2.26; 95 % confidence interval for the control group = 1.51–1.88).
Post hoc analysis of the level of Cu (M ASD = 20.6 μmol/L, SD ASD = 3.3 μmol/L, M control = 19.9 μmol/L, SD control = 4.1 μmol/L) and Zn (M ASD = 10.7 μmol/L, SD ASD = 1.8 μmol/L, M control = 12.1 μmol/L, SD control = 1.5 μmol/L) showed that there was a difference between the two groups in the ratio between Cu and Zn, which was a consequence of the differences in Zn levels between the groups (Mann-Whitney U = 267.5, p = 0.007), but not Cu (Mann-Whitney U = 398.5, p = 0.327). In the group of children with ASD, the elevated ratio between Cu and Zn was also associated with lower general cognitive abilities (Spearman ρ =−0.373, df = 47 p = 0.010), whereas this was not found in the control group (Spearman ρ =−0.022, df = 20, p = 0.482).
Porphyrins in the Urine
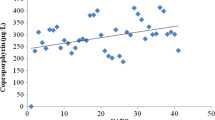
Analysis of porphyrins in the urine was performed in 18 children with ASD and 19 children in the control group. Statistical analysis of the first set of porphyrin fractions (uroporphyrin I, heptacarboxyporphyrin I, hexacarboxyporphyrin I and pentacarboxyporphyrin I) did not show any statistically significant difference according to group (Wilks’ Λ = 0.734, df 1 = 4, df 2 = 14, p = 0.328, η 2 = 0.266), gender (Wilks’ Λ = 0.740, df 1 = 4, df 2 = 14, p = 0.343, η 2 = 0.260) or age (Wilks’ Λ = 0.914, df 1 = 4, df 2 = 14, p = 0.942, η 2 = 0.050). Multivariate analysis of covariance showed a trend towards statistical correlation between the presence of ASD and the concentration of coproporphyrin I and III (Wilks’ Λ = 0.833, df 1 = 2, df 2 = 19, p = 0.176, η 2 = 0.167). Additional analyses performed using generalized linear models showed a marginally lower concentration of coproporphyrin III (Wald χ 2 = 3.71, df = 1, p = 0.054) in the group of children with ASD; the concentration of coproporphyrin I (Wald χ 2 = 2.81, df = 1, p = 0.094) however did not differ between the two groups, as shown in Fig. 1.
Discussion
In the present study, no statistically significant differences in the serum levels of individual metals were found between the group of children with ASD and children with other neurological disorders. However, there was a significantly elevated Cu/Zn ratio, mainly as a result of differences in the level of Zn. An elevated Cu/Zn ratio has also been found in other studies of people with ASD [44–46, 74]. But according to the literature, this change is not specific for ASD. It is mentioned in connection with a variety of health problems, in the field of developmental and behavioural disorders most frequently with attention deficit hyperactivity disorder (ADHD) [66, 67, 81]. In the present study, there were only two children with ADHD in the control group, so that comparison was not possible. It would therefore be reasonable to upgrade the study to include more participants with ADHD.
The reasons for the disturbed Cu/Zn ratio can be found at different levels of the metabolism of the two trace elements, with the result that, among other things, can lead to reduced activity of the detoxification mechanism of metallothionein and/or Cu/Zn SOD1, resulting in excessive oxidative stress and damage to brain cells [70, 82]. On the other hand, the reduced level of Zn can be the consequence of increased oxidative stress, which increases the activity of the metallothionein system and the binding of Zn and Cu to it, resulting in reduced availability of these two elements for activity in other enzyme pathways [44]. Regardless of the mechanism of its origin, considering the results of the present study, it would be reasonable to add Zn to the food in all those children with ASD who have reduced levels of Zn or elevated Cu/Zn ratio in the blood. The normal ratio between these two elements is approximately 1:1, and the lower limit of normal is 1.4 [44, 45]. Russo and deVito cite the positive effect of treatment with Zn in certain children with ASD [74].
In the present study, the group of children with ASD and the control group did not differ according to the serum levels of Hg. Also in the context of the CHARGE study, where analysis of the blood level of Hg was carried out in 452 children (249 with autism, 143 with developmental delay and 60 healthy), taking into account the nutritional, medical, pharmaceutical and dental sources of Hg, there were no significant differences between the groups. In terms of the CHARGE, the main factor influencing the Hg level was the quantity of fish consumed, which was less in the group of children with ASD, so that the blood level of Hg was also lowest in this group [83]. In addition to the fish consumption, the release of Hg from dental amalgam fillings and vapour makes the predominant contribution to human exposure to Hg [84]. No exposure to Hg vapour can be considered totally harmless since Hg vapour has no toxic threshold [85].
The amounts of Hg in blood can be low relatively short time after the exposure has ceased, even though the amounts of Hg in critical organs (brain and kidneys) are high. Blood Hg shows only exposure in recent weeks, while urinary Hg shows exposure the last few months [48], so the serum analysis of Hg cannot confirm or deny in full the potential harmful effect of Hg in children with neurodevelopmental disorders, including children with ASD.
Because specific porphyrins, especially coproporphyrin and precoproporphyrin, in urine, can be a more subtle indicator of heavy metal poisoning [50–52], the level of these compounds was also determined in our study. As found, the coproporphyrin III in the urine was in fact marginally lower in the group of children with ASD. Because researchers who demonstrate the link between the development of ASD and the increased concentration of the porphyrin fraction in the urine as a marker of the poisoning of the organism with heavy metals, especially Hg, in their studies do not generally control the results with participants with other neurodevelopmental/neurological disorders [51, 52], as was done in the present study, it can be that those results are not so specific for children with ASD, as have been thought, but rather represent some common aetiopathological mechanisms across different neurodevelopmental disorders.
Also, the fact that the level of porphyrins in urine can be influenced by factors other than toxic heavy metals should be considered in the interpretation of the results. Among others, the disturbance of porphyrins metabolism may be due to haemochromatosis [55, 86], hepatitis C [55], HIV disease [55] and poisoning by various organic molecules, including ethanol [54, 55, 87] and hexachlorobenzene [54, 88].
So, regarding environmental factors, including Hg, that possibly influenced the development of ASD, the very complex nature of this relationship should be taken into account. Studies have been carried out on additional factors that could, together with low dose exposure to Hg, constitute a potentially harmful effect on the developing brain, e.g. certain immune deficiencies, unsuitable and poor quality food, particularly a deficiency of long-chain unsaturated fatty acids, the amino acid methionine and the trace elements Zn and Se, in conjunction with the expression of certain genes and the reduced ability of the organism to effectively excrete these heavy metals [82, 89–91]. In their study, Thompson and Boekelheide cite older age, consumption of fish and increased alcohol consumption as those factors that increase the burden on the organism in women of childbearing age of some environmental toxins, including Hg [92]. Since the majority of studies still focus on the potentially damaging effect of individual environmental toxins on health, some researchers emphasize the need for new methodological approaches, which would take into account the cumulative risk of several environmental toxins [93].
Conclusions
In the present study, the possible influence of metals was studied, which has been for many years the subject of conflicting explanations of the possible causes of ASD. Among the results, the significantly elevated Cu/Zn ratio in children with ASD compared to the control group of children with other neurological disorders stood out. According to the literature, such a result is not specific only for people with ASD as it is most frequently mentioned in association with ADHD. Irrespective of the cause, the result of the present study supports the need for measuring Zn and Cu in the blood of all children with ASD and the recommendation for the addition of Zn to food in the case of its insufficiency.
References
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Washington
Autism and Developmental Disabilities Monitoring Network Surveillance Year (2008) Principal Investigators; Centers for Disease Control and Prevention (2012) Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 61(3):1–19
Fombonne E (2009) Commentary: on King and Bearman. Int J Epidemiol 38:1241–1242
King M, Bearman P (2009) Diagnostic change and the increased prevalence of autism. Int J Epidemiol 38:1224–1234
Sinzig J, Walter D, Doepfner M (2009) Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Atten Disord 13:117–126
Anholt GE, Cath DC, van Oppen P, Eikelenboom M, Smit JH, van Megen H, van Balkom AJ (2010) Autism and ADHD symptoms in patients with OCD: are they associated with specific OC symptom dimensions or OC symptom severity? J Autism Dev Disord 40:580–589
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T et al (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68:1095–1102
Rutter M (2005) Aetiology of autism: findings and questions. J Intellect Disabil Res 49:231–238
Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R et al (2012) A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum Genet 131:565–79
Devlin B, Scherer SW (2012) Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev 22:229–237
Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN (2006) The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 114:1119–1125
Stoltenberg C, Schjølberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C et al (2010) The autism birth cohort (ABC): a paradigm for gene-environment-timin research. Mol Psychiatry 15:676–680
Schendel DE, Diguisseppi C, Croen LA, Fallin MD, Schieve LA, Wiggins LD (2012) The study to explore early development (SEED): a multisite epidemiologic study of autism by the centers for autism and developmental disabilities research and epidemiology (CADDRE) network. J Autism Dev Disord 42:2121–2140
King MD, Fountain C, Dakhlallah D, Bearman PS (2009) Estimated autism risk and older reproductive age. Am J Public Health 99:1673–1679
Shelton JF, Tancredi DJ, Hertz-Picciotto I (2010) Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 3:30–39
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL et al (2012) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129:1121–1128
Wickramasinghe SN, Fida S (1994) Bone marrow cells from vitamin B12- and folate-deficient patients misincorporate uracil into DNA. Blood 83:1656–1661
Duthie SJ, Hawdon A (1998) DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J 12:1491–1497
Duthie SJ (1999) Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 55:578–592
Kapiszewska M, Kalemba M, Wojciech U, Milewicz T (2005) Uracil misincorporation into DNA of leukocytes of young women with positive folate balance depends on plasma vitamin B12 concentrations and methylenetetrahydrofolate reductase polymorphisms. A pilot study. J Nutr Biochem 16:467–478
Fenech M (2010) Folate, DNA damage and the aging brain. Mech Ageing Dev 131:236–241
Nakao LS, Augusto O (1998) Nucleic acid alkylation by free radical metabolites of ethanol. Formation of 8 (1-hydroxyethyl) guanine and 8-(2-hydroxyethyl)guanine adducts. Chem Res Toxicol 11:888–894
Nakao LS, Fonseca E, Augusto O (2002) Detection of C8-(1-hydroxyethyl)guanine in liver RNA and DNA from control and ethanol-treated rats. Chem Res Toxicol 15:1248–1253
Hori K, Miyamoto S, Yukawa Y, Muto M, Chiba T, Matsuda T (2012) Stability of acetaldehyde-derived DNA adduct in vitro. Biochem Biophys Res Commun 423:642–646
Singh R, Gromadzinska J, Mistry Y, Cordell R, Juren T, Segerbäck D, Farmer PB (2012) Detection of acetaldehyde derived N(2)-ethyl-2’-deoxyguanosine in human leukocyte DNA following alcohol consumption. Mutat Res 737:8–11
Kopf PG, Walker MK (2010) 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol 245:91–99
Beedanagari SR, Taylor RT, Hankinson O (2010) Differential regulation of the dioxin-induced Cyp1A1 and Cyp1B1 genes in mouse hepatoma and fibroblast cell lines. Toxicol Lett 194:26–33
Stejskalova L, Pavek P (2011) The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbonreceptor (AhR) in the placenta. Curr Pharm Biotechnol 12:715–730
Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M (2002) Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis 23:1199–1207
Spink BC, Pang S, Pentecost BT, Spink DC (2002) Induction of cytochrome P450 1B1 in MDA-MB-231 human breast cancer cells by non-ortho-substituted polychlorinated biphenyls. Toxicol in Vitro 16:695–704
Brown DJ, Van Overmeire I, Goeyens L, Denison MS, De Vito MJ, Clark GC (2004) Analysis of Ah receptor pathway activation by brominated flame retardants. Chemosphere 55:1509–1518
Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R (2011) Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect 119:873–877
Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hertiala J, Allayee H et al (2012) Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE case-control study. Am J Clin Nutr 96:80–89
Shelton JF, Hertz-Picciotto I, Pessah IN (2012) Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect 120:944–951
Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R (2013) Traffic-related air pollution, particulare matter, and autism. JAMA Psychiatry 70:71–77
Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I (2013) Is maternal influenza or fever during pregnancy associated with autism or developmetal delay? Results from the CHARGE study. J Autism Dev Disord 43:25–33
Aschwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, van de Walter J (2011) Association of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol 232:196–199
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J (2011) Altered T cell responses in children with autism. Brain Behav Immun 25:840–849
Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J (2012) Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 42:1435–1445
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56:304–316
Kern JK, Geier DA, Audhya T, King PG, Sykes LK, Geier MR (2012) Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol Exp (Wars) 72:113–153
Landrigan PJ (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 22:219–225
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp 73:225–236
Faber S, Zinn GM, Kern JC II, Kingston HMS (2009) The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 14:171–180
Yasuda H, Yoshida K, Yasuda Y, Tsutsui T (2011) Infantile zinc deficiency: association with autism spectrum disorders. Sci Rep 1:129. doi:10.1038/srep00129
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80:1611–1617
Bjørklund G (1991) Mercury in the dental office. Risk evaluation of the occupational environment in dental care (in Norwegian). Tidsskr Nor Laegeforen 111:948–951
Stejskal V (2013) Mercury-induced inflammation: yet another example of ASIA syndrome. Isr Med Assoc J 15:714–715
Brester MA (1988) Biomarkers of xenobiotic exposures. Ann Clin Lab Sci 18:306–317
Geier DA, Geier MR (2006) A prospective assessement of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotox Res 10:57–64
Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R (2006) Porphyrinuria in childhood autistic disorder: implications for enviromental toxicity. Toxicol Appl Pharmacol 214:99–108
Wang L, Angley MT, Gerber JP, Sorich MJ (2011) A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers 16:537–552
Kimbrough RD (1987) Porphyrins and hepatotoxicity. Ann N Y Acad Sci 514:289–296
Van Meter JR, Tierney KR, Pittelkow MR (2011) Iron, genes, and viruses: the porphyria cutanea tarda triple threat. Cutis 88:73–76
Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M (2013) Porphyrin and heme metabolism and the porphyrias. Compr Physiol 3:365–401
Chauhan A, Chauhan V, Brown T (2010) Autism: oxidative stress, inflammation, and immune abnormalities. CRC Press, Boca Raton
Sarkany RP (1999) Porphyria. From Sir Walter Raleigh to molecular biology. Adv Exp Med Biol 455:235–241
Gross U, Hoffmann GF, Doss MO (2000) Erythropoietic and hepatic porphyrias. J Inherit Metab Dis 23:641–661
Woods JS (2005) The association between genetic polymorphisms of coproporphyrinogen oxidase and an atypical porphyrinogenic response to mercury exposure in humans. Toxicol Appl Pharmacol 206:113–120
Cohen DJ, Johnson WT, Caparulo BK (1976) Pica and elevated blood lead level in autistic and atypical children. Am J Dis Child 130:47–48
Prasad AS (2012) Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol 26:66–69
Johnson F, Giulivi C (2005) Superoxide dismutases and their impact upon human health. Mol Aspects Med 26:340–352
Nozik-Grayck E, Suliman HB, Piantadosi CA (2005) Extracellular superoxide dismutase. Int J Biochem Cell Biol 37:2466–2471
Zimnicka AM, Maryon EB, Kaplan JH (2007) Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282:26471–26480
DiGirolamo AM, Ramirez-Zea M (2009) Role of zinc in maternal and child mental health. Am J Clin Nutr 89:940S–945S
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365
Suhy DA, Simon KD, Linzer DI, O’Halloran TV (1999) Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J Biol Chem 274:9183–9192
Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P (2010) Affinity gradients drive copper to cellular destinations. Nature 465:645–648
Kang YJ (2006) Metallothionein redox cycle and function. Exp Biol Med (Maywood) 231:1459–1467
Underwood EA (1977) Trace elements in human and animal nutrition, 4th edn. Academic, New York
Davis GK, Mertz W (1987) Copper. In: Mertz W (ed) Trace elements in human and animal nutrition, vol 1, 4th edn. Academic, San Diego, pp 301–364
Wyatt AR, Wilson MR (2013) Acute phase proteins are major clients for the chaperone action of α2-macroglobulin in human plasma. Cell Stress Chaperones 18:161–170
Russo AJ, deVito R (2011) Analysis of copper and zinc plasma concentration the efficacy of zinc therapy in individuals with Asperger’s syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS) and autism. Biomark Insights 6:127–133
Carver PL (2013) Metal ions and infectious diseases. An overview from the clinic. Met Ions Life Sci 13:1–28
Rainsford KD (1998) Copper and zinc in inflammatory and degenerative diseases. Kluwer Academic Publishers, Dordrecht
Goggs R, Vaughan-Thomas A, Clegg PD, Carter SD, Innes JF, Mobasheri A, Shakibaei M, Schwab W, Bondy CA (2005) Nutraceutical therapies for degenerative joint diseases: a critical review. Crit Rev Food Sci Nutr 45:145–164
Strauss E, Shernan EM, Spreen O (2006) A compendium of neuropsychological tests, 3rd edn. Oxford University Press, New York
Parker SK, Schwartz B, Todd J, Pickering LK (2004) Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics 114:793–803
Boben D (2003) Slovenska standardizacija Ravenovih progresivnih matric: norme za CPM. SPM in APM. Center za psihodiagnostična sredstva, Ljubljana
Kiddie JY, Weiss MD, Kitts DD, Levy-Milne R, Wasdell MB (2010) Nutritional status of children with attention deficit hyperactivity disorder: a pilot study. Int J Pediatr 2010:767318. doi:10.1155/2010/767318
Dufault R, Schnoll R, Lukiw WJ, Leblanc B, Cornett C, Patrick L, Wallinga D, Gilbert SG, Crider R (2009) Mercury exposure, nutritional deficiencies and metabolic disruptions may affect learning in children. Behav Brain Funct 5:44. doi:10.1186/1744-9081-5-44
Hertz-Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah IN (2009) Blood mercury concentrations in CHARGE Study children with and without autism. Environ Health Perspect 118:161–166
Clarkson TW, Friberg L, Nordberg GF, Sager P (1988) Biological monitoring of metals. Plenum Press, New York
IPCS—International Programme on Chemical Safety (1991) Environmental Health Criteria 118: inorganic mercury. WHO, Geneva
Vieira FM, Nakhle MC, Abrantes-Lemos CP, Cançado EL, dos Reis VM (2013) Precipitating factors of porphyria cutanea tarda in Brazil with emphasis on hemochromatosis gene (HFE) mutations. Study of 60 patients. An Bras Dermatol 88:530–540
Liu SW, Lien MH, Fenske NA (2010) The effects of alcohol and drug abuse on the skin. Clin Dermatol 28:391–399
Peters HA, Gocmen A, Cripps DJ, Bryan GT, Dogramaci I (1982) Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol 39:744–749
Hornig M, Chian D, Lipkin WI (2004) Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry 9:833–845
Gerecht M, Austin DW (2011) The plausibility of a role for mercury in the etiology of autism: a cellular perspective. Toxicol Environ Chem 93:1251–1273
Stamova B, Green PG, Tian Y, Hertz-Picciotto I, Pessah IN, Hansen R et al (2011) Correlations between gene expression and mercury levels in blood of boys with and without autism. Neurotox Res 19:31–48
Thompson MR, Boekelheide K (2013) Multiple environmental chemical exposures to lead, mercury and polychlorinated biphenyls among childbearing-aged women (NHANES 1999-2004): body burden and risk factors. Environ Res 121:23–30
Sarigiannis DA, Hansen U (2012) Considering the cumulative risk of mixtures of chemicals – a challenge for policy makers. Environ Health 11 Suppl 1:S18. doi:10.1186/1476-069X-11-S1-S18
Acknowledgments
This study was financed by the Slovenian Research Agency (J3-9470-0312-06). The authors thank the children who participated in the study and their parents. We also thank the staff of the Clinical Department of Child, Adolescent and Developmental Neurology for their cooperation in the study and the Kobis Company for performing the analysis of urinary porphyrins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macedoni-Lukšič, M., Gosar, D., Bjørklund, G. et al. Levels of Metals in the Blood and Specific Porphyrins in the Urine in Children with Autism Spectrum Disorders. Biol Trace Elem Res 163, 2–10 (2015). https://doi.org/10.1007/s12011-014-0121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0121-6