Abstract
Central regulatory mechanisms for food intake regulation vary among animals. Evidence from animal studies suggests central opioids and dopamine have prominent role on appetite regulation but their interaction(s) have not been studied in layer-type chicken. Thus, in this study six experiments designed to investigate intracerebroventricular (ICV) administration of SCH23390 (D1 like receptors antagonist), Sulpride (D2 like receptors antagonist), DAMGO (μ-opioid receptors agonist), DPDPE (δ-opioid receptors agonist), U-50488H (κ-opioid receptors agonist) on feeding behavior in 3 h food deprived neonatal layer-type chickens. In experiment 1, chicks ICV injected with control solution, SCH23390 (2.5 nmol), DAMGO (125 pmol) and their combination (SCH23390 + DAMGO). In experiment 2: control solution, SCH23390 (2.5 nmol), DPDPE (δ-opioid receptors agonist, 40 pmol) and SCH23390 + DPDPE were applied to the birds. In experiment 3, injections were control solution, SCH23390 (2.5 nmol), U-50488H (30 nmol) and SCH23390 + U-50488H. In experiments 4–6 were similar to experiments 1–3 except Sulpride (2.5 nmol) applied instead of SCH23390. Then, cumulative food intake was recorded until 120 min after injection. According to the results, ICV injection of DAMGO (125 pmol) significantly decreased food intake but co-injection of DAMGO + SCH23390 diminished DAMGO-induced hypophagia (P < 0.05). Also, SCH23390 was not able to decrease the DPDPE- and U-50488H-induced hyperphagia (P > 0.05). Furthermore, Sulpride had no role on DAMGO, DPDPE and U-50488H-induced food intake (P > 0.05). These results suggest there is an interaction between opioidergic and dopaminergic systems via μ and D1 receptors in appetite regulation in chicken.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Appetite regulation is a complex physiologic phenomenon which interacts via diverse signals from central and peripheral tissues. In the brain, neurotransmitters by neurological mechanisms are responsible for food intake regulation (Zendehdel et al. 2014). It is important to note, appetite regulates by a wide distributed neural network with variety of peptides (Alizadeh et al. 2015; Hassanpour et al. 2015). Dopamine (DA) is the predominant catecholamine neurotransmitter in the central nervous system (CNS). To date, at least five distinct subtypes of DA receptors identified (D1–D5), belong to G protein coupled receptor subtypes (GPCRs). D1 like receptor subtypes (D1 and D5) couples to the stimulatory G protein (Gs) via adenylyl cyclase pathway while D2 like subfamily (D2, D3 and D4) acts through inhibiting adenylyl cyclase and activation of K+ channels (Ikemoto 2007). D1 and D2 receptors are more abundant than the other dopamine receptors in the brain (Cadet et al. 2010). DA controls several physiological functions such as emotion, locomotor activity, cognition and food intake (Ikemoto 2007).
Opioids are known as inhibitory neurotransmitters. Three opioid receptors classified mainly as mu (µ), delta (δ) and kappa (κ) which are homologous to GPCRs (Fichna et al. 2007; Erbs et al. 2014). Endogenous opioid peptides exist abundant in the CNS and have an important role in the regulation of pain mechanisms, respiration, immune system (Le Merrer et al. 2009) and food intake (Bodnar 2014).
Feeding behavior is modulated in several parts of the brain, such as striatum, hypothalamus, amygdala, orbitofrontal cortex (OFC), nucleus ventral tegmental area (VTA), nucleus accumbens (NAcc), tractus solitaries (NTS) and arcuate nucleus (ARC) (Parker et al. 2014). Several neurotransmitters, including DA, cannabinoids, opioids, GABA and serotonin are implicated in the rewarding effect of food (Chen et al. 2006; Le Merrer et al. 2009). DA is a key anorexigenic neurotransmitter modulating reward which acts mainly through its projections from the VTA into the NAcc and ARC (Volkow et al. 2011). The inhibitory effect of DA on food intake was decreased by SCH 23390 pretreatment in chicken (Bungo et al. 2010; Zendehdel et al. 2014). The same observation reported in mammals which ICV injection of SKF 38393 (D1 receptors agonist) and apomorphine (D2 receptors agonists) decreased cumulative food intake in rats (Kuo 2002).
Reports suggest the endogenous opioidergic system is involved in food intake regulation. Central µ and δ receptors agonists exert orexigenic effects in mammals (Taha 2010; Kaneko et al. 2012; Kozlov et al. 2013). There is evidence food intake regulation pathways are dissimilar between mammals and avian (Zendehdel and Hassanpour 2014). For instance, ICV injection of [D-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO) and β-casomorphin (µ-opioid receptor agonists) decreased while [D-Pen2,5]-enkephalin (DPDPE) (δ-opioid receptor agonist) and U-50488H (κ-opioid receptor agonist) increased feeding behavior in chicks (Bungo et al. 2004, 2005).
There are extensive functional interactions between the DA and opioid systems within the reward circuitry (Vucetic et al. 2010). For example, opioidergic and DAergic systems are involved in stress modified feeding behavior in rat (Samarghandian et al. 2003). Moreover, it is reported excessive sugar intake sensitized D1 and μ receptors in rat (Colantuoni et al. 2001). As well, μ opioid receptors in the VTA have a crucial role in the stimulant effects of food on mesolimbic DA transmission (Tanda and Di Chiara 1998). Most of the present knowledge regarding on behavioral effects of opiates and DA on food intake has been derived from studies in mammalian species. Central opioidergic and DAergic systems play crucial roles in physiological and pathological conditions in animals and human (Volkow et al. 2011).
No report exists on the interconnection of opioidergic and DAergic systems on central food intake in avian. On the basis of comparative physiology it is important to determine the role of neurotransmitters in other species (Zendehdel and Hassanpour 2014). So, the purpose of the present study was to investigate the role of ICV injection of D1 and D2 receptors antagonists on μ, δ and κ induced feeding in 3 h food deprived (FD3) neonatal layer-type chicken.
Materials and Methods
Animals
In this study, to assume interaction of opioidergic and dopaminergic systems on central food intake, 288 one-day-old female layer chickens were purchased from a local hatchery (Morghak Company, Tehran, Iran) and kept for 2 days as flocks and then birds randomly allocated into transferred into their individual cages. Birds were maintained in stabilizing electrically heated batteries at a temperature of 32 °C ± 1, kept at 40–50 % relative humidity and 23:1 lighting/dark period (Olanrewaju et al. 2006). During the study birds had ad libitum access to a commercial starter diet containing 21 % crude protein and 2850 kcal/kg metabolizeable energy (Animal Science Research Institute Co. Iran). 3 h prior to the injections, birds were food deprived (FD3) but had free access to water. ICV injections were done at 5 days of age. Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care.
Experimental Drugs
Experimental drugs include SCH23390 (D1 like receptors antagonist), Sulpride (D2 like receptors antagonist), [D-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO, μ-opioid receptors agonist), [D-Pen2,5]-enkephalin (DPDPE, δ-opioid receptors agonist), U-50488H (κ-opioid receptors agonist) and Evans blue were purchased from Sigma Co. (Sigma, USA). Drugs at first dissolved in absolute dimethyl sulfoxide and then diluted with 0.85 % control solution containing Evans blue at a ratio of 1:250.
ICV Injection Procedures
In this study, 6 experiments designed, each includes 4 treatment groups (A–D) with 12 replicates in each group (n = 48 birds per experiment). In each experiment, the birds were weighed and based on their body weight allocated into experimental groups so the average weight between treatment groups was as uniform as possible. In each experiment birds were injected intracerebroventriculary once using a microsyringe (Hamilton, Switzerland) without anesthesia in accordance with Davis et al. (1979) and Furuse et al. (1997). Briefly, in this technique, head of the birds was held with an acrylic device in which the bill holder was 45º and the calvarium was parallel to the surface of the Table 1 (Van Tienhoven and Juhasz 1962). An orifice was made in a plate that immediately located over the skull of the right lateral ventricle. Microsyringe was inserted into the ventricle via this orifice and tip of the needle perforated 4 mm below the skin of the skull (Jonaidi and Noori 2012). There is no injection-induce physiologic stress using this technique in neonatal chicken (Saito et al. 2005). Each chick received one injection during the study (control or drug solution) in a volume of 10 µL. At the end of the experiments, to recognize the accuracy of injection, the chicks were sacrificed by decapitation and direct placement of injection (in the lateral ventricle) was verified by the presence of Evans blue followed by slicing the frozen brain tissue. In each experiment 12 birds received injections, but only data from individuals were used for analysis, which dye was present in their lateral ventricle (9–12 chickens per group). All experimental procedures were done from 8:00 a.m. until 15:30 p.m.
Food Intake Measurement Procedure
In experiment 1, chicks in group (A) received ICV injection of control solution, (B) SCH23390 (D1 like receptors antagonist, 2.5 nmol), (C) DAMGO (μ-opioid receptors agonist, 125 pmol) and group (D) their combination (SCH23390 + DAMGO). In experiment 2, ICV injection of (A) control solution (distilled water contained Evans blue), (B) SCH23390 (2.5 nmol), (C) DPDPE (δ-opioid receptors agonist, 40 pmol) and group (D) SCH23390 + DPDPE were applied to the birds. In experiment 3, ICV injections were (A) control solution, (B) SCH23390 (2.5 nmol), (C) U-50488H (κ-opioid receptors agonist, 30 nmol) and (D) SCH23390 + U-50488H. In experiments 4–6 were similar to experiments 1–3 except Sulpride (D2 like receptors antagonist, 2.5 nmol) applied instead of SCH23390. Each bird was injected once only. Right away after injection, chickens were returned to their individual cages and provided ad libitum food (pre-weighed) and water. Then the cumulative food intake was recorded at 30, 60 and 120 min post injection. Food consumption was calculated based on percent of body weight (% BW) to adjust the differences between body weights. Drug dosage was determined according to the previous (Steinman et al. 1987; Bungo et al. 2004, 2005; Yanagita et al. 2008; Zendehdel et al. 2014, 2015; Alimohammadi et al. 2015) and pilot studies (un-published).
Statistical Analysis
Cumulative food intake (% BW) was analyzed by two-way analysis of variance (ANOVA) for repeated measurement using SPSS 16.0 for Windows and is presented as mean ± SEM. For treatments showing a main effect by ANOVA, means were compared using post hoc Bonferroni test. P < 0.05 was considered as significant differences between treatments.
Results
Effects of central opioidergic and DAergic systems on cumulative food intake in FD3 neonatal layer-type chicks are shown in Figs. 1, 2, 3, 4, 5 and 6.
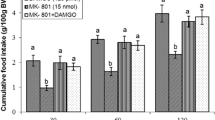
Effect of ICV injection of SCH23390 (2.5 nmol), DAMGO (125 pmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. SCH23390: D1 like receptors antagonist. DAMGO: μ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
Effect of ICV injection of SCH23390 (2.5 nmol), DPDPE (40 pmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. SCH23390: D1 like receptors antagonist. DPDPE: δ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
Effect of ICV injection of SCH23390 (2.5 nmol), U-50488H (30 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. SCH23390: D1 like receptors antagonist. U-50488H: κ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
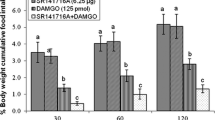
Effect of ICV injection of Sulpride (2.5 nmol), DAMGO (125 pmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. Sulpride: D2 like receptors antagonist. DAMGO: μ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
Effect of ICV injection of Sulpride (2.5 nmol), DPDPE (40 pmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. Sulpride: D2 like receptors antagonist. DPDPE: δ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
Effect of ICV injection of Sulpride (2.5 nmol), U-50488H (30 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens is presented in mean ± SEM. Sulpride: D2 like receptors antagonist. U-50488H: κ-opioid receptors agonist. There are significant differences between groups with different superscripts in a column (a and b; P < 0.05)
In experiment 1, ICV injection of sub effective dose of SCH23390 (D1 like receptors antagonist, 2.5 nmol) had no significant effect on cumulative food intake (% BW) in comparison with control group at 30, 60, 120 and 180 min post-injection [F(l,43) = 2.44, 1.98, 4.71; 2.36; P = 0.91, 0.59, 0.52; 0.73 respectively]. Also, the ICV injection of an effective dose of DAMGO (μ-opioid receptors agonist, 125 pmol) significantly diminished food intake at time-points 30, 60, 120 and 180 min after injection [F(l,43) = 139.17, 127.18, 144.73; 126.44; P < 0. 01, respectively]. Co-injection of SCH23390 (2.5 nmol) and DAMGO (125 pmol) lessened hypophagic effect of μ-opioid receptors at time-points 30, 60, 120 and 180 min after injection [F(l,43) = 171.09, 145.12, 197.84; 163.42; P < 0.001, respectively]. These results imply an interaction exists between the D1 and μ-opioid receptors on food intake in layer-tye chicken.
In experiment 2, ICV injection of DPDPE (δ-opioid receptors agonist, 40 pmol) significantly increased food intake at 30, 60, 120 and 180 min post-injection [F(l,43) = 164.11, 128.97, 142.31; 173.12; P < 0.01, respectively]. Additionally, co-administration of SCH23390 + DPDPE was not able to attenuate δ-opioid receptors-induced hyperphagia compared to control group at time-points 30, 60; 120 and 180 min after injection [F(l,43) = 1.26, 2. 42, 3.09; 1.97; P = 0.61, 0.49, 0.53; 0.78; respectively]. Perhaps, there is no interconnection between D1 and δ-opioid receptors on feeding behaviour in birds.
In experiment 3, ICV administration of SCH23390 (2.5 nmol) had no effect on cumulative food intake in layer-type chicken (P > 0.05). Hence, U-50488H (κ-opioid receptors agonist, 30 nmol) amplified food intake in comparison to control group at time-points 30, 60;120 and 180 min after injection [F(l,43) = 137.11, 159.71, 151.02; 163.22; P < 0.01, respectively]. Furthermore, co-injection of SCH23390 + U-50488H had no effect on κ-opioid receptors-induced food intake in FD3 neonatal layer-type chicks at 30, 60, 120 and 180 min post-injection [F(l,43) = 3.19, 2.73, 5.01; 2.53; P = 0.59, 0.64, 0.71; 0.63; respectively]. It seems, there is no interaction between D1 and κ-opioid receptors on appetite regulation in birds.
In experiment 4, ICV injection of sub effective level of Sulpride (D2 like receptors antagonist, 2.5 nmol) had no significant effect on food intake (P > 0.05). Also, DAMGO (125 pmol) had anorixigenic effect in FD3 neonatal layer-type chicks compared to control group at time-points 30, 60, 120 and 180 min after injection [F(l,43) = 184.15, 193.62, 129.75; 149.13; P < 0.001, respectively]. Likewise, co-injection of Sulpride and DAMGO was not able to diminish μ-opioid receptors- induced hypophagia in FD3 chicks at 30, 60, 120 and 180 min post-injection [F(l,43) = 1.04, 3.11, 2.46; 3.64; P = 0.93, 0.47, 0.51; 0.57; respectively]. We thus hypothesize that the suppressive effect of μ-opioid receptors on cumulative food intake is not mediated by D2 receptors.
In experiment 5, ICV administration of DPDPE (δ-opioid receptors agonist) at a dose of 40 pmol significantly amplified food consumption at 30, 60, 120 and 180 min post-injection [F(l,43) = 157.01, 149.75, 118.32; 161.72; P < 0.01, respectively]. As well, no significant effect observed on food intake after ICV injection of Sulpride (2.5 nmol) (P > 0.05). Moreover, co-injection of Sulpride + DPDPE had no effect on δ-opioid receptors induced food intake in in FD3 chicks at 30, 60, 120 and 180 min post-injection injection [F(l,43) = 2.37, 1.93, 4.05; 2.17; P = 0.62, 0.95, 0.48; 0.72; respectively]. This implies the effect of δ-opioid receptors on food intake is not mediated by D2 receptors.
In experiment 6, no significant effect on food consumption observed after ICV injection of 2.5 nmol of Sulpride compared to control group (P > 0.05). Additionally, U-50488H at a dose of 30 nmol amplified food intake at 30, 60, 120 and 180 min post-injection [F(l,43) = 125.06, 127.84, 140.12; 152.38; P < 0.01, respectively]; but the hyperphagic effect of U-50488H was not fluctuate by co-injection of Sulpride + U-50488H at 30, 60, 120 and 180 min post-injection [F(l,43) = 3.06, 3.47, 2.19; 1.24; P = 0.80, 0.46, 0.51; 0.67; respectively]. Perhaps D2 receptors have no role on κ-opioid receptors-induced hyperphagia in neonatal layer-type chicks.
Discussion
To our knowledge this paper is the first report on interactions between DAergic and opioidergic systems on food intake in FD3 neonatal layer-type chickens. Obtained data in experiments revealed ICV injection of DAMGO (µ-opioid receptors agonist) decreased (Figs. 1 and 4) while DPDPE (δ-opioid receptors agonist) and U-50488H (κ-opioid receptors agonist) increased cumulative food intake in FD3 neonatal layer-type chicks (Figs. 2, 3, 5 and 6).
Opioids are known as orexigenic system which acts through nucleus accumbens (NAc) and NTS in rodents (Fichna et al. 2007). ICV injection of morphine or DAMGO into hypothalamus has hyperphagic effect in rat (Browning et al. 2006; Zheng et al. 2007). According to our previous research (Zendehdel et al. 2015) and results of the current study, effect of µ-opioid receptors is completely different in layer-type chicks compared to the mammals. Few studies investigated the effects of opioidergic system in domestic fowls. For instance, ICV injection of DAMGO inhibited ingestion in neonatal broiler (McCormack and Denbow 1989; Bungo et al. 2004). Effects of neurotransmitters such as ghrelin, leptin and adiponectin on feeding behavior regulation are somewhat dissimilar between mammals and avian (Novoseletsky et al. 2011; Zendehdel and Hassanpour 2014). It is suggested genetic selection for egg production in layers might alter their responsiveness to physiological appetite regulation mechanisms and/or pathways (Denbow 1994).
ICV injection of DPDPE and U-50488H (δ and κ receptors agonists, respectively) reinforced food consumption in layer-type neonatal chicken. ICV injection of DPDPE elevated feeding behavior in rats (Levine 2006; Kaneko et al. 2012) and neonatal broiler chicken (Yanagita et al. 2008; Khan et al. 2009; Alimohammadi et al. 2015). It seems, δ and κ receptors have orexigenic effect in both mammalian and domestic fowl.
DAergic system modulates food intake in both mammals and avian. ICV injection of DA and L-DOPA decreased food intake in FD3 broiler cockerels (Zendehdel et al. 2014). Blockade of various DA receptor subtypes is associated with reduced feeding response in rats. Also, a dose dependent hypophagia reported after ICV injection of D1 and D2 receptors agonists in rat (Terry and Katz 1992). D1 knockout mice showed a decrease in the operant performance to obtain sucrose under different schedules of reinforcement. Similarly, D2 deficient mice presented a delayed acquisition of an operant task to obtain reward. In fact, the DA may be involved in the translation of food motivation into adapted behaviors to obtain food (Barbano and Cador 2007).
In our previous research (Zendehdel et al. 2014) and pilot studies no significant effect observed using other DA receptors antagonist AMI-193 (D2 receptor antagonist), NGB2904 (D3 receptor antagonist) and L-741, 742 (D4 receptor antagonist) on reward regulation in FD3 broiler cockerels. Perhaps, these receptors are not involved in appetite regulation in birds. As discussed above, genetic variations between animals might responsible for observed discrepancy. New findings of this study are important as comparative physiology. So, based on the available information, here we investigated interconnection of D1 and D2 like receptors on opioidergic system induced feeding.
We used sub-effective dose of DA antagonists, which blocks receptor without effect on food intake to assay possible interaction(s) of DAergic system with opioid induced food intake. Of particular interest of the present study was that the inhibitory effect of μ-opioid receptors on food intake decreased by administration of a D1 receptors antagonist in chicken. Our data indicates there is a relationship between DAergic and opioidergic systems in neonatal layer type chicken. An interconnection exists between DAergic and opioidergic systems where excessive sugar intake sensitized D1 and μ-receptors in rat (Colantuoni et al. 2001). In mammals, µ-opioid receptor is subdivided into two subtypes: µ1 and µ2 but their roles have not been studied in chickens. Further researches will be needed to establish the hetero receptors in layers.
To date researches were done to investigate effect of opiates on the binding characteristics of D1 receptors in specific rat brain regions. Following repeated morphine or naloxone treatment, Kd values were significantly decreased in both hypothalamus and midbrain of morphine and naloxone-treated animals. These researchers employed 3H-SCH-23390 for D1 receptor binding ligand. So they conclude interaction exist between opiates and dopaminergic system where repeated intermittent treatment with opiates induces alterations in D1 receptors binding properties (Elwan and Soliman 1995). Beyond the limitation of this study, we were not able to determine kd value for µ- and D1-receptors in chickens. We think, merit studies need to distinguish kd value in avian species.
Anatomical evidence suggesting both receptors involved in feeding regulation centers in the brain (Volkow et al. 2011; Parker et al. 2014). DA is released in the olfactory tubercle and NAcc in the brain. Furthermore, DA supplies projections from the VTA into the NAcc and ARC (Zendehdel et al. 2014). μ-opioid receptor is present in DAergic neurons of the substantia nigra (Samarghandian et al. 2003). Additionally, μ-opioid receptors impress the orexigenic effect on food intake via NAcc and NTS in rat (Browning et al. 2006). ICV injection of naloxonazine into VTA impairs morphine- and nicotine-induced stimulation of DA release in the NAc shell suggests these effects are the result of an activation of µ1 receptors (Tanda and Di Chiara 1998).
There are interesting evidences which recommends there is interaction between DA and opioid-related gene expression in response to reward where stimulation of the opioidergic system decreased expression of D1 and D2 receptors as well as reuptake of DA within NAcc and PFC in rat (Vucetic et al. 2010). To date several researches done to identify pathway(s) underlying between these two systems, however, direct mechanism is not fully elicited. It is suggested DAergic and opioidergic systems might interact by effect on other appetite regulatory neurotransmitters. It seems, µ-opioid receptors are involved in the orexigenic effect of neuropeptide Y (NPY)/agouti related protein (AgRP) neurons in the ARC (Barnes et al. 2006) however, neural pathway between NPY and opioidergic system is not identified in poultry’s hypothalamus (Dodo et al. 2005).
Other hypotheses imply DAergic and opioidergic systems interact via corticotropin-releasing factor (CRF) on food intake. CRF and its receptors, play crucial effects on energy intake and body weight regulation where known as hypophagic neurotransmitter. DA is known to stimulate the release of CRF from the paraventricular nucleus of the hypothalamus. Opioid peptides also affect the hypothalamic CRF release. However, in this study, we were not able to measure CRF levels after ICV injection of DAergic and opioidergic drugs (Samarghandian et al. 2003). We think merit study is required to clarify the precise mechanism by which CRF, DA and opioids increase food intake. GPCRs are abundant, widely expressed and involved in major physiological responses. GPCR heteromerization refers to the direct interaction among at least 2 different functional receptors forming a complex with specific functional and biochemical properties, dissimilar from those of its component receptor units. Several heteromerization reported for D1 receptors with the other receptor heteromers (Albizu et al. 2010). In a study, Juhasz et al. (2008) heterooligomer formation reported between and D1 receptors. We think further studies needed to investigate heteromerization between μ and D1 receptors (Rozenfeld and Devi 2010).
As observed, in the current study, there was no interaction between D1 and D2 receptors with δ- and κ-opioid receptors on food intake regulation in neonatal layer type chicken. In our recent work, we observed co-injection of δ-opioid receptor agonist and CB1 receptors antagonist was not able to diminish the hyperphagic effect of DPDPE. Furthermore, ICV injection of U-50488H (κ-opioid receptors agonist) and CB1 and CB2 receptors antagonists was not able to attenuated hyperphagic effect of U-50488H (Zendehdel et al. 2015). So, we think, presumably δ- and κ-opioid receptors have few interactions with other neurotransmitters on feeding behavior in layer-type chicken. To our knowledge, there was no previous study on the role of central DAergic and opioids on food intake in avian. So, we were not able to compare our results with it. It seems, other neurotransmitters such as cannabinoids, glutamate, serotonin and GABA might responsible for interaction between these systems on feeding behavior regulation (Volkow et al. 2011). Most research on central food intake regulation has done with rat models, whereas considering few investigations done in birds. These observations can be used as base information on central food intake regulation in birds. Finally, the authors recommend merit further investigation need to clarify direct cellular and molecular signaling pathways of DAergic and opioidergic systems with other receptors in physiology of food intake regulation in poultry.
References
Albizu L, Moreno JL, González-Maeso J, Sealfon SC (2010) Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics. CNS Neurol Disord Drug Targets 9(5):636–650
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun. doi:10.1007/s11259-015-9631-8
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun. doi:10.1007/s11259-015-9636-3
Barbano MF, Cador M (2007) Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology 191:497–506
Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA (2006) Increased expression of mu opioid receptors in animals susceptible to dietinduced obesity. Peptides 27:3292–3298
Bodnar RJ (2014) Endogenous opiates and behavior: 2013. Peptides 62:67–136
Browning KN, Zheng Z, Gettys TW, Travagli RA (2006) Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol 575:761–776
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2004) Feeding responses to µ-, δ- and κ-opioid receptor agonists in the meat-type chick. Pharmacol Biochem Behav 78:707–710
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2005) Effects of various µ-, δ- and κ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85:519–523
Bungo T, Yanagita K, Shiraishi J (2010) Feed intake after infusion of noradrenalin, dopamine or its precursor into the lateral ventricles in neonatal chicks. J Anim Vet Adv 9(4):760–763
Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disorders-Drug Targets 9:526–538
Chen RZ, Frassetto A, Fong TM (2006) Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking l-opioid receptors. Brain Res 1108:176–178
Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG (2001) Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport 12(16):3549–3552
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Dodo K, Izumi TH, Bungo T (2005) Response of neuropeptide Y-induced feeding to µ-, δ-Ueda and κ-opioid receptor antagonists in the neonatal chick. Neurosci Lett 373:85–88
Elwan MA, Soliman MR (1995) Alteration of D1 and D2 dopaminergic receptor kinetics in specific rat brain regions following repeated administration of opiates. Pharmacology 51(2):73–83
Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J, Koch M, Kessler P, Hentsch D, Birling M, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D (2014) A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. doi:10.1007/s00429-014-0717-9
Fichna J, Janecka A, Costentin J, Do Rego J (2007) The endomorphin system and its evolving neurophysiological role. Pharmacol Rev 59:88–123
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci. doi:10.1080/00071668.2015.1059407
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56(1):27–78
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198:827–832
Juhasz JR, Hasbi A, Rashid AJ, Rashid AJ, George SR, George SR (2008) Mu-opioid receptor heterooligomer formation with the dopamine D1 receptor as directly visualized in living cells. Eur J Pharmacol 581(3):235–243
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Khan MSI, Ohkubo T, Masuda N, Tachibana T, Ueda H (2009) Central administration of metastin increases food intake through opioid neurons in chicks. Compd Biochem Physiol Part A 153:209–212
Kozlov AP, Nizhnikov M, Kramskaya TA, Varlinskaya EI, Spear NE (2013) μ-opioid blockade reduces ethanol effects on intake and behavior of the infant rat during short-term but not long-term social isolation. Pharmacol Biochem Behav 103:773–782
Kuo D (2002) Co-administration of dopamine D1 and D2 agonists additively decreases daily food intake, body weight and hypothalamic neuropeptide Y level in rats. J Biomed Sci 9(2):126–132
Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Levine AS (2006) The animal model in food intake regulation: examples from the opioid literature. Physiol Behav 89:92–96
McCormack JF, Denbow DM (1989) Ingestive responses to mu and delta opioid receptor agonists in the domestic fowl. Br Poult Sci 30:327–340
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J poultry Sci 5(4):301–308
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res 260:131–138
Rozenfeld R, Devi LA (2010) Receptor heteromerization and drug discovery. Trends Pharmacol Sci 31(3):124–130
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Samarghandian S, Ohata H, Yamauchi N, Shibasaki T (2003) Corticotropin-releasing factor as well as opioid and dopamine are involved in tail-pinch-induced food intake of rats. Neurosci 116:519–524
Steinman JL, Fujikawa DG, Wasterlain CG, Cherkin A, Morley JE (1987) The effects of adrenergic, opioid and pancreatic polypeptidergic compounds on feeding and other behaviors in neonatal leghorn chicks. Peptides 8:585–592
Taha SA (2010) Preference or fat? Revisiting opioid effects on food intake. Physiol Behav 100:429–437
Tanda G, Di Chiara G (1998) A dopamine-μ1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci 10:1179–1187
Terry P, Katz JL (1992) Differential antagonism of the effects of dopamine D1-receptor agonists on feeding behavior in the rat. Psychopharmacology 109(4):403–409
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Volkow ND, Wang GJ, Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15(1):37–46
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM (2010) Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151:4756–4764
Yanagita K, Shiraishi J, Fujita M, Bungo T (2008) Effects of N-terminal fragments of β-endorphin on feeding in chicks. Neurosci Lett 442:140–142
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Hasani K, Babapour V, Seyedali Mortezaei S, Khoshbakht Y, Hassanpour S (2014) Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Vet Res Commun 38:11–19
Zendehdel M, Hassanpour S, Babapour V, Charkhkar S, Mahdavi M (2015) Interaction between endocannabinoid and opioidergic systems regulates food intake in neonatal chicken. Int J Pept Res Ther. doi:10.1007/s10989-015-9457-9
Zheng H, Patterson LM, Berthoud HR (2007) Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27:11075–11082
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Morteza Zendehdel, Elham Ghashghayi, Shahin Hassanpour and Ali Baghbanzadeh declare that they have no conflict of interest.
Informed consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and animal rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Zendehdel, M., Ghashghayi, E., Hassanpour, S. et al. Interaction Between Opioidergic and Dopaminergic Systems on Food Intake in Neonatal Layer Type Chicken. Int J Pept Res Ther 22, 83–92 (2016). https://doi.org/10.1007/s10989-015-9486-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-015-9486-4