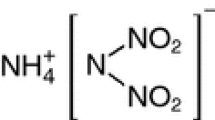
Abstract
Basic copper nitrate [Cu(NO3)2·3Cu(OH)2, BCN] is a widely used oxidizer for gas-generating compounds. The oxidizers that replace some BCN with ammonium nitrate (NH4NO3, AN) have been investigated to increase the performance of the gas-generating agents. The purpose of this study was to understand the thermal behavior and stability of AN/BCN mixtures. To this end, mixtures prepared by two kinds of methods, with and without heat treatment, were analyzed by X-ray powder diffraction to investigate composition of samples, and differential scanning calorimetry and thermogravimetry–differential thermal analysis with mass spectrometry (TG–DTA–MS) to investigate the thermal behavior and evolved gases. It was found that [Cu(NH3)2](NO3)2 was formed in the sample with thermal treatment. The samples with and without heating exhibited different decomposition processes. It is considered that the residual AN and the amount of [Cu(NH3)2](NO3)2 in the mixture affected the decomposition behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The composition of BCN and guanidine nitrate (GN) has been widely used for gas generation in automobile airbags because it is a large gas-producing agent and reduces the combustion temperature [1]. BCN is used as the oxidizer in these compositions [2–5]. It is known that BCN is produced as an intermediate during the decomposition of hydrated copper nitrate [6–9]. BCN decomposes according to the following reaction [10]:
The theoretical mass loss according to Eq. (1) is 33.75 %. Gas-generating agents require a high ratio of gasification per unit mass for the reduction in size and mass of automobile airbag systems. To increase the ratio of gasification per mass, some BCN is typically replaced with other oxidizers that become gases after the reaction such as ammonium dinitramide (ADN) [11–13] and ammonium nitrate (AN) [14, 15]. ADN is a high energy oxidizer and a promising oxidizer for solid propellants, so many studies on its properties in terms of burning rate, stability, and other important performance parameters have been performed [16–24]. However, it has too low of a melting temperature to function as the gas-generating agent in automobile airbag systems.
In contrast, AN is promising in propellants and gas generators, due to its high oxygen balance (+20.0 %) and cost-effectiveness. It is a halogen-free, smokeless oxidizer and has been widely used as an oxidizer in energetic compositions. However, the uses of AN in propellants and gas generators are restricted due to its high hygroscopicity, the solid-state-phase transitions at temperatures below 130 °C, and low combustion performance. Many combinations of combustible contents and additives with AN have thus been explored in an attempt to improve these properties [25–39].
This study focuses on oxidizers that replace some BCN with AN to increase the gasification ratio of gas-generating agents. Wada et al. [40] has studied the combustion mechanism of mixtures of AN, BCN, and GN for developing AN as an oxidizer in gas-generating agents in automobile airbag systems. They found that combustion conditions and mixing ratios of samples affected the type of combustion residuals. However, thermal analyses of AN and BCN were insufficient.
Understanding the thermal behavior and stability is necessary for the performance of gas-generating agents. In this study, to mix AN and BCN homogeneously, mixtures of AN and BCN were prepared by powder mixing and melt mixing. The samples were analyzed with X-ray powder diffraction (XRD) to investigate compositions, and with differential scanning calorimetry (DSC) and thermogravimetry–differential thermal analysis with mass spectrometry (TG–DTA–MS) to investigate the thermal behavior and evolved gases.
Experimental
Materials
Samples of AN (Wako Pure Chemical Industries, Ltd.) and BCN (Nihon Kagaku Sangyo Co., Ltd.) were used for the experiments. Samples were dried by silica gel in a glove box in which the relative humidity was kept lower than 15 % at room temperature under an air atmosphere. Pure AN and BCN were ground in a mortar (under 5.5 μm) and mixed in a one to four mass ratio. Approximately 8 g of the mixture was placed into a stainless container with an inner diameter of 68 mm and height of 170 mm. The samples were mixed at a rotation rate of 35 rpm for 1 h by a rotating mixing system. One of the samples was loaded into a test tube and heated in the electronic furnace and oven at 170 °C near the melting point of AN.
Composition analysis
To determine compositions of AN/BCN mixtures after thermal treatment, X-ray diffraction patterns were measured using a powder X-ray diffractometer (XRD, Rigaku RINT-2500). A 100-mg sample on a glass plate was measured at room temperature. Diffraction patterns were recorded in the angular range of 10°–90° 2θ with a scanning rate of 2° min−1.
Thermal property and evolved gas
Thermal behaviors of non-thermal and thermal-treated samples of AN and BCN mixtures, pure AN and pure BCN were characterized using sealed cell differential scanning calorimetry (SC-DSC, Mettler Toledo HP DSC827e). For SC-DSC measurements, approximately 2 mg of sample was loaded into a stainless steel container (SUS303), sealed in air and then heated from 30 to 400 °C at a heating rate of 10 K min−1. The thermal characteristics and decomposition gases of the AN and BCN mixtures were also evaluated using a thermogravimetry–differential thermal analyzer (TG–DTA, Rigaku TG/DTA-8120) connected with a mass spectrometer (MS, Shimadzu GC/MS-QP2010). The samples (2 mg) were heated from room temperature to 350 °C at a heating rate of 10 K min−1 under helium flow (200 mL min−1) in an open aluminum cell. The mass spectrometer was operated in electron impact ionization mode, with selected ion monitoring for m/z = 17, 18, 28, 30, 44 and 46 (NH3, H2O, N2, N2O, NO2 and HNO3) from reaction (1) and Ref. [35].
Results and discussion
Composition analysis
The samples prepared by the two methods (a. without heating, b. with heating at 170 °C for 1 h) are shown in Fig. 1. Blue products were formed in the sample after heating about 170 °C for 1 h.
The XRD patterns of the pure AN, pure BCN, and AN/BCN mixtures recorded at room temperature are shown in Fig. 2. Pure AN and BCN were detected in the XRD pattern of the AN and BCN mixture without thermal treatment. However, in the sample with thermal treatment at 170 °C for 1 h, diamine copper nitrate [Cu(NH3)2](NO3)2 was present in addition to AN and BCN. The peaks at about 15°, 19°, 22°, and 24° were identified as the peaks of [Cu(NH3)2](NO3)2, which is likely the blue product formed in the sample with thermal treatment (Fig. 1b), according to reaction (2).
The theoretical mass ratio of the reaction between AN and BCN according to reaction (2) is AN/BCN = 4/3. In this study, it is suspected that all the AN reacted with BCN to form the copper complex. In fact, AN was observed in the mixture with thermal treatment because [Cu(NH3)2](NO3)2 hydrolyzed to form AN and BCN when allowed to stand in air [41].
Thermal property and evolved gas
The DSC curves of the pure AN, pure BCN, and AN/BCN mixtures at a heating rate of 10 K min−1 under sealed condition are shown in Fig. 3. In the sample without thermal treatment, three endotherms were observed below 170 °C. The endotherms at 50 and 120 °C were due to a solid-state-phase transition of AN. It is considered that endotherm at 150 °C was due to melting of the AN and BCN mixture from visual observe using open vessel. The exothermal reaction was observed at 260 °C. The endotherm at range of 310–360 °C was owing to the decomposition of substance generated from reaction between AN and BCN. On the other hand, in the sample with thermal treatment, the endotherm at 50 °C was not observed. This means the [Cu(NH3)2](NO3)2 formed during thermal treatment inhibited the solid-state-phase transition of AN at 50 °C. The broad endotherm around 120 °C is eutectic of AN, BCN, and [Cu(NH3)2](NO3)2. The exothermal reaction of the sample with thermal treatment also occurred at 260 °C. The endotherm at range of 310–360 °C was observed as well as the powder mixing sample. Under the sealed condition, there was no significant difference in thermal behavior above 260 °C between the mixtures prepared by powder mixing and melt mixing.
The TG and DTA curves of the samples are shown in Fig. 4. From the TG curves, mass loss of AN and BCN mixtures was higher by about 10 % than that of pure BCN. The DTA curves showed different behavior between the samples with and without thermal treatment. The mixture without thermal treatment lost mass in two stages at 125 and 200 °C. The TG curve of the mixture with thermal treatment showed mass loss in three stages, at 120, 210, and 230 °C. Comparison with the SC-DSC results, DTA showed that thermal behavior above 200 °C was different in terms of onset temperature. The difference seemed to result from the open condition.
Real-time analysis of the evolved gases was carried out using TG–MS. Figures 5 and 6 show the total ion current (TIC) curves for the AN/BCN mixtures in addition to the MS curves for mass-to-charge (m/z) ratios of 17, 18, 28, 30, 44 and 46. The peaks are assigned to NH3 (m/z = 16, 17), H2O (m/z = 17, 18), N2 (m/z = 28), NO (m/z = 30), N2O (m/z = 30, 44), NO2 (m/z = 30, 46) and HNO3 (m/z = 30, 44, 46). These gases were detected after the decomposition of AN, BCN, and [Cu(NH3)2](NO3)2 [10, 33, 41]. The TIC curve for AN/BCN without thermal treatment displayed a broad peak (130–170 °C) and a sharp peak (200–250 °C). The broad peak from gas generation of m/z = 18 and 46 began at the same time as the melt endotherm of the mixture (Fig. 4). This indicates that H2O and HNO3 were generated due to the reaction between BCN and molten AN [reaction (2)]. The sharp peak (200–250 °C) corresponded to the endotherm (200–220 °C) and the exotherm (220–250 °C) of the DTA curve (Fig. 4). The m/z = 18, 28, 30 and 46 derived from H2O, N2 and NO2 in the range of 200–220 °C were observed. This indicates that the endotherm was decomposition of AN and BCN. Gases such as NH3, N2 and N2O generated from decomposition of [Cu(NH3)2](NO3)2 [40], and [Cu(NH3)2](NO3)2 decomposed with an exotherm at 240 °C [35, 42]. Therefore, the exotherm (220–250 °C) resulted from decomposition of [Cu(NH3)2](NO3)2. On the other hand, the TIC curve of the sample with thermal treatment exhibited one broad peak and two sharp peaks. In the range of 110–120 °C, m/z = 18 and 46 began at the same time as the eutectic endotherm of the mixture (Fig. 4). AN and BCN formed by hydrolysis of [Cu(NH3)2](NO3)2 reacted with [Cu(NH3)2](NO3)2 again to generate H2O and HNO3. The m/z = 18, 30, and 46 were detected in the peak (210–230 °C). The endotherm (210–230 °C) of the thermal-treated mixture was due to decomposition of BCN without generating N2 [reaction (2)]. The TIC peak (230–250 °C) was considered the decomposition of [Cu(NH3)2](NO3)2 as in the sample without thermal treatment. It is likely that BCN and [Cu(NH3)2](NO3)2 of the thermal-treated mixture decomposed without interacting, considering the separate TIC curves (200–250 °C). In contrast, components of the mixture without thermal treatment decomposed with interaction. The amounts of AN and [Cu(NH3)2](NO3)2 were different between the powder mixed sample and the thermal-treated sample because AN changed to [Cu(NH3)2](NO3)2 during heating at 170 °C for 1 h. The difference in decomposition was caused by the residual AN and [Cu(NH3)2](NO3)2 in the mixture. From the results of DSC and TG–DTA–MS, the difference in thermal behavior above 200 °C was deemed to be derived from the vaporization and decomposition of each sample.
Conclusions
AN/BCN mixtures prepared by powder mixing and melt mixing were analyzed by X-ray powder diffraction (XRD) to investigate compositions, and with differential scanning calorimetry (DSC) and thermogravimetry–differential thermal analysis with mass spectrometry (TG–DTA–MS) to determine thermal behavior and evolved gases. From the XRD results, it was shown that [Cu(NH3)2](NO3)2 was formed from the reaction between molten AN and BCN. [Cu(NH3)2](NO3)2 formed during thermal treatment inhibited the solid-state-phase transition of AN at 50 °C. Thermal analysis indicated the measurement conditions affected the decomposition process of the mixtures. Under the sealed condition, the gases vaporized and decomposition occurred in the reactions with solid or liquid AN, BCN, and [Cu(NH3)2](NO3)2. It is considered that the same reaction occurred in the samples with and without thermal treatment. From the results of TG–DTA–MS, the gasification ratio of the mixtures was higher than in pure BCN. In the case of the sample without heating, H2O and HNO3 were generated due to the reaction between BCN and molten AN [reaction (2)] at 130 °C. The endotherm in the range of 200–220 °C was due to decomposition of AN and BCN. In the range of 220–250 °C, [Cu(NH3)2](NO3)2 decomposed with an exotherm generating gases such as NH3, N2 and N2O. On the other hand, with the sample with thermal treatment, reaction (1) occurred from AN and BCN formed by hydrolysis of [Cu(NH3)2](NO3)2 at 110 °C. BCN decomposed in the range of 210–230 °C, and [Cu(NH3)2](NO3)2 decomposed with an exotherm in the range of 230–250 °C. It is considered that the amount of AN and [Cu(NH3)2](NO3)2 affected the decomposition process of mixtures under the measurement conditions of this study.
References
Mei X, Cheng Y, Li Y, Zhu X, Yan S, Li X. Thermal decomposition properties of guanidine nitrate and basic curip nitrate. J Therm Anal Calorim. 2013;111:131–5.
Bucerius KM, Schmid HM. US Patent 5,542,999; 1996.
Barnes MW, Taylor RD, Hock C. US Patent 5,635,668; 1997.
Barnes MW, Taylor RD. US Patent 5,608,183. 1997;03:04.
Taylor RD, Mendenhall IV. Burn rate enhancement of basic copper nitrate-containing gas generant compositions: U. S. Patent 7,998,292 B2. 2011;08:16.
L’vov BV, Novichikhin AV. Mechanism of thermal decomposition of hydrated copper nitrate in vacuo. Spectrochim Acta B. 1995;50:1459–68.
Ghose J, Kanungo A. Studies on the thermal decomposition of Cu(NO3)2·3H2O. J Therm Anal. 1981;20:459–62.
Badica P, Aldica G, Crisan A. Decomposition of Ca:Cu = 1:1 nitrate powder. Thermal analysis and structural studies. J Mater Sci. 2002;37:585–94.
Ryu SK, Lee WK, Park SJ. Thermal decomposition of hydrated copper nitrate [Cu(NO3)2·3H2O] on activated carbon fibers. Carbon Sci. 2004;5:180–5.
Ilcheva L, Maneva M, Bozadziev P. Thermal investigation of the basic copper (II) nitrate Cu(OH)1.5(NO3)0.5. J Therm Anal. 1979;16:205–7.
Bottaro JC, Penwell PE, Schmitt RJ. 1,1,3,3-tetraoxo-1,2,3-triazapropene anion, a new oxy anion of nitrogen: the dinitramide anion and its salts. J Am Chem Soc. 1997;119:9405–10.
Pak Z. Some ways to higher environmental safety of solid rocket propellant application. In: Proc AIAA/SAE/ASME/ASEE 29th joint propulsion conf exhibition. Monterey; 1993.
Östmark H, Bemm U, Langlet A, Sanden R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energ Mater. 2000;18:123–8.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;67:253–81.
Oxley JC, Smith JL, Rogers E, Yu M. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45.
Oxley JC, Smith JL, Zheng W, Rogers E, Coburn MD. Thermal decomposition studies on ammonium dinitramide (ADN) and 15N and 2H isotopomers. J Phys Chem A. 1997;101:5642–52.
Sinditskii VP, Egorshev Y, Levshenkov AI, Serushkin VV. Combustion of ammonium dinitramide, part 1: burning behavior. J Propul Power. 2006;22:769–76.
Matsunaga H, Yoshino S, Kumasaki M, Habu H, Miyake A. Aging characteristics of the energetic oxidizer ammonium dinitramide. Sci Tech Energ Mater. 2011;72:131–5.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Matsunaga H, Habu H, Miyake A. Thermal behavior of new oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;111:1183–8.
Matsunaga H, Habu H, Miyake A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim. 2014;116:1227–32.
Fujisato K, Habu H, Hori K. Condensed phase behavior in the combustion of ammonium dinitramide. Propellant Explos Pyrotech. 2014;39:714–22.
Fujisato K, Habu H, Hori K. Role of additives in the combustion of ammonium dinitramide. Propellant Explos Pyrotech. 2014;39:518–22.
Sugie Y, Miyake A. Effects of temperature on nitration of sulfamates. J Therm Anal Calorim. 2014;116:1213–7.
Sinditskii VP, Egorshev VY, Levshenkov AI, Serushkin VV. Ammonium nitrate: combustion mechanism and the role of additives. Propellant Explos Pyrotech. 2005;30:269–80.
Wada Y, Arai M. A study on ammonium nitrate-metal nitrate double salts as oxidizers for gas generating agent. Sci Tech Energ Mater. 2010;71:39–43.
Miyata Y, Hasue K. Burning characteristics of aminoguanidinium 5,5′-Azobis- 1H tetrazolate/ammonium nitrate mixture-effects of particle size and composition ratio on burning rate. J Energ Mater. 2011;29:344–59.
Kohga M, Okamoto K. Thermal decomposition behaviors and burning characteristics of ammonium nitrate/polytetrahydrofuran/glycerin composite propellant. Combust Flame. 2011;158(578–82):15.
Nakamura H, Saeki K, Akiyoshi M, Takahasi K. The reaction of ammonium nitrate with carbon powder. Kayaku Gakkaishi. 2002;63:87–93 (In Japanese).
Pandey M, Jha S, Kumar R, Mishra S, Jha RR. The pressure effect study on the burning rate of ammonium nitrate-HTPB-based propellant with the influence catalysts. J Therm Anal Calorim. 2012;107:135–40.
Golovina N, Nechiporenko G. Ammonium nitrate phase state stabilization with small amounts of some organic compounds. Cent Eur J Energ Mater. 2009;6:45–56.
Golovina N, Nechiporenko G. Phase state stabilization of ammonium nitrate for creating an oxidizing agent for smokeless gas-generating formulations yielding no toxic combustion products. Russ J Appl Chem. 2007;80:24–30.
Vorozhtsov A, Archipov V, Bondarchuk S, Popok N, Klyakin G, Babuk V, Luca LTD, Galfetti L. Ballistic characteristics of solid propellants containing dual oxidizer. In: Proc 1st Europ Conf. Aerospace Sci Moscow; 2005.
Sudhakar AOR. Mathew S. Thermal behavior of CuO doped phase-stabilised ammonium nitrate. Thermochim Acta. 2006;451:5–9.
Kajiyama K, Izato Y, Miyake A. Thermal characterristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;113:1475–85.
Izato Y, Miyake A, Date S. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propellant Explos Pyrotech. 2013;38:129–35.
Izato Y, Kajiyama K, Miyake A. Thermal decomposition mechanism of ammonium nitrate and copper(II) oxide mixtures. Sci Tech Energ Mater. 2014;75:128–33.
Nagayama S, Katoh K, Higashi E, Nakano K, Kumagae K, Habu H, Wada Y, Arai M. Differential scanning calorimetry analysis of crystal structure transformation in spray-dried particles consisting of ammonium nitrate, potassium nitrate, and a polymer. J Therm Anal Calorim. 2014;118:1215–19.
Nagayama S, Katoh K, Higashi E, Nakano K, Habu H. Effect of polymer addition amount and type on thermal decomposition behavior of spray-dried particles comprising ammonium nitrate, potassium nitrate, and polymer. J Therm Anal Calorim. 2014;118:1221–27.
Wada Y, Hori K, Arai M. Combustion mechanism of mixtures of guanidine nitrate, ammonium nitrate, and basic copper nitrate. Sci Technol Energ Mater. 2010;71:83–7.
Dyukarev SS, Morozov IV, Reshetova LN, Guz OV, Arkhangel’skii IV, Korenve YM, Spiridonov FM. Copper(II) nitrate ammoniates Cu(NH3)4(NO3)2 and Cu(NH3)2(NO3)2 and their thermolysis under reduced pressure. Russ J Inorg Chem. 1999;44:883–8.
Southern TM, Wendlandt WW. The thermal decomposition of metal complexes—XX: some amine copper(II) nitrate complexes. J Inorg Nucl Chem. 1970;32:3783–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiota, K., Matsunaga, H. & Miyake, A. Thermal analysis of ammonium nitrate and basic copper(II) nitrate mixtures. J Therm Anal Calorim 121, 281–286 (2015). https://doi.org/10.1007/s10973-015-4536-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4536-x