Abstract
The mechanism of copper (II) oxide and molybdenum (VI) oxide co-reduction by Mg + C mixture was investigated at non-isothermal conditions by carrying out simultaneous differential thermal (DTA) and thermogravimetric (TG) analyses combined with X-ray diffraction (XRD) analysis of intermediate and final products. The whole process was found to involve several phenomena: interaction between oxides with CuMoO4 salt formation, high-exothermic reactions occurring directly between metal oxides and magnesium, as well as low-exothermic carbothermal reactions. In order to better understand the complex nature of calorimetric and TG curves, the behaviour of each single reagent as well as that of binary, ternary and quaternary mixtures was studied at linear heating. It was revealed that the simultaneous reduction of Cu and Mo oxides proceeds more easily by carbon than by magnesium. Only due to the decisive role of carbon on the reaction pathway, the combined and complete reduction of oxides by Mg/C reducing mixture becomes possible at relatively low temperatures. The sequence of chemical reactions possibly occurring during the heating process on the basis of DTA/TG curves and XRD analyses results of quenched reaction products has been proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During last two decades, an increased interest has been paid to the so-called pseudoalloys based on Cu–refractory metal system (such as Cu–Mo). Their unique properties and multiple functionalities make them ideal for numerous high-tech applications, e.g. instrumentation and portable equipment industries [1, 2]. The development of new preparation methods of Cu–Mo composite materials for thermal management with high physicomechanical properties and bulk density is in the focus of modern research, as their characteristics highly depend on microstructure and phase composition of alloys [3–10].
A number of novel technologies have been developed to enhance Mo–Cu composite densification ability by using finer precursors, such as homogeneously mixed molybdenum and copper oxides/salts. Generally, MoO3–CuO powder mixtures were ground in a ball mill and reduced at 600–1000 °C up to 10 h in the hydrogen atmosphere [6–8, 11, 12], which is a energy-consuming process and accompanied with undesirable growth of Cu grains, as well as results in drastic decrease in process efficiency.
In our previous work [13], molybdenum and copper oxides joint reduction was performed by energy-saving combustion synthesis approach using Mg + C mixture as combined reducer. The use of such reducing mixture allows to control the reaction temperature in a wide range and to synthesize Mo–Cu composite powders in a controllable combustion mode. It should be underlined that it is highly challenging to monitor and reveal mechanism of the combustion reaction due to its high velocity. Note that according to the available literature data, the mechanism of joint reduction of MoO3 and CuO was not studied at all. To fill the gap, one of the approaches is the modelling of the process at “soft” conditions (e.g. low heating rates and tuning the process within the time) by using the DTA/TG method. This approach provides an enhanced opportunity to reveal the stepwise nature of complex reactions in the multicomponent systems.
In this work, the mechanism and kinetics of molybdenum and copper oxides joint reduction by Mg + C mixture were studied by DTA/TG method combined with XRD analysis of intermediate products quenched from various stages.
Materials and methods
The following powders were used as raw materials: MoO3 (high grade, Pobedit Company, Russia, particle size less than 15 μm), CuO (high grade, STANCHEM, Poland, particle size less than 40 μm), magnesium powder (MPF-3, Russia, 150- to 300-μm particle size) and carbon black (P-803, Russia, particle size less than 1 μm).
Differential thermal (DTA) and thermogravimetric (TG) analyses were carried out using DTA/TG, Q-1500 instrument (“Derivatograph Q1500” MOM, Hungary) which is connected to multichannel acquisition system, and output signals are recorded by a computer. Differential thermogravimetric (DTG) and DTA points were registered in every 1 s, samples 50–200 mg were placed in Al2O3 crucibles with 1 mL volume, and Al2O3 powder was used as reference material. Measurements were taken in argon atmosphere at flow rate of 120 mL min−1. Heating rate was programmed to be 2.5, 5, 10 and 20 °C min−1. The thermoanalytical curves were recorded up to a temperature 1000 °C. In order to stop the reaction and quench the intermediate and final products for further examinations, the heater power was switched off automatically at preset temperatures; then, the furnace was removed, and sample was cooled down by inert gas flow. The cooling rate in the temperature range from 1000 to 600 °C (more interesting area) was around 300 °C min−1. The samples obtained in this way were examined by XRD method with monochromatic CuKα radiation (diffractometer DRON-3.0, Burevestnik, Russia) operated at 25 kV and 10 mA. The microstructure of the initial reagents and obtained products was evaluated using scanning electron microscope (BS-300, Tesla, CZ).
Results and discussion
To clarify the interaction laws and mechanism in the complex quaternary (CuO–MoO3–Mg–C) system, it was expedient firstly to explore the interaction patterns in binary (CuO–MoO3, CuO–Mg, CuO–C, MoO3–Mg, MoO3–C) and ternary (CuO–C–Mg, MoO3–C–Mg, CuO–MoO3–C, CuO–MoO3–Mg) systems at the identical conditions of linear heating.
Binary systems
CuO–MoO3 system
As it can be seen from DTA/TG curves (Fig. 1), at heating the mixture of CuO and MoO3 oxides with 1:1 molar ratio (V h = 20 °C min−1), low-endothermic process takes place in a 470–650 °C temperature range, which corresponds to the formation of copper molybdate, CuMoO4, according to the X-ray analysis results. Note that five different modifications for CuMoO4 have been reported in the literature (α-CuMoO4, β-CuMoO4, γ-CuMoO4, CuMoO4-II, CuMoO4-III). In our investigations, up to 700 °C α-modification is stable and endothermic peak at that temperature (D point) corresponds to the change in copper molybdate modification (α-CuMoO4 to CuMoO4-III) [14–16]. According to the literary data [17, 18], at 820 °C copper molybdate incongruent melting takes place.
Thus, in the case of the absence of reducers, copper molybdate, CuMoO4 formation occurs from the MoO3 and CuO oxide equimolar mixture. The quenched sample at 420 °C (A point) contains only initial oxides, but at 470 °C (B) partial formation of CuMoO4 is observed; some amount of initial oxides are also present. At 650 °C (C point), product consists solely of CuMoO4 (Fig. 1; see also Table 1). Experiments also showed that the change in oxides ratios brings the formation of copper molybdates with various compositions (e.g. Cu3Mo2O9).
CuO–Mg, CuO–C, MoO3–Mg, MoO3–C systems
In the CuO–Mg binary system, the exothermic interaction begins before the melting of magnesium (T s = 570 °C, V h = 20 °C min−1), the process is single stage, with the maximum temperature of DTA peak T max = 600 °C, and is accompanied by the formation of metallic copper and magnesium oxide (Fig. 2a).
The carbothermal reduction of copper oxide is a low-exothermic process with two sequential stages (Fig. 2b). The first stage is the CuO interaction with carbon by the formation of Cu2O suboxide (440–530 °C), and then, Cu2O is reduced to copper at 530–650 °C temperature interval. The mass change in the TG curve corresponds to the removal of carbon dioxide either during the first or second stage and makes about 11.9 and 14.8 %, respectively (according to reactions’ equations: 13.3 and 15.0 %).
According to our previous studies [19], the reduction of molybdenum trioxide by magnesium is a single-step reaction, with high-exothermic effect, which starts at about the same temperature as the reduction of copper oxide by magnesium (T s = 580 °C, T max = 620 °C, V h = 20 °C min−1). On the other hand, the carbothermal reduction of molybdenum trioxide proceeds in two stages: first stage (MoO3 → MoO2) is low exothermic (560–650 °C) and the second stage is endothermic (>850 °C) [19]. The latter is accompanied by the formation of molybdenum and molybdenum carbide.
According to these data obtained, during the reduction of complex mixtures, it should be taken into account the fact that the second stage of carbothermal reduction of CuO (Cu2O + C → Cu + CO2) and the first stage of molybdenum trioxide carbothermal reduction (MoO3 + C → MoO2 + CO2) take place at the same temperature range, which coincides also with their separate reduction processes by magnesium.
Ternary systems
CuO–C–Mg system
The reduction regularities of copper oxide have been explored for different ratios of Mg/C reducer’s mixture. Therefore in the case of carbon-poor mixtures, when carbon amount is less than 0.5 mol, which is insufficient for full reduction of CuO (e.g. CuO + 0.25C + 0.5Mg mixture), it has been shown that as it was expected, in the 430–540 °C temperature range, the carbothermic reduction of copper oxide takes place up to Cu2O formation (Fig. 3a). Mass loss according to TG curve makes about 8.3 % (according to the CuO + 0.25C + 0.5Mg = 1/2Cu2O + 0.25CO2 + 0.5Mg reaction equation—11.6 %). Then, magnesiothermic reduction occurs at higher temperature. It means that for this type of mixtures, carbothermic and magnesiothermic reduction stages are fully separated. Moreover, in contrast to pure magnesiothermic reduction process (carbon absence case), in this case, the reduction stage by magnesium moves to the higher temperature range and proceeds after magnesium melting (at about 730 °C).
In the carbon-rich mixtures, the first stage of carbothermal reduction of copper (II) oxide to copper (I) oxide takes place at the same temperature range as that for carbon-poor mixtures. At higher carbon content, when it is sufficient for complete reduction of copper oxide (≥0.5 mol), it was supposed that magnesiothermic reduction stage will be absent. However, this stage is still displayed (Fig. 3a, DTA curve), indicating that at the presence of magnesium, the carbothermic reduction of Cu2O is shifted to higher temperatures: it starts at ~550 °C and is drawn up to a temperature above the melting point of magnesium (see Fig. 3a, TG curve). As a result, magnesium also participates in the reduction process. In fact, in this case, there is overlapping of carbothermal with magnesiothermal reduction stages of Cu2O.
Total mass loss is about 12 % (full carbothermic reduction of CuO must be 21 %): during the first stage, copper (II) oxide reduces by carbon up to copper (I) oxide (Δm = 8.5 %), and the second stage begins with carbothermic reduction of Cu2O but did not manage to complete (Δm = 3.5 %). Then, it continues by magnesium after its melting. The foregoing is confirmed by the XRD analysis results of quenched samples (Fig. 3b) from the different characteristic temperatures [590 °C (A), 840 °C (B)].
MoO3–Mg–C system
According to the results of our previous studies [19] (Fig. 4, curve 2, 2′), in this system, the interaction starts with carbothermal reaction: molybdenum trioxide is reduced by carbon to molybdenum dioxide, MoO2 at 560–630 °C. Then, the process is continued after the melting of magnesium, and molybdenum dioxide is reduced by magnesium up to metallic molybdenum at 700–800 °C [19]. In fact, in this case also, the first carbothermal stage of reduction (MoO3 → MoO2) occurs approximately at the same temperature range as that in the absence of magnesium. On the other hand, the magnesiothermal stage of reduction, as in the case of copper oxide, moves to higher temperature range and takes place after the melting of magnesium.
In fact, the participation of carbon during the first stage of interaction leads to the significant shift of the magnesiothermic reduction temperature (compare curves 1 and 2). Note that similar phenomenon was observed also in [20] at tungsten (VI) oxide reduction by Mg + C mixture, although in that case, carbon was implicated in low extent.
CuO–MoO3–C system
DTA/TG analyses performed for the CuO + MoO3 + 2C mixture showed that at heating of this mixture, sequential reduction of oxides occurs (Fig. 5; Table 1). At the first stage, copper oxide reduces up to Cu2O; moreover, the first step of the molybdenum trioxide’s reduction (MoO3 to MoO2) coincides with the second stage of copper oxide reduction (Cu2O to Cu), as it was mentioned above (see “CuO–Mg, CuO–C, MoO3–Mg, MoO3–C systems” section). The foregoing is confirmed by the XRD analyses of the quenched samples obtained from characteristic temperatures (Fig. 5b). Thus, carbon addition into the oxides’ mixtures prevents the formation of CuMoO4 (please see “CuO–MoO3 system” section), because the carbothermal reduction of CuO starts earlier and occurs faster (Fig. 5a) than the salt formation process.
CuO–MoO3–Mg system
DTA/TG results obtained for the CuO–MoO3–Mg ternary system (Fig. 6) showed that up to 520 °C, interaction has not occurred (point A), but at T max = 600 °C, low-exothermic reaction was accompanied with the formation of CuMoO4, as well as MgMoO4 according to the XRD analysis of quenched sample at the point B (630 °C). After the melting point of magnesium (650 °C), another low-exothermic reaction occurs. The recent corresponds to the partial reduction of CuMoO4 up to Cu2Mo3O10 and Mo, and formation of MgO·MoO2 mixed oxide (see Fig. 6b, curve C). Thus, one may state that the salt formation from oxides at low heating conditions prevents the magnesiothermal reduction processes of oxides. As a result, the simultaneous reduction of oxides proceeds more easily by carbon than by magnesium.
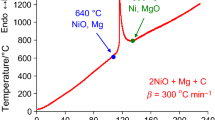
Quaternary CuO–MoO3–C–Mg system
As it can be seen in Fig. 7, at 480 °C (A point), interaction has not been started yet (initial mixture is immutable); then, up to 600 °C two-step reduction of copper oxide to copper and the molybdenum trioxide reduction to MoO2 occur. In the above-listed reactions, only carbon figures as a reducer. This is confirmed both by the mass change in TG curve and by XRD analysis of quenched sample. The mass losses correspond to the CuO + C → Cu + CO2 and MoO3 + C → MoO2 + CO2 interactions. Besides, magnesium oxide has not been found in the samples quenched at 600 °C (B point). The latter proves that magnesium has not been entered into interaction yet. The magnesiothermal reaction starts after magnesium melting at 720 °C and is accompanied by the formation of molybdenum, magnesium oxide and composite oxide of molybdenum and magnesium (C point). Most likely, molybdenum and magnesium mixed oxide (MoO2·MgO) formed at insufficient amounts of reducers by interaction of magnesia and MoO2 [19]. The mass change in TG curve (Fig. 7a) is missing above 600 °C which also proves that only magnesium has participated as a reducer in the second stage.
Based on DTA/TG/DTG and the XRD analyses results of quenched samples (Fig. 7a, b), the following scheme may be presented for the reactions pathway:
-
1.
CuO + C → Cu2O + CO2(CO)
-
2.
Cu2O + C → Cu + CO2(CO) and MoO3 + C → MoO2 + CO2(CO)
-
3.
Melting of Mg
-
4.
MoO2 + Mg → Mo + MgO;
-
5.
MoO2 + MgO → MoO2·MgO
Thus, according to DTA/TG and XRD analysis results of the CuO–MoO3–C–Mg system, the reduction of oxides occurs sequentially; therefore, process starts with low-exothermic reaction (carbothermal reduction) and then continued with high-exothermic one (reduction by magnesium) (Fig. 7a).
The proceeding of the reactions by suggested mechanism facilitates the fact that in the presence of carbon, temperature shift of magnesiothermal reduction takes place, which is conditioned by several factors:
-
1.
The carbothermal reaction precedes both CuMoO4 salt formation process and magnesiothermal interaction. Due to this, it becomes possible to realize reduction process in mild conditions. On the other hand, in this case, instead of initial oxides, the intermediate products (in this case Cu2O and MoO2) participate in magnesiothermal reduction, but at different temperature range, than the initial oxides.
-
2.
The addition of carbon to the MoO3–CuO–Mg initial mixture and subsequent CO/CO2 gas release loosens the sample, leading to the weakening of contact of reactants’ particles and, as a result, moves the subsequent reaction stages to a higher temperature range.
-
3.
The addition of carbon strongly affects the direct contact of reagents (regardless of possible gas releasing) and makes spatial difficulties for interacting particles.
Finally, it should be noted that only due to such pathway of reduction reactions, which excludes the possibility of salt formation process between oxides, the combined reduction of oxides at relatively low temperatures by Mg/C reducing mixture becomes possible.
Conclusions
The interaction pathway in the MoO3–CuO–Mg–C complex system was explored by thermal analysis method combined with XRD analyses of quenched intermediate and final products. It was shown that the whole process involves several phenomena: interaction between oxides with salt formation, high-exothermic reactions occurring directly between oxides and magnesium, as well as low-exothermic carbothermal reactions. It has been demonstrated that in the presence of magnesium as a reducer, salt formation process takes place and prevents the magnesiothermal reduction of oxides. In contrast to magnesium, the addition of carbon into the oxides’ mixtures prevents the formation of CuMoO4, because the carbothermal reduction of oxides occurs earlier and faster than the salt formation process. As a conclusion, one may state that the simultaneous reduction of oxides proceeds more easily by carbon than by magnesium. And finally, only due to such pathway of reduction reactions becomes possible the combined and complete reduction of oxides at relatively low temperatures by Mg/C reducing mixture.
References
Davis JR. Copper and copper alloys. USA: ASM International; 2001.
Chen G, Wu G, Jiang L, Zhu D, Sun D. Microstructure and mechanical properties of high densification Mo/Cu composites. Key Eng Mater. 2007;353–358:2883–6.
Johnson JL, German RM. Role of solid-state skeletal sintering during processing of Mo–Cu composites. Metall Mater Trans A. 2001;32:605–13.
Hu B-Q, Bai P-K, Wang Y-Z. Sintering and processing nanocomposite powder of Mo-8 wt% Cu. J Funct Mater. 2012;43(8):1031–3.
Li Z, Han S-L, Jiang Y-L, Zhao D-F, Meng F. Fabrication and sintering property of Mo–Cu composite powder by mechano-thermochemical method. Mater Sci Eng Powder Metall. 2011;16(5):774–80.
Peng S, Jigui C, Lei W, Jingsong Z, Yifang W, Yanbo C. Preparation and characterization of Mo–15Cu superfine powders by a gelatification-reduction process. J Alloy Compd. 2009;476:226–30.
Aokui S, Wang Dezhi W, Zhuangzhi ZX. Synthesis of ultra-fine Mo–Cu nanocomposites by coreduction of mechanical-activated CuMoO4–MoO3 mixtures at low temperature. J Alloy Compd. 2010;505:588–91.
Li Z, Zhai Y. Preparation of Mo60Cu40 composite nano-powder by hydrogen reaction. Rare Metal Mater Eng. 2010;39(1):6–9.
Popa F, Chicinaş I, Isnard O, Pop V. Heat-treatment influence on Ni–Fe–Cu–Mo nanocrystalline alloy obtained by mechanical alloying. J Therm Anal Calorim. 2012;110(1):295–9.
Adorno AT, Silva RAG, Carvalho TM. (α + γ1) Complex phase formation in the Cu-10 mass% Al-6 mass% Ag alloy. J Therm Anal Calorim. 2009;97(1):127–30.
Zhao M, Wang J, Liu W, Zhou M. Preparation and reduction behavior of Mo–Cu powders by Sol–Gel. J Phys Conf Ser. 2009;188(1):2–6.
Kang Z, Chen W, Ding B. Fabrication of composite nanopowders of MoCu by sol–gel. Rare Metal Mater Eng. 2005;34(6):990–3.
Aydinyan SV, Manukyan KHV, Kharatyan SL. Combustion synthesis of Mo–Cu nanocomposites by co-reduction of molybdenum and copper oxides. In: XII International Symposium on SHS, South Padre Island, TX, USA. 2013. pp 100–101.
Wiesmann M, Ehrenberg H, Miehe G, Peun T, Weitzel H, Fuess H. p-T phase diagram of CuMoO4. J Solid State Chem. 1997;132:88–97.
Ehrenberg H, Weitzel H, Paulus H, Wiesmann M, Wltschek G, Geselle M, Fuess H. Crystal structure and magnetic properties of CuMoO4 at low temperature (g-phase). J Phys Chem Solids. 1997;58:153.
Tali R, Tabachenko VV, Kovba LM, Dem’yanets LN. The crystal strucure of CuMoO4-III. Russ J Inorg Chem. 1991;36:927.
Nassau K, Shiever JW. Cupric oxide-molybdenum oxide phase diagram in air and in oxygen. J Am Ceram Soc. 1969;52(1):36–40.
Tadeusz M, Jacek Z. Subsolidus phase diagram of Cu2O–CuO–MoO3 system. J Solid State Chem. 1980;31(2):135–43.
Manukyan K, Aydinyan S, Aghajanyan A, Grigoryan Y, Niazyan O, Kharatyan S. Reaction pathway in the MoO3 + Mg + C reactive mixtures. Int J Refract Metals Hard Mater. 2012;31:28–32.
Baghdasaryan AM, Niazyan OM, Khachatryan HL, Kharatyan SL. DTA/TG study of tungsten oxide and ammonium tungstate reduction by (Mg + C) combined reducers at non-isothermal conditions. Int J Refract Metals Hard Mater. 2014;43:216–21.
Acknowledgements
The authors acknowledge the financial support of the State Committee of Science of the Republic of Armenia (Project #13_1D192).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirakosyan, H., Minasyan, T., Niazyan, O. et al. DTA/TG study of CuO and MoO3 co-reduction by combined Mg/C reducers. J Therm Anal Calorim 123, 35–41 (2016). https://doi.org/10.1007/s10973-015-4919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4919-z