Abstract
Characterized with a large gas production and low combustion temperature, the guanidine nitrate (GN) gas-generating agents are studied and applied widely. The determination factors of thermal decomposition properties of guanidine nitrate and basic cupric nitrate (GN/BCN) gas-generating agents for airbag application was investigated by the thermogravimetry–differential scanning calorimetry–mass spectrmetry–Fourier transform infrared spectroscopy (TG-DSC-MS-FTIR) and automatic calorimeter. Five different mass ratios were concerned. Our study showed that the onset reaction temperatures of GN/BCN mixtures were lower than that of individual GN and BCN. The thermal decomposition of GN/BCN mixtures could be divided into three stages, including the dissociation and escape of crystal water, solid (GN)-solid (BCN) phase reaction, and liquid (GN)-solid (BCN) phase reaction. When mass ratio of GN/BCN was 62.24/37.73, the largest value of the reaction heat was measured to 3152.7 J g−1, with N2 and H2O as the major gases during thermal decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, the gas-generating agents are constituted by fuel and oxidizing agents. GN contains rich nitrogen element (49 wt%), and low oxygen demanded [1, 2]. BCN can provide more oxygen, and reduce the combustion heat of gas-generating agent [3, 4]. As containing merits of large gas production and low combustion temperature, the GN/BCN gas-generating agents are being studied and applied widely [3–5]. In 2009, R.S. Damse [6] has studied the mechanism of thermal decomposition of GN, and found out that slow breakage of the relatively strong C–N bond of GN was the major cause for the slow burning behavior of GN. Jimmie C. Oxley et al. [7] have envisioned three possible decomposition routes of GN, and confirmed that nitric acid and NH3 were dissociated from GN. In 2010, Satoru Yoshino and Atsumi Miyake [8] have researched the thermal decomposition properties of GN and 1,2,4-triazole-3-one (TO) mixtures. They thought that the interaction between TO and nitric acid from the dissociation of GN was proposed for the thermal decomposition of TO/GN mixtures.
For a better understanding of thermal decomposition properties of GN/BCN mixtures, thermogravimetry–differential scanning calorimetry-mass spectrmetry–Fourier transform infrared spectroscopy (TG/DSC-MS-FTIR) and automatic calorimeter were performed.
Experimental
Materials
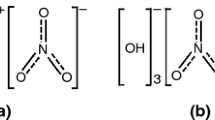
The purity of GN (Sanming Coffer Fine Chemical Industrial Co., Ltd) was 98.26 %, and the average particle size was 5.52 μm. The Cu-content and Nitrate-content of BCN (Sanming Coffer Fine Chemical Industrial Co., Ltd) were 52.83 and 24.58 %, respectively. The 50 % accumulative size (D50) was 1.63 μm. Molecular structure of GN was showed as Fig. 1.
Reagents were mixed for sixty minutes using chopsticks on parchment paper carefully [9]. Samples were prepared by mixing a stoichiometric composition and a positive and negative oxygen balance based on Eq. 1. Table 1 shows the mixture compositions.
Apparatus and method
The heat of reaction was measured using Hunan YouXin YX-ZR Type automatic calorimeter. Benzoic acid was used to calibrate the thermal capacity of automatic calorimeter. Because nickel chrome ignition wire could not ignite GN/BCN mixtures directly, black powder was used to ignite GN/BCN mixtures. 2.5 g of GN/BCN mixtures and 0.3 g of black powder were weighed. Each of the mixtures was tested three times, and took the average.
TG/DSC-MS-FTIR can provide not only the thermal analysis date but also the information of the decomposition gases evolved from the pyrolysis of GN/BCN mixtures. The thermal decomposition process was monitored by MS and FTIR on-line. The apparatus is constituted of three parts: TG/DSC (NETZSCH STA449C) systems, MS (NETESCH-QMS403C) systems, and FTIR (NICOLET6700 FTIR) system. NETESCH-QMS403C conditions: ionizing electron energy of 70 eV, quartz capillary gas connector, pressure injection 1,000 mbar, and capillary temperature 200 °C. NICOLET6700 FTIR conditions: The spectra were integrated 12 times, with a resolution of 4 cm−1, the gas cell and the gas tube between the TG/DSC and the FTIR stayed at 200 °C. All samples were placed in alumina crucibles (80 μL). Samples 1–7 were weighted at 0.30 ± 0.05 mg for the TG/DSC-MS-FTIR tests by heating them at 5 K min−1 from 30 to 600 °C. High-purity argon was used as purge gas with a gas flow rate of 20 mL min−1.
Results and discussion
Heat of reaction
In Fig. 2 the heat of reaction results are plotted against the oxygen balance (OB) of GN/BCN mixtures. When the oxygen balance of GN/BCN decreases, the heat of reaction of GN/BCN increases and almost establishes after OB < 0. When oxygen balance of GN/BCN mixtures becomes −5 (mass ratio GN/BCN = 62.64/37.36), then heat of reaction reaches a maximum value (3152.7 J g−1).
Based on Eq. 1, Hess’s Law and the relationship between the reaction heat at constant pressure (\( Q_{\text{p}} \)) and the reaction heat at constant volume (\( Q_{\text{V}} \)), the reaction heat of sample 4 could be calculated.
where i is the resultant, p is the reactants, and \( \Updelta_{\text{f}} H_{\text{m}}^{{{\theta}}} \) is the standard molar enthalpy of formation.
And the reaction heat at constant volume (\( Q_{\text{V}} \))
where \( {P}_{0} \) is the standard atmospheric pressure, equals 101.325 kPa, V is the volume of all resultants at the standard atmospheric pressure, and \( V_{0} \) is the volume of oxygen bomb.
The volume of oxygen bomb could be ignored and the volume of solid phase in resultants also could be ignored. So
where\( V_{\text{g}} \) is the volume of gas resultants at the standard atmospheric pressure, N is the molar quantity of gas resultants, R is the molar gas constant, equals 8.314 J K−1 mol−1, and T is the ambient temperature.
So the calculated value of the reaction heat of GN/BCN is 3006 J g−1 (the calculated result is negative, meaning exothermic), and the experimental value is 2964 J g−1, the relative error is only 1.40 %. When oxygen balance is positive (sample 5 and 6), the decomposition of excessive BCN in mixtures need to absorb vast heat, so the heat of reaction is lower than that in zero oxygen balance. When oxygen balance is negative (sample 2 and 3), the combustion of excessive GN in mixtures would emit some heat, so the heat of reaction is slightly higher than that in zero oxygen balance.
Thermal analysis
Figure 3 shows the results of the DSC and TG curves at 5 K min−1 heating rates for the pure GN (sample 1), the pure BCN (sample 7) and the mixtures GN/BCN (sample 2–6) with different mass ratios.
Table 2 lists the temperatures at 5 % mass loss (T TG5 %), onset reactive temperatures (T DSC), the temperature of endothermic peak (T p1), the temperature of first exothermic peak (T p2), and the temperature of second exothermic peak (T p3).
The T TG5 % of GN/BCN mixtures is between 160 and 180 °C, the T DSC is between 150 and 170 °C, the T p1 is between 170 and 180 °C, the T p2 is between 180 and 190 °C, the T p3 is near 200 °C. There are no observable difference between each of GN/BCN mixtures about T TG5 %, T DSC, T p1, T p2, and T p3. But the T TG5 % and T DSC of GN/BCN mixtures are lower than those of individual GN and BCN.
Individual GN (sample 1) has three peaks. The first endothermic peak at 213 °C in the DSC curve corresponds to the phase change from solid to liquid. After this phase change, the main decomposition of GN begins at about 278 °C and completed at 320 °C (72.8 % mass loss). Ref. [10] considered that the endothermic decomposition is caused by the decomposition of NH4NO3 or the dissociation and escape of HNO3 + NH3. Meanwhile, a kind of polymer production called Melon is prepared from the intermediate (H2N–C ≡ N) by multi-step polymerization. And the third endothermic peak at higher temperature is the decomposition of Melon [6, 7, 10].
DSC/TG curves of the pure BCN (sample 7) show that the decomposition reaction starts at about 219 °C and ends with a 34 % loss in mass. The mass ratio of CuO in BCN is about 66.25 %, and the mass ratio of Cu2O in BCN is about 59.58 %. It indicated that most of the solid residue of thermal decomposition of BCN is CuO.
There are three main peaks in the decomposition process of GN/BCN mixtures. So the thermal decomposition of GN/BCN mixtures could be divided into three stages. The first endothermic peak between 170 and 180 °C is thought to be the dissociation and the escaping of crystal water (DSC-MS curve could explain it). The second peak is an exothermic peak at about 185 °C. The ratio between the value of the second peak and the value of the third peak becomes larger with an increase of BCN in GN/BCN mixtures. And the peak temperature is significantly lower than the onset melting temperature (198 °C) of pure GN and the onset decomposition temperature (204 °C) of BCN. Hence, the reactive of GN/BCN mixtures is considered to be a solid (GN)-solid (BCN) phase reaction. The third peak is still an exothermic peak at about 200 °C. The value of third peak becomes lower with an increase of BCN in GN/BCN mixtures. The exothermic peak near 200 °C which is slightly higher than the onset melting temperature (198 °C) of GN and lower than the onset decomposition temperature (204 °C) of BCN is thought to be a liquid (GN)-solid (BCN) phase reaction, which increases the contact area of GN and BCN and making the reaction more intense. When the oxygen balance is positive, the weak exothermic peak is thought to be a combined effect of the exothermic reaction of GN/BCN and the endothermic decomposition of excess BCN.
Gas products analysis
Evolved gas analyses are performed using TG/DSC-MS-FTIR. Figure 4 represents the DSC-MS of the gas products of sample 3. It can be found that the m/z = 14(N), 16(O or NH2), 17(OH or NH3), 18(H2O), 28(CO or N2), 30(NO), and 44(CO2 or N2O) are detected. Figure 5 shows the FTIR spectra of gas products of sample 3 at 200 °C. The IR spectral peaks are assigned to νN2O: ~2,238 cm−1, νCO2: ~2,368 cm−1, δH2O: 3,500–3,800 cm−1 [11]. Comparing Fig. 4 with 5, it is certain that there are H2O, N2, N2O, and CO2, and almost no NO (1,965–1,762 cm−1) and CO (2,176 cm−1). Figure 4 shows only m/z = 17 and 18 have corresponding weak peak compared with endothermic peak of DSC at about 180 °C, so the initial endothermic reaction of GN/BCN mixtures in the range of 150–180 °C is thought to be dissociation and escape of chemical water.
Conclusions
Based on the investigation of the thermal decomposition properties of GN/BCN mixtures, the following conclusions were drawn.
- 1:
-
The heat of reaction for OB (GN/BCN) = −5 is larger than those of other mixing ratios.
- 2:
-
The initial reactive temperatures of GN/BCN mixtures are significantly lower than the decomposition temperature of BCN and the melting temperature of GN.
- 3:
-
The reaction of GN/BCN mixtures could be divided into three stages. And the main stages include the dissociation and escape of crystal water, solid (GN)-solid (BCN) phase reaction and liquid (GN)-solid (BCN) phase reaction.
- 4:
-
The gases evolved from GN/BCN mixtures are determined to be H2O, N2, and N2O. H2O and N2 are the major gases for the thermal decomposition of GN/BCN mixtures.
References
Mendenhall IV, Smith RG. Microgas-generating agent [P]. CN Patent 03812220.0. 2003.
Ulas A, Risha GA, Kuo KK. Ballistic properties and burning behaviour of an ammonium perchlorate/guanidine nitrate/sodium nitrate airbag solid propellant. Fuel. 2006;85:1979–86.
Jianzhou W. Gas-generating agent composition [P]. CN Patent 200410086968.7. 2003.
Jianzhou W. Gas-generating agent composition [P]. CN Patent 03801083.6. 2003.
Seo YD, Chung SH, Yoh JJ. Automotive airbag inflator analysis using the measured properties of modern propellants. Fuel. 2011;90:1395–401.
Damse RS. Studies on the decomposition chemistry of triamino guanidineazide and guanidine nitrate. J Hazard Mater. 2009;172:1383–7.
Oxley JC, Smith JL, Naik S, Moran J. Decompositions of urea and guanidine nitrate. J Energ Mater. 2009;27:17–39.
Yoshino S, Miyake A. Thermal decomposition properties of 1,2,4-triazole-3-one and guanidine nitrate mixtures. J Therm Anal Calorim. 2010;102:513–6.
Akiyoshi M, Nakamura H, Hara Y. The thermal behavior of the zinc complexes as a non-azide gas generant for safer driving—Zn complexes of the carbohydrazide and semicarbazide. Propellants, Explos Pyrotech. 2000;25:41–6.
Liu Z. Thermal analyses for energetic materials. Beijing: National Defense Industry Press; 2008. p. 184–6.
Li Y, Cheng Y. Investigation on the thermal stability of nitroguanidine by TG/DSC-MS-FTIR and multivariate non-linear regression. J Therm Anal Calorim. 2010;100:949–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mei, X., Cheng, Y., Li, Y. et al. Thermal decomposition properties of guanidine nitrate and basic cupric nitrate. J Therm Anal Calorim 114, 131–135 (2013). https://doi.org/10.1007/s10973-012-2851-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2851-z