Abstract
Ammonium dinitramide (ADN) is a promising new oxidizer for solid propellants because it possesses both high oxygen balance and high energy content, and does not contain halogen atoms. A necessary characteristic of solid propellants is chemical stability under various conditions. This study focused on the thermal decomposition mechanism of ADN under pressurized conditions. The pressure was adjusted from 0.1 to 6 MPa, while ADN was heated at a constant rate. The exothermal behavior and the decomposition products in the condensed phase during heating were measured simultaneously using pressure differential scanning calorimetry (PDSC) and Raman spectrometry. PDSC analyses showed the multiple stages of exotherms after melting. The exothermal behavior at low temperatures varied with pressure. Analysis of the decomposition products indicated that ammonium nitrate (AN) was generated during decomposition of ADN at all pressures. At normal pressure, AN was produced at the same time as start of exotherm. However, the temperature at which the ratio of ADN in chemical species in the condensed phase began to decrease under high pressure was higher than that at atmospheric pressure despite the existence of significant exotherm. At initial stage, thermal decomposition of ADN that does not generate AN was thought to be promoted by increased pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increasing focus on environmental issues is occurring worldwide. Energetic materials are now evaluated not only from their performance but also their current or potential environmental impact. Ammonium perchlorate (AP, NH4ClO4) has been used as a solid rocket propellant oxidizer for a long time due to its superior oxygen balance, energy density, and stability, as well as its relatively ease of handling. However, AP contains Cl, and so its combustion generates significant quantities of HCl as a reaction product. Because HCl is a toxic, corrosive gas, such emissions are of environmental concern. In addition, the potential toxicity of trace levels of perchlorate contamination in drinking water has become a public health issue. To eliminate these problems, AP must be replaced with a new halogen-free energetic oxidizer. Many studies pursued the development of new, environmentally safe (“green”) propellants containing less toxic or halogen-free propellants that provide performance in terms of burning rate, specific impulse, stability, and other important performance parameters [1–12].
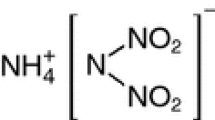
A promising new oxidizers considered as a replacement for AP is ammonium dinitramide [ADN, NH4N(NO2)2] [13–15], shown in Fig. 1. ADN possesses a high oxygen balance, can provide high energy, and is halogen-free, which makes it a candidate for an environmentally-friendly oxidizer in propellants and other energetic systems.
Like many highly energetic materials, ADN can degrade during storage. This has the potential not only to reduce its performance but also to reduce the safety of energetic devices. Analyses on samples that were stored for 11 years in the dark determined that it degrades to ammonium nitrate (AN) during storage, and that the thermal reactivity of ADN is reduced with aging [16, 17]. The incorporation of AN into an ADN/HTPB propellant increased the ignition delay of the propellant [18], decreased the specific impulse of the ADN/HTPB propellant [19], and significantly reduced the ADN burning rate [20]. Therefore, understanding the factors that affect the chemical stability of ADN, such as decomposition mechanisms, kinetics, and the influence of aging on performance, is important. Such information provides valuable data that can help to estimate a safe storage life, help choose suitable storage conditions (such as a safe temperature range), and the application of appropriate chemical stabilizers.
The present study focuses on the thermal decomposition mechanism of ADN. Analyses at various conditions such as heating rates, sample masses, atmospheres, and additives are effective to get better information about thermal decomposition behavior. Many studies concerning the thermal decomposition mechanisms have been conducted [10, 17, 19, 21–29]. These studies have reported that ADN decomposes into numerous products, including N2O, NO2, NO, NH4NO3, HNO3, N2, HONO, H2O, and NH3. However, few data exist about the thermal decomposition mechanism of ADN under pressurized conditions. Measurements under pressure enable a greater understanding of the influence of volatile and reactive species such as NO2 and HNO3. The goal of the present study was to improve understanding of this unique oxidizer by studying the thermal decomposition behavior of ADN under pressure. Thermal behavior and decomposition products of ADN were measured simultaneously using a combination of thermal analysis and spectrometry.
Experimental
ADN (Hosoya Pyro-Engineering Co., Ltd.), and AN (Wako Pure Chemical Industries, Ltd.) were used as received without further purification. AN was included in these studies because it is a main product of ADN decomposition [16].
The thermal behavior of ADN was characterized using pressure differential scanning calorimetry (PDSC, Mettler Toledo, DSC27HP). Samples weighing approximately 5 mg were loaded into a gold plate cell, and then heated from 30 to 350 °C at 5 K min−1. The atmosphere was 200 mL min−1 of nitrogen, and surrounding pressure was 0.1, 1, 2, 4, or 6 MPa.
Decomposition behavior in the condensed phase was measured using DSC and Raman spectrometry (DSC-Raman). For Raman spectroscopy, a Kaiser Raman RXN system coupled to a 785-nm semiconductor laser was used. The sample was heated from 95 to 350 °C at a constant rate under the conditions described above. During heating, the sample in the pan was illuminated by a focused laser beam through a glass porthole in the DSC cover.
Results and discussion
Thermal behavior of ADN under pressurized condition
The PDSC curves of ADN at each pressure value are shown in Fig. 2. The endotherm from melting of ADN was observed at approximately 92 °C. After melting, two exothermic events occurred between 135 and 220 °C at each pressure. The heat values and onset temperatures at this range, Q DSC and T DSC, are summarized in Table 1. T DSC was defined as the point when the exothermic power reached 0.2 W g−1 from baseline. The values of Q DSC and T DSC were similar at all pressures. However, the shape of the exothermic peak depended on pressure. From 0.1 to 2.0 MPa, the exothermic peak at low temperatures became clearer as the surrounding pressure increased. These results indicated that some exothermic reaction was promoted by pressurization. In contrast, at pressures greater than 2 MPa, the reaction was inhibited with increasing pressure. This suggests that the reaction mechanism changed between 2 and 4 MPa. In addition, other exothermic events were observed from 220 °C at pressure greater than 2 MPa. This is thought to come from exothermic reaction involving materials which is easy to volatilize at normal pressure.
The products in condensed phase during ADN decomposition
The Raman spectra of species in the condensed phase with time at 0.1 and 2 MPa are shown in Figs. 3 and 4, respectively. Note that the vertical axis of each spectrum is scaled differently. At 95 °C, the Raman bands from the NO2 group in ADN were observed at 1,520, 1,340, 1,170, 1,040, 830, 760, and 480 cm−1, and that from the N3 group in ADN was observed at 950 cm−1. Between 145 and 155 °C, the ratio of the Raman scattering values at 1,040 cm−1 began to increase, although those of other Raman scatterings decreased with the same behavior. Upon further heating, the only Raman band present was at 1,040 cm−1, and then this scattering also decreased and no Raman band was observed at a sufficiently high temperature. In addition, no other Raman band was formed during the experiments. This behavior was observed not only under atmospheric pressure but also under pressurized conditions.
A Raman band at 1,040 cm−1 is also a typical band from an NO −3 group. Possible decomposition products of ADN that contain an NO −3 group include HNO3 and AN. However, HNO3 also has other Raman bands at 710 and 640 cm−1, which have a same intensity level as that at 1,040 cm−1 [30]. In contrast, typical bands for AN (shown in Fig. 5) are observed only at 1,280, 1,040, and 710 cm−1, and the intensity of the band at 1,040 cm−1 is much greater than that of the other peaks. Thus, the products observed in this DSC-Raman results indicate a product that contains an NO −3 group, which was thought to be AN. The main product in the condensed species was AN during thermal decomposition of ADN; the AN decomposed further at higher temperature.
When the surrounding pressure was high, AN persisted until higher temperatures. Therefore, the exothermic event beginning at 220 °C and high pressure resulted from the decomposition of AN.
The details of the exothermic reaction at low temperatures also were examined because notable changes in thermal behavior occurred with the increasing pressure. Figure 6 shows the extended figure of thermal behavior and molar ratio of ADN in chemical species in the condensed phase with an increase in temperature. Since only ADN and AN were observed in the condensed phase during heating by Raman spectrometry, a standard curve was constructed between mixture ratio of ADN to AN and intensity of Raman scattering come from ADN at 1,340 cm−1 to from ADN and AN at 1,040 cm−1, and the change of the ADN ratio in condensed phase during heating was calculated. Under atmospheric pressure, the ratio of ADN in chemical species in the condensed phase was decreased at temperatures lower than 150 °C. However, the temperature of the onset of ADN decrease increased with the increase of surrounding pressure, and finally became nearly constant at 2 MPa, even though the exothermic peak in this range became clearer as surrounding pressure at high pressure. Two possible reasons exist for this behavior.
A first possible explanation is the progression of the decomposition of ADN that does not produce AN. In previous studies [23, 24], the following reactions were proposed as such reaction.
The second possibility is that AN produced from ADN decomposition was further decomposed immediately. The AN dissociates to NH3 and HNO3, and the decomposition of HNO3 can be catalyzed in the presence of acid [31, 32].
Influence of AN on thermal decomposition of ADN
Since AN was the main decomposition product in the condensed phase, the effects of AN on the decomposition behavior of ADN were investigated. Figure 7 shows the thermal behavior of pure ADN and ADN/AN (9.5/0.5, 8/2, and 2/8) at 2 MPa. The exothermic peaks at 135–220 °C (first exotherms) indicated that the main reaction of ADN decomposition, and the peak at temperatures greater than 220 °C (second exotherm) showed that AN came from mixing before the tests, or from ADN decomposition at the first exotherms.
From these results, AN affected on the first exotherms. The exotherm at low temperature shifted to the high temperature side, and finally overlapped that of at high temperatures. Under the ADN-rich conditions, i.e., ADN/AN = 9.5/0.5 and 8/2, the exotherm at high temperatures become clearer. The heat values of the exotherms, Q DSC, are summarized in Table 2. The Q DSC-1st decreased with an increase of the AN content; however, the amount of ADN did not correlate with Q DSC-1st. In addition, the Q DSC-2nd value was not adequate if all of the AN from ADN decomposition remained at the second exotherm. Therefore, AN was thought to contribute to ADN decomposition at high temperatures.
Figure 8 shows the ADN ratio in chemical species in the condensed phase during heating at 2 MPa using PDSC-Raman. The decrease in the amount of ADN occurred in a manner similar to that during pure ADN decomposition at temperatures greater than 175 °C. This result indicates that the same reactions occurring during pure ADN decomposition at temperatures greater than 175 °C occurred in the ADN/AN mixture.
Conclusions
The thermal decomposition behavior of ADN under pressurized condition was investigated using PDSC and Raman spectrometry. Exothermic reactions at low temperatures became more significant with an increase in pressure. Composition analysis of the condensed phase showed that AN was an intermediate formed during decomposition of ADN at all pressures. At normal pressure, AN was produced at the same time as start of exotherm. At high pressures, however, the ADN ratio in chemical species in the condensed phase was not decreased at low temperature despite the existence of marked exotherm. Two possible reasons exist for this effect. One is that it results from either the reaction that does not produce AN, and the second is that further decomposition of AN occurs from ADN decomposition at initial stage of decomposition. The thermal decomposition behavior of an ADN/AN mixture showed the effects of AN on the decomposition behavior of ADN. AN affected on the first exotherms of ADN decomposition. The AN inhibits the decomposition of ADN at low temperatures, and contributed to exothermal reactions at high temperatures.
References
Guerya JF, Chang IS, Shimada T, Glick M, Boury D, Robert E, Napior J, Wardle R, Perut C, Calabro M, Glick R, Habu H, Sekino N, Vigier G, Andrea BD. Solid propulsion for space applications: an updated roadmap. Acta Astronaut. 2010;66:201–19.
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD. A review of energetic materials synthesis. Thermochim Acta. 2002;384:187–204.
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, Gandhe BR, Rao AS. Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater. 2009;161:589–607.
Okamoto K, Kohga M, Hasue K. Thermal behavior and tensile property of PTHF/HTPB blend. Sci Tech Energ Mater. 2009;70:87–93.
Wada Y, Seike Y, Tsuboi N, Hasegawa K, Kobayashi K, Nishioka M, Hori K. Combustion mechanism of tetra-ol glycidyl azide polymer. Sci Tech Energ Mater. 2008;69:143–8.
Pandey M, Jha S, Kumar R, Mishra S, Jha RR. The pressure effect study on the burning rate of ammonium nitrate-HTPB-based propellant with the influence catalysts. J Therm Anal Calorim. 2012;107:135–40.
Pourmortazavi SM, Rahimi-Nasrabadi M, Kohsari I, Hajimirsadeghi SS. Non-isothermal kinetic studies on thermal decomposition of energetic materials KNF and NTO. J Therm Anal Calorim. 2012;110:857–63.
Xu KZ, Chen YS, Wang M, Luo JA, Song JR, Zhao FQ, Hu RZ. Synthesis and thermal behavior of 4,5-dihydroxyl-2-(dinitromethylene)-imidazolidine (DDNI). J Therm Anal Calorim. 2011;105:293–300.
Venkatachalam S, Santhosh G, Nian KN. An overview on synthetic routes and properties of ammonium dinitramide (ADN) and other dinitramide salts. Propel Explos Pyrotech. 2004;29:178–87.
Santhosh G, Ghee AH. Synthesis and kinetic analysis of isothermal and non-isothermal decomposition of ammonium dinitramide prills. J Therm Anal Calorim. 2008;94:263–70.
Heinz T, Pontius H, Aniol J, Birke C, Leisinger K, Reihard W. Ammonium dinitramide (ADN)-prilling, coating, and characterization. Propel Explos Pyrotech. 2009;34:231–8.
Teipel U, Heintz T, Krause HH. Crystallization of spherical ammonium dinitramide (ADN) particles. Propel Explos Pyrotech. 2000;25:81–5.
Bottaro JC, Penwell PE, Schmitt RJ. 1,1,3,3-Tetraoxo-1,2,3-triazapropene anion, a new oxy anion of nitrogen: the dinitramide anion and its salts. J Am Chem Soc. 1997;119:9405–10.
Pak Z. Some ways to higher environmental safety of solid rocket propellant application. In: Proc. AIAA/SAE/ASME/ASEE 29th joint propulsion conf and exhibition. Monterey; 1993.
Östmark H, Bemm U, Langlet A, Sanden R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energ Mater. 2000;18:123–8.
Matsunaga H, Yoshino S, Kumasaki M, Habu H, Miyake A. Aging characteristics of the energetic oxidizer ammonium dinitramide. Sci Tech Energ Mater. 2011;72:131–5.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Takahashi K, Matsumoto K, Nakadai K, Oide S, Kuwahara T, Yu X, Shibamoto H, Habu H. Ignition characteristics of AN/ADN composite propellants. Proc. Int. lst symp. on energetic materials and their applications (ISEM2011). Okinawa; 2011.
Matsunaga H, Habu H, Miyake A. Thermal behavior of new oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;111:1183–8.
Starunin VA, D’Yakov AP, Manelis GB. Combustion of ammonium dinitramide. Combust Flame. 1999;117:429–34.
Brill TB, Brush PJ, Patil DG. Thermal decomposition of energetic materials 58. Chemistry of ammonium nitrate and ammonium dinitramide near the burning surface temperature. Combust Flame. 1993;92:178–86.
Oxley JC, Smith JL, Zheng W, Rogers E, Coburn MD. Thermal decomposition studies on ammonium dinitramide (ADN) and 15N and 2H isotopomers. J Phys Chem A. 1997;101:5642–52.
Vyazokin S, Wight CA. Ammonium dinitramide: kinetics and mechanism of thermal decomposition. J Phys Chem A. 1997;101:5653–8.
Löbbecke S, Krause H, Pfeil A. Thermal analysis of ammonium dinitramide decomposition. Propel Explos Pyrotech. 1997;22:184–8.
Kazakov AI, Rubtsov YI, Manelis B. Kinetics and mechanism of thermal decomposition of dinitramide. Propel Explos Pyrotech. 1999;24:37–42.
Pavlov AN, Grebennikov VN, Nazina LD, Nazin GM, Manelis GB. Thermal decomposition of ammonium dinitramide and mechanism of anomalous decay of dinitramide salts. Russ Chem Bull. 1999;48:50–4.
Tompa AS. Thermal analysis of ammonium dinitramide (ADN). Thermochim Acta. 2000;357–8:177–93.
Mishra IB, Russell TP. Thermal stability of ammonium dinitramide. Thermochim Acta. 2002;384:47–56.
Shmakov AG, Korobenichev OP. Bol’shova TA. Thermal decomposition of ammonium dinitramide vapor in a two-temperature flow reactor. Combst Explos. Shock Waves. 2002;38:284–94.
Lucas H, Petitet JP. High pressure Raman spectroscopy of nitric acid. J Phys Chem A. 1999;103:8952–8.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Matter. 1999;67:253–81.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Matter. 2005;127:204–10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsunaga, H., Habu, H. & Miyake, A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim 116, 1227–1232 (2014). https://doi.org/10.1007/s10973-013-3626-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3626-x