Abstract
The state-of-art of polyaluminosialates is reviewed in terms of inorganic 〈mers〉 showing the composition, degree of netting, function of modifying atoms and the role of non-bridging oxygen as well as hydroxyl groups (biocompatibility). The polymeric condensation is compared with the vitrification of glasses upon cooling. The replacement of Si by P is discussed as well as the analogous precipitation process of amorphous hydrous silica (opal). Progress of geopolymers and biopolymers usefulness is shown within the framework of generalized world of macromolecules screening hundred contemporary citations. Pultrusion technology is presented, capable to produce composite geopolymers reinforced by basalt fibers staying suitable for mechanical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Reviewing geopolymers and their links with the classical state of quenched glasses
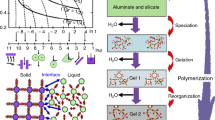
As a matter of curiosity the novel so-called geopolymeric materials were introduced about 10 years ago on the pages of this journal [1, 2] by Davidovits (cf. Fig. 1).
Geopolymers—X-ray-amorphous inorganic polysialates [1–5] are cementitious composites, which are commonly produced by idiosyncratic wet copolymerization (i.e., synthesis via solution) of the individual alumina and silica components [1–14]. Such a room-temperature synthesis process takes place when aluminosilicate source materials are dissolved in aqueous solution at very high pH yielding thus mostly non-crystalline zeolitic-like precursor, which is also termed as copolymer. The reactive Si–OH-based easy-soluble monomers in water-glass rather easily penetrate into the structure of inserted solid Al-compounds. This can be compared with the case of a high-temperature melting process of precursor glass batch, where conversely the rigid structure of quartz sand is diffusion-disintegrated by the penetration of Ca, Na, Al atoms from their more easily melted compounds thus braking up the original –Si–O–Si– structure. Both routes factually represent a mutually comparable type of vitrification reactions equally facilitating ionic interactions by either the movable hydrated ions or the diffusible atoms in the melted viscous state.
Aluminosilicate gels (zeolite precursors) are mostly synthesized [14–23] with the composition characterized by general formula M m [–(Si–O)z–Al–O] n ·wH2O, where M m are modifying cations (mostly Na, K, Ca, Mg), n is the degree of polycondensation and z is structural ranking (1, 2, 3,…). Configurationally tetrahedrons SiO4 and AlO4 are mutually bonded by oxygen bridges forming thus Si–O–Al-based chains and rings. The positively charged M m ions ought to be compensated by the negative charge of four coordinated Al. Generated gel-like structure is partially amorphous or nano-crystalline depending on both the amount of initial solid matter and its nature (character of raw materials) as well as on the condition of the reaction conditions (pH). The amorphous state is primarily favored for a higher concentration of solid precursor in the preparatory suspension.
The gel subsequently hardens into rigid geopolymers (resembling the glass formation upon the melt solidification), which may be characterized in a number of ways, correspondingly applicable to classical glasses. For example, the description in terms of principal constituents (alumina and silica) [22–26], their structure (tetrahedral Al–O and Si–O units in random three-dimensional framework), charge-balancing role of the tuning metallic (often alkali) ions, thermal glass-formation characteristics [27, 28] and their macroscopic properties (moderately strong and hard, stable up to 1000 °C, etc.). The specific circumstances of the low temperature synthesis [14–28] such as the condensation temperature of alumina and silica resources at high pH and distinctiveness of various sorts of water-glasses [29] are worth of a further clarification. XRD patterns of commercial melted glass and wet synthesized inorganic polymer were compared [29] showing broad peaks between 17° and 34° 2θ (typical for amorphous phases) which for inorganic polymer is slightly shifted to the higher 2θ revealing questioningly its relatively more “dense” structure than that for analogous commercial glass. Further deconvolution of such broad peaks in XRD patterns can enable a more quantitative estimation of the degree of amorphicity.
Certain formalism was developed in order to investigate structural units involved, mostly in the terms of fragments such as [–Si–O–Al–O–] called sialate units [1–14] (or polysialate when condensed concurrently). Further suggested units contain different Si:Al ratios, such as [–Si–O–Al–O–Si–O–] (sialate-siloxo [1–4, 8, 14–16]) and [–Si–O–Al–O–Si–O–Si–O–] (sialate-disiloxo). The Si:Al atomic ratio implies 1, 2, and 3, however, non-integer ratios intermediate between 1:1 and 1:3 may be anticipated as changeable combinations of basic units, provided that the content of charge-balancing cations is appropriate (often water content controlled). The units with Si:Al > 3 are designated as sialate and polysialate geopolymers. In the sense of majority of the Earth’s crust, which is composed of siloxo-sialates and sialates, the common feldspar series are albite–anorthite (NaAlSi3O8–CaAl2Si2O8) describable as poly(sialate-disiloxo) for albite to poly(disialate) for anorthite.
Hypocrystalline materials and their ‘mers’ framework
So far not enough attention has been paid to the basic structural disposition though detailed studies on reaction mechanism are available [30–39]. The inherent stoichiometry can be understood analogously to organic and/or inorganic –mers (known in classical polymeric chains) [39–47]. Such generalized spheres of the so-called hypocrystalline materials (an newly coined term more appropriate for an approved terminology) are specific of possessing the regular polyhedrons AO n (such as SiO4, AlO3, AlO4, BO4, BO3, PO4, etc.) [33, 47]. Important is the linking function of bridges formed by all n atoms of oxygen. If oxygen is bridged with two central cations, A- (e.g., {SiO4/2} as AO n/2) then i atoms of oxygen become bridged with A by a double bond (the so called terminal bonds, known from a single component glass composed, for example, of phosphorus oxides containing –mers of {O=P(–O–)3}. The adequate coordination formula ensues as {AO i/1O(n-i)/2}, where {PO1/1O3/2} may serve as an illustrative example of coinciding multifaceted stoichiometry. In the analogy with organic polymers, the group {AO i/1O(n-i)/2} can be considered as (n−i)-functional—mer. Similar attitude can be applied to geopolymers based on aluminosilicates composed of tetrahedral alumina and silica units. When condensed at ambient temperature the mer—units of AO n/2 become repositionable because of their concoction long lifetime this is comparable with the degree of immovability at the high temperature state of melts. Similarly to oxide glasses of a randomly interconnected web (continuous random network [40–47]) a relatively more complex copolymer system (containing supplements of moderating electropositive elements typically alkaline oxides, M2O, or other metallic oxides) persevere the function of modifying oxides. In oxide systems (melts/glasses/macromolecules) such additions to a single-component (tetragonally netted) solution result in the breakdown of bridging bonds A–O–A and formation of the so-called non-bridging oxygen. They can be described by set of equations: M2O ⇒ 2 M+ + O2− and M′O ⇒ M′2+ + O2− and A–O–A + O2− ⇒ 2A–O− , which are factually responsible for the dissociation concerning a single molecule of modifying oxide and the subsequent breakdown of bridging bonds (A–O–A). In the case of typical modifying oxides the equilibrium [42, 46] shifts toward the products and the arrangement can be easily derivable from melt/glass/polymer initial stoichiometry.
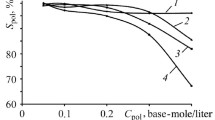
The cation distribution affects the species in the silicate solution, i.e., the amount of monomers, dimers, etc. [19, 29, 32, 33, 48–50]. Water-glass enforced by few percent of Al-ions [50] or partly substituted by P2O5 increases its penetrating reactivity. The study of vitrification of silicate solution indicates that the reaction commencement becomes associated with a concentration decrement as a result of separate phase growth [29, 30]. Comparing the molecular structure of reactants (metakaolinite and silicate solution) with the geopolymers, a complete rearrangement of molecular environment (short-range order of Al and Si) is evident [33]. Alumina is likely altered from their distorted crystal order via certain intermediate species (associated with valences IV, V, and VI) and linked to one SiO4 unit in metakaolinite forming the regular AlIV{Q 4(4Si)} [33, 44–46] where Q’s are the standard representation of Si-based configurationally motives [44–46], see below. However, the Al atoms seem to attain a more symmetrical environment in final geopolymers than in the original metakaolinite. The most reactive species in silicate solution is likely the hydroxyl anion OH− (or H3SiO4 −), which can draw parallel thoughts toward the basis of bioactivity of inorganic materials [51–56] and the coupled effect of non-bridging oxygen [47, 54]. It may even catch the attention of an innovative conception toward life creation on the Earth [51, 52] providing geopolymers as a new target biomaterial [57].
Accounting for the specificity of geopolymers, the charge-balancing metal ions factually make feasible the crucial polymerization in (–Si–O–Al–O–Si–O–) sialate-silixo chains where the atomic ratio remains Si:Al = 1:1 (however, capable to increase up to 3:1). When reaching the value above 3, generalized polysilicates became comparable to melted (counter-partner) glass. This approach, however, has not been applied to a geopolymeric state due to yet unacquainted methodology. The difference between melted glass and condensed sialate-silixo polymers endures in the subsistence of a certain coordination of (–Si–O–Al–O–Si–O–) with both Si4+ and Al3+ cations in the fourfold coordination, which is unavoidably balanced by the presence of modifying cations. It is ranging from fully amorphous up to partly organized (modulated) compositions of hyper-crystalline states thus exhibiting definite nano-crystalline regions. Single and multiple Al–O–Al bridging cannot carry on to subsist, nevertheless, alternatively can survive in minerals such as above-mentioned albite (NaAlSi3O8) and anortite (CaAl2Si2O8).
Worth another attention is still an apparent analogy with generalized (even organic) polymers mentioning the so-called mean degree of netting. Structural motives, Q, which appear throughout the melt–glass–polymers [33, 44, 45, 47] can be characterized by a coordination formula {AO(i+j)/1O(n-i-j)/2}, where j is the number of bridging oxygen atoms associated with a single central atom being ripped away by the action of oxygen produced through the dissociation of modifying oxides (j ≤ n−i). In the case of silica glasses (i = 0, n = 4) there is a direct link between the concept of the so called Q-notation (i.e., Q k, k = 0, 1, 2, 3, 4) and a coordination formula, Q k ≡ {SiO(4-k)/1O k/2}. Similar approach may become welcome for its alternative implementation to the circumstances of geopolymers, which fashion is still under prospect. Nonetheless, it was shown from NMR data [58, 59] that Q 4Si(2Al) and Q 4Si(3Al) components can exist in alkaline geopolymers, the latter being the highest for the Si/Al ratio of 1.5. For higher concentration of Na and consequently lower Si/Na ratio in the activating water-glass (and with Si/Al ratio measured in the polymer support) a feasible scheme points up that the hydrated sodium aluminosilicates bears a three-dimensional structure in which Q 4Si(3Al) predominates.
Some prospective studies
An alternative attitude to geopolymers can be anticipated when assuming a wider stoichiometry range by, e.g., the incorporating phosphates in addition or even instead silicates (where Si is totally or partially replaced by P) and/or borates as an alternative to aluminates. On the contrary to the needed alkaline environment for polyaluminosialates the phosphates are formed by an acid–base reaction between a metal oxide and an acid phosphate. Virtually any divalent or trivalent oxide that is sparingly soluble may be used to form these phosphate geopolymers. Berlinite (AlPO4) may serve as a good example, which is formed by the reaction between alumina and phosphoric acid (Al2O3 + 2H3PO4 ⇒ 2AlPO4 + 3H2O). It was also demonstrated that phosphate geopolymers of trivalent oxides such as Fe2O3 and Mn2O3 may possibly be produced by the oxide reduction and then acid–base reaction of the reduced oxide with phosphoric acid. Such wide-ranging phosphate materials represent another variety of mineral geopolymers [60–63] possibly promoting the incorporation of Fe cations for magnetic applications [48, 63, 82].
Relevance of bridging and non-bridging oxygen [45–47] to polysialate–polyphosphate geopolymers overlapping tetrahedral alumina/silicate/phosphate units has not been so far studied nor analysed so that this would become a target of future investigations. Notwithstanding the addition of acids, which occasionally accelerates the formation of gels, may support an idea that the gelling mechanism involves a cross-linking of preexisting (often linear) polymers someway analogous to organic polymeric systems. A range of other activation processes were also investigated [63, 64], however, any indistinct integration of theory of organic polymers, occasionally applied even to soluble silicates, may not assist a better interpretation of such aqueous inorganic systems.
Strategy for geopolymers applicability as a matrix for reinforced composites (fibers, foam, and/or ceramic) is different from the practice in similarly activated cements [25, 65] producing, however, analogous materials that possesses ‘cementitious’ property. It is foreseeable that the structures of these alkali-activated cement, alkali-activated slag, fly ash, etc., are different resulting from different chemical-mechanistic paths. The calcium silicate hydrate is a major binding phase in Portland cements holding also somewhat unstable ettringite. In dissimilarity the binding property of geopolymers results from the formation of a three-dimensional (mostly amorphous) aluminosilicate network containing tetrahedral unit with diminishing transport properties (causing thus a low mobility of particles). Therefore, the viscosity of such dispersal heterogeneous system tolerates systematical formation of a required shape by slow rearrangement. The rates of partial polymerization reactions are in agreement with the rate of structure ordering processes. Depolymerization requires the formation of zeolite nuclei within a geopolymer micelle in the hydrous aluminosilicate gel. The time needed for such crystallization varies from a few hours to several days; aging time at room temperature is about one day and crystallization time at 100 °C up to 100 h (for the relevant glassy Na2O/K2O–Al2O3–SiO2 system the crystallization temperature is in the range from 150 to 230 °C). The structure of amorphous silica is of a more open arrangement than that of closely related cristobalite. Spaces/sites on the reaction surface are worth of extra attention being capable to accommodate hydroxyl ions. Such a reactive surface bears an ionic charge and particularly silica is under continual switching process of equilibration between solution and interfaces, which thus enables better physical–chemical interaction even with the enforced fillers.
Some practical aspects of geopolymeric composites can be seen from the matrix primary properties exhibiting the compressive strength of 100 MPa, which is about twice that of cement but having only one-half its density. Filling with all-length polypropylene fibers increases its strength up to about 200 MPa. For a plausible technological application we brought in a special so-called pultrusion technology [66] in which the enforcing material is a yarn bundle, better a rowing of basalt fibers [67, 68] (2520 TEX, ϕ = 13 μm), which is enforced through an extrusion nozzle to harden, see Fig. 2. For a weight ratio of rowing of about 80%, with commercial matrix (Baucis FG), the resulting banding strength reaches 360 MPa, which becomes reliable for mechanical applications (when expensive classical epoxy analogous composite is not more than twice stronger) [67–69]. The advantage of this geopolymeric composite is its excellent fire resistance (so-called fire–smoke-toxicity).
There are some unclear points as the determinability of glass transition temperature of polysialates. It is often accomplished from the position of loss modulus apex (resolved by DMA) [39] but for many amorphous materials the glass transition temperature becomes overlapped by crystallization upshots [70]. Better solid phase identification is needed [70–74]. So far there were no attempts to look for a glass-formation coefficient, common in other types of glassy materials [75], which may provide a better insight to the structure of geopolymers (and their modeling challenge [76–79]) as well as for some new applicability [80, 81].
Further interrelation aspects of far-reaching polymeric materials
Another extension of a generalized understanding of inorganic polymeric materials can be located in the sphere of amorphous hydrous silica—opals [82–88], which is a remarkable material with the general formula SiO2·nH2O, but is precipitated as mono-dispersed colloidal particles (from 150 to 400 nm in diameter). In the most common weathering model [83–85, 87, 88] it is formed analogously to the gel process [67, 68] via geopolymers-like formation through gelatinous state of approximate stoichiometry (M m (SiO2) Z (AlO2) Y nMOH·wH2O). Sandstones are a common source of silica where the process of chemical weathering (of relatively soluble silicates such as the feldspars contained in these sediments) results in the formation of an alkaline silica solution. An example of such a mineral is potassium feldspar which weathers through the idealized stoichiometry (e.g., 2KAlSi3O8 + 3H2O ⇒ Al2Si2O5(OH)4 + 2KOH + 4SiO2) by the permeation of ground water through the sediments resulting in kaolin, dissolved silica, and an increase in pH through the release of potassium hydroxide.
The process of cross-linking via sol–gel processes is characteristic of possible incorporating a substantial amount of organic compounds as additives [88]. Thus, the characteristic property of a more broadly viewed naturally occurring geopolymers can involve a substantial amount of organic/humid materials. The term geopolymers can thus be understood by geochemical communities as macromolecular organic-containing polymers (or biopolymers) indicating the geological transformation of various geo-molecules through geochemical processes [89–93] during diagenesis. Consequently, such a mineralization path provides a stable material as the final (alternating) products in the Earth, such as kerogen, asphalt, etc. Such an attitude is also cross-boundary to an assortment of geo- and bio-polymers along with a generalized comprehension of existence of various biomaterials (for example biological glasses [94–97]) representing innumerable organic macromolecules accountable for life, their nucleation and transformation records, where an important role plays common states of low ordering, either forming glasses, amorphous matter, modulated, and nano-crystalline structures [98]. It incorporates basic questions of the determinability of such glassy and amorphous states [70–75, 98], adequate structure analysis [76–78, 99], search for a wide-ranging material applicability [11, 61, 99], etc. It involves a huge sphere of biological life, its diverseness, and also its curiosity to ever appear [51, 52].
References
Davidovits J. Geopolymers and geopolymeric materials. J Thermal Anal. 1989;35:429–41.
Davidovits J. Geopolymers: inorganic polymeric materials. J Thermal Anal. 1991;37:1633–56.
Davidovits J. Geopolymer chemistry and applications. 2008. Saint Quentin: Geopolymer Institute; 2008 (ISBN 2951482012).
Glukovsky VD. Gruntosilikaty. Kiev: Grosstrojizdat; 1959 (in Russian).
Brandštetr J. Slag-alkaline concretes, Stavivo. 1984;64:110–4 (in Czech).
Douglas E, Biloodeau A, Brandštetr J, Malhota M. Activated ground granulated blast-furnace slag: preliminary investigation. Cem Concr Res. 1991;21:101–8.
Douglas E, Bilodeau A, Malhotra VM. Properties and durability of alkali-activated slag concrete. ACI Mater J. 1992;89:509–16.
Xu H, Van Deventer JSJ. The geopolymerization of alumino-silicate minerals. Int J Miner Proc. 2000;59:247–66.
F. Šoukal T. Opravil P, Ptáček B, Foller B, Brandštetr J, Roubíček P. Geopolymers—amorphous ceramics via solution. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating noncrystalline states. Plzeň: OPS-ZČU; 2009, pp. 556–584 (ISBN 978-80-87269-06-0, available on request at sestak@fzu.cz).
Fletcher RA, MacKenzie KJD, Nicholson CL. The composition range of aluminosilicate geopolymers. J Eur Ceram Soc. 2005;25:1471–7.
Shi C, Krivenko VP, Roy D. Alkali-activated cements and concretes. Bristol: Taylor and Francis; 2006 (ISBN 978-0-415-70004-7)
Duxson P, Fernandez-Jimenez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ. Geopolymers: the current state of the art. J Mater Sci. 2007;42:2917–33.
Škvára F. Alkali activated materials or geopolymers? Ceram Silik. 2007;51:173–8.
Kriven WM. Inorganic polysialates or geopolymers. Am Ceram Soc Bul. 2010;89:31–4.
Rahier H, Simons W, VanMele B. Low-temperature synthesized aluminosilicate glasses. 3: Influence of the composition of the silicate solution on production, structure and properties. J Mater Sci. 1997;32:2237–47.
Barbosa VFF, MacKenzie KJD. Synthesis and thermal behavior of potassium sialate geopolymers. Mater Lett. 2003;57:1477–82.
O’Connors SJ, MacKenzie KJD. Synthesis, characterization and thermal behavior of lithium aluminosilicate inorganic polymers. J Mater Sci. 2010;45:3707–13.
Rahier H, Denayer JF, Van Mele B. Low-temperature synthesized aluminosilicate glasses—Part IV—modulated DSC study on the effect of particle size of metakaolinite on the production of inorganic polymer glasses. J Mater Sci. 2003;38:3131–6.
Duxson P, Mallicoat SW, Lukey GC, Kriven WM, van Deventer JSJ. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolinite-based geopolymers. Colloid Surf Physcochem Eng Asp. 2007;292:8–20.
Duxson P, Provis JL. Designing precursors for geopolymer cements. J Am Ceram Soc. 2008;91:3864–9.
Bel JL, Driemeyer PE, Kriven WM. Formation of ceramics from metakaolin based geopolymers. J Am Ceram Soc. 2009;91:607–15.
Yip CK, Lukey GC, Provis JL. Effect of calcium silicate sources on geopolymerization. Cem Concr Res. 2008;38:554–64.
O’Connor SJ, MacKenzie KJD, Smith EM, Hanna JV. Ion exchange in the charge-balancing sites of aluminosilicate inorganic polymers. J Mater Chem. 2010;20:10234–40.
Barbosa VFF, MacKenzie KJD, Thaumaturgo C. Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers. Int J Inorg Mater. 2000;2:309–17.
Tailby J, MacKenzie KJD. Structure and mechanical properties of aluminosilicate geoplolymer composites with Portland cement and its constituent minerals. Cem Concr. 2010;40:787–94.
Cui XM, Liu LP, Zheng GJ. Characterization of chemosynthetic Al2O3–2SiO2 geopolymers. J Non-cryst Sol. 2010;356:72–6.
Bernal SA, Rodriguez ED, Mejia de Gutierrez R. Mechanical and thermal characterization of geopolymers based on silicate-activated metakaolin/slag blends. J Mater Sci. 2011;46:5477–86.
Barbosa VFF, MacKenzie KJD. Thermal behavior of inorganic geopolymers and composites derived from sodium polysialate. Mater Res Bull. 2003;38:319–31.
Zaharaki D, Kommitsas K, Perdikatsis V. Use of analytical techniques for identification of inorganic polymer gel composition. J Mater Sci. 2010;45:2715–24.
Lobbus M, Vogelsberger W, Sonnefield J, Seidel A. Current considerations for the dissolution kinetics of solid oxides with silica. Langmuir. 1998;14:3023–33.
Trish TT, Jansen API, van Santen RA. Mechanism of oligomerization reactions of silica. J Phys Chem. 2006;110:23099–106.
Hlae D, Chandhary R. Mechanism of geopolymerization and factors influencing its development—a review. J Mater Sci. 2007;42:729–46.
Rahier H, Wastiels J, Biesemans M, Williem R, van Assche G, van Mele B. Reaction mechanism, kinetics and high temperature transformation of geopolymers. J Matter Sci. 2007;42:2982–96.
Provis JL, van Deventer JSJ. Geopolymerisation kinetics. 2. Reaction kinetic modelling. Chem Eng Sci. 2007;62(9):2318–29.
John L, Walls Philip A, van Deventer Jannie S. J. Geopolymerization kinetics 3—effects of Cs and Sr salts. Chem Eng Sci. 2008;63:4480–9.
De Silva P, Sagoe-Crenstil K, Sirivivatnanon V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem Concr Res. 2007;37:512–8.
Buchwald A, Vicent M, Kriegel R. Geopolymeric binders with different fine fillers—phase transformations at high temperatures. Appl Clay Sci. 2009;46:190–5.
Lloyd RR, Provis JL, van Deventer JSJ. Quantitative mechanistic modeling of silica solubility and precipitation during the initial period of zeolite synthesis. Cem Concr. 2010;40:1386–92.
Tian H, Zhang C, Wu L, XChen Y. Studies of mechanism of silica polymerization reactions in the combination of silica sol and potassium sodium water glass via isothermal heat conduction microcalorimetry. J Thermal Anal Calorim. 2010;101:064–959.
Mysen BO, Richet P. Silicate glasses and melts: properties and structure, developments in geochemistry. Amsterdam: Elsevier; 2005 (ISBN 0-444-52011-2).
Virgo D, Mysen BO, Kushiro I. Anionic constitution of silicate melts. Science. 1979;208:1371–7.
Murduch JB, Stebinm JF, Carmicheal ISE. Effect of network-modifying cations in silicate and aluminosilicate melts and glasses. Am Mineral. 1985;70:332–9.
Těmkin M. Mixtures of fused salts as ionic solutions. Acta Physicochem URSS. 1945;20:4511–9 (in Russian).
Macháček J, Gedeon O, Liška M. Group connectivity in binary silicate glasses. J Non Cryst Sol. 2006;352:2173–9.
Liška M, Macháček J, Perichta P, Gedeon O, Pilát J. Thermochemical modeling and molecular dynamics simulations of calcium aluminate glasses. Ceram Silik. 2008;52:61–5.
Greaves GN, Sen S. Inorganic glasses, glass-forming liquids and amorphizing solids: a review. Adv Phys. 2007;56:1–166.
Šesták J, Koga N, Strnad Z. Non-bridging oxygen in silica biocompatible glass ceramics and magnetic properties of Fe2O3 added borates. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating non-crystalline states, Plzeň: OPS-ZČU; 2009. pp. 354–386 (ISBN 978-80-87269-06-0, available on request at sestak@fzu.cz).
Nishida T, Oku H. Local structure and chemical durability of FeOOH-fixed sodium silicate glass prepared from water glass. J Radioanal Nucl Chem. 2002;253:303–6.
Tsai MS, Huang PY, Yang CH. Formation mechanism of colloidal silica via sodium silicates. J Nanopart Res. 2006;8:943–9.
Gianopoulosu I, Pantas D. Hydrolitic stability of sodium silicite gels in the presence of aluminium. J Mater Sci. 2011;45:5370–7.
Hench LL. Glasses and genes: a forecast for the future. Glastech Ber Glass Sci Tech. 1997;70:439–48.
Hench LL. Life and death: the ultimate phase transformation. Thermochim Acta. 1996;280/281:1–14.
Strnad Z, Šesták J. Biocompatible glass-ceramics. Invited lecture at the 2nd international conference on intelligent processing and manufacturing of materials, Honolulu; 1999. pp. 123–129.
Koga N, Strnad Z, Šesták J. Thermodynamics of non-bridging oxygen in silica bio-compatible glass-ceramics for bone tissue substitution. J Thermal Anal Calorim. 2003;71:927–41.
Šesták J, Strnad Z, Strnad J, Holeček M, Koga N. Biomedical thermodynamics and implantology aspects of biocompatible glass-ceramics and otherwise modified inorganic materials and surfaces. Advanced Mater Res. 2008;39(40):329–42.
Strnad Z, Strnad J. Physicochemical properties, healing capacity of inorganic endosteal biomaterials used for mimetic bone substitution in implantology. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating non-crystalline states, Plzeň: OPS-ZČU; 2009. pp. 538–584 (ISBN 978-80-87269-06-0, available on request at sestak@fzu.cz).
MacKenzie KJD, Rahner N, Smith ME, Wong A. Calcium-containing inorganic polymers as potential bioactive materials. J Mater Sci. 2010;45:999–1007.
Granizo ML, Alonso S, Blanco-Varela MT, Martinaz-Ramirez S. Alkali activation of metakaolin: parameters affecting mechanical, structural and microstructural properties. J Mater Sci. 2007;42:2934–43.
Duxson P, Provis JL, Lukey GC, Seaprovic P, van Deventer JSJ. Si-NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir. 2005;21:3028–37.
Wagh AS, Jeong SY. Chemically bonded phosphate ceramics: dissolution model of formation. J Am Ceram Soc. 2003;86:1838–44.
Wagh AS. Chemically bonded phosphate ceramics: a 21st century materials with diverse application. Amsterdam: Elsevier; 2004 (ISBN: 0-08-044505-5).
Wagh AS. Phosphate geopolymeric materials. Invited lecture at the 35th international conference on advanced ceramics and composites, Dayton; 2011.
Gomes KC, Lima GST, Torres SM. Iron distribution in geopolymers with ferromagnetic rich precursor in functional and structural materials. Book Ser Mater Sci Forum. 2010;643:131–8.
Granizo ML, Alonso S, Blanco-Varela MT, Martinaz-Ramirez S. Alkaline activation of metakaolin: effect of calcium hydroxide in the products of reaction. J Am Cer Soc. 2002;85:225–31.
Poděbradská J, Černý J, Drchalová J, Rovnaníková P, Šesták J. Analysis of glass fiber reinforced cement composites regarding their thermal and hygric/moist material parameters. J Thermal Anal Calorim. 2004;77:85–97.
Foller B. Systematic classification of pultrusion technology. In proc Polym Compos. Plzeň: JECMAGAZINE ZČU; 2011. pp. 22–26.
Deak T, Czigany T, Marsalkova M, Militký J. Manufacturing and testing of long basalt fiber rein forcing thermoplastic matrix composites. Polym Eng Sci. 2010;50:2448–56.
Militký J, Kovačič V, Křemenáková D. Basalt filaments—properties and applications. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating noncrystalline states. Plzeň: OPS-ZČU, pp. 499–520.
Foller B, Šesták J. Composite geopolymers and their research study at NTC laboratory for rheological and thermal research. In: Dubaj T, Cibulková Z, editors. The proceedings of the 3rd joint Czech–Hungarian–Polish–Slovak thermoanalytical conference—TERMANAL. Bratislava: Slovak Chemical Society; 2011. p. SL7
Šesták J. Use of phenomenological enthalpy versus temperature diagram (and its derivative-DTA) for a better understanding of transition phenomena in glasses. Thermochim Acta. 1996;280/281:175–90.
Wunderlich B. Glass transition as a key to identifying solid phases. J Appl Polym Sci. 2007;105:49–59.
Wunderlich B. The three reversible crystallization and melting processes of semicrystalline macromolecules. Thermochim Acta. 2001;396:33–41.
Queiroz C, Šesták J. Aspects of the non-crystalline state. Phys Chem Glasses Eur J Glass Sci Technol B. 2010;51:165–72.
Hutchinson JM. Determination of the glass transition temperature: methods correlation and structural heterogeneity. J Therm Anal Calorim. 2009;98:11–579.
Kozmidis-Petrovic A, Šesták J. Forty years of the Hrubý glass-forming coefficient via DTA when comparing other criteria in relation to the glass stability and vitrification ability. J Thermal Anal Calorim; 2012. doi:10.1007/s10973-011-1926-6 (in press).
Duxson P, Provis JL, Lukey GC. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloid Surf Phys Chem Eng Asp. 2005;269:47–58.
Provis JL, Duxson P, van Deventer JSJ. The role of mathematical modeling and gel chemistry in advancing geopolymer technology. Chem Eng Res Des. 2005;83:853–60.
Conrad CF, Icopini GA, Yasubara H, Nandstra JZ, Brantly SL. Modeling of kinetics of nanaocolloid formation and precipitation of silica in geologically relevant aqueous solutions. Geochem Cosmochim Acta. 2007;71:531–42.
White CE, Provis JL, Proffen T. Quantitative mechanistic modeling of silica solubility and precipitation during the initial period of zeolite synthesis. J Phys Chem C. 2011;115:9879–88.
Foller B. Dynamic mechanical analysis of composites with a defined magnetic permeability. In: Proc Polym Compos. Plzeň: JECMAGAZINE, ZČU; 2011, pp. 7–10.
Feng D, Tan H, Van Deventer JSJ. Ultrasound enhanced geopolymerisation. J Mater Sci. 2004;39:571–80.
Iler RK. The chemistry of silica, solubility, polymerization, colloid and surface properties, and biochemistry. New York: Wiley; 1979 (ISBN 0-471-02404-X).
Devison B. The origin of precious opal: a new model. Aust Gemmol. 2004;22:50–8.
Williams LA, Crerar DA. Silica diagenesis, I. Solubility controls. J Sedim Petrol. 1985;55:301–11.
Williams LA, Crerar DA. II. General mechanisms. J Sedim Petrol. 1985;55:312–21.
Thomas P, Heide K, Šesták J, Šimon P (2009) Properties of some natural glasses: Australian opals and Czech tektite Moldavites. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating noncrystalline states, Plzeň: OPS-ZČU; 2009. pp. 200–248 (ISBN 978-80-87269-06-0, available on request at sestak@fzu.cz).
Thomas P, Šesták J, Heide K, Füeglein E, Šimon P. Thermal properties of Australian sedimentary opals and Czech Moldavites. J Therm Anal Calorim. 2010;99:861–7.
Kozuka H, editor. Handbook of sol-gel science and technology, I: sol-gel processing. Almeida RM, editor. II: Characterization of sol-gel materials and products and Sakka S, editor. III: Applications of sol-gel technology. Berlin: Springer; 2005 (ISBN 978-1-4020-7969-6).
Perry CC, Keeling TT. Biosolidification: the role of the organic matrix in structure control. J Biolog Inorg Chem. 2000;5:537–50.
Kim D, Petrisor IG, Yen TFY. Geopolymerizartion of biopolymers: a preliminary inquiry. Carbohyd Polym. 2004;56:213–7.
Kim D, Lai H-T, Chilingar GV, Yen TFY. Geopolymer formation and its unique properties. Environ Geol. 2006;51:103–11.
Coradin T, Livage J. Aqueous silicates in biological sol-gel applications: new perspective of old precursors. Acta Chem Res. 2007;40:819–26.
Granja PL, Barbosa MA, Pouysegu L, de Joso B, Rouvais F, Baquey C. Cellulose phosphates and biomaterials, mineralization of chemically modified regenerated cellulose hydrogels. J Mater Sci. 2011;36:2163–72.
Zámečník J, Bilavčík A, Faltus M, Šesták J. Water state in plants at low and ultra-low temperatures. CryoLetters 2003;24:412.
Šesták J, Zámečník J. Can clustering of liquid water and thermal analysis be of assistance for better understanding of biological glasses exposed to ultra-low Temperatures. J Thermal Anal Calorim. 2007;88:411–6.
Zámečník J, Šesták J. Biological glasses and their formation during overwintering and cryopreservation of plants. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral properties of materials accentuating noncrystalline states. Plzeň: OPS-ZČU; 2009. pp. 176–198 (ISBN 978-80-87269-06-0, available on request at sestak@fzu.cz).
Šesták J, Mareš JJ, Hubik P, editors. Glassy, amorphous and nano-crystalline materials: thermal physics, analysis, structure and properties. Berlin: Springer; 2011 (ISBN 978-90-481-2881-5).
Provis JL, Lukey GC, van Deventer JSJ. Do geopolymers actually contain nanocrystalline zeolites? Chem Mater. 2005;17:3075–85.
Zhang J, Provis JL, Feng D, Van Jannie JSJ. Geopolymers for immobilization of heavy metals. J Hazard Mater. 2008;157:587–98.
Acknowledgements
This study has been carried out by NTC ZČU Pilsen under the support of the Project No. FR-TI 1/335 “Geopolymeric composites with high technical parameters” provided by the Ministry of Industry and Business of the Czech Republic and within the CENTEM Project, No. CZ.1.05/2.1.00/03.0088 that is co-funded from the ERDF within the OP RDI program of the Ministry of Education, Youth and Sports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šesták, J., Foller, B. Some aspects of composite inorganic polysialates. J Therm Anal Calorim 108, 511–517 (2012). https://doi.org/10.1007/s10973-011-2037-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2037-0