Abstract
Geopolymerization is a developing field of research for utilizing solid waste and by-products. It provides a mature and cost-effective solution to many problems where hazardous residue has to be treated and stored under critical environmental conditions. Geopolymer involves the silicates and aluminates of by-products to undergo process of geopolymerization. It is environmentally friendly and need moderate energy to produce. This review presents the work carried out on the chemical reaction, the source materials, and the factor affecting geopolymerization. Literature demonstrates that certain mix compositions and reaction conditions such as Al2O3/SiO2, alkali concentration, curing temperature with curing time, water/solid ratio and pH significantly influences the formation and properties of a geopolymer. It is utilized to manufacture precast structures and non-structural elements, concrete pavements, concrete products and immobilization of toxic metal bearing waste that are resistant to heat and aggressive environment. Geopolymers gain 70% of the final strength in first 3–4 h of curing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialization leads to the generation and release of undesirable pollutants into the environment. In order to keep pace with the rapid industrialization there is a necessity to select such process, which would cause minimum pollution in environment.
In recent years, there is an increasing awareness on the quantity and diversity of hazardous solid waste generation and its impact on human health. Increasing concern about the environmental consequences of waste disposal has led to investigation of new utilization avenues [1].
The greatest problem faced by industries, as far as waste disposal is concerned is the safe and effective disposal of its effluent, sludge and by-products such as large quantities of fly ash that are produced during the combustion of coal used for electricity generation. It is estimated that by the year 2010, the amount of the fly ash produced will be about 780 million tones annually [2]. Most of this ash is disposed in landfills at suitable sites [1, 3]. Landfilling is not a desirable option because it not only causes huge financial burden to the foundries, but also makes them liable for future environmental costs and problems associated with landfilling regulations [4]. The increasing load of toxic metals in the landfill potentially increases the threat to ground water contamination.
Increasing economic factors also dictate that industry should look forward to recycling and reuse of waste material as a better option to landfilling and discarding.
Need exists for a technology that can easily and cheaply handle large quantities of waste materials and by-products containing heavy metals as an alternative to OPC (ordinary Portland cement).
Disposal of hazardous waste must meet at least two conditions [5]
-
(1)
Safe chemical encapsulation i.e. control their release into ground water and seepage water.
-
(2)
Structural stability with respect to adverse environmental condition.
Production of one ton of Portland cement requires about 2.8 ton raw materials, including fuel and other materials and generates 5 to 10 % of dusts. Altogether 6000–14000 m3 dust-containing air-streams are generated per ton cement manufacture, which contain between 0.7 to 800 g/m3 of dust and accounts for about one ton of green house gas CO2 released to the atmosphere as a result of de-carbonation of lime in the kiln during manufacturing of cement (Eq. 1) [2, 6, 7].
A technology was therefore sought as an alternative to the afore-mentioned standards and, further more, the cost figures must not be intolerable [5]. To overcome these problems, geopolymers emerged as a possible solution for using the by-products and could be utilized to manufacture precasts structure and non-structural elements, concrete pavements, concrete products and immobilization of toxic waste that are resistant to heat and aggressive environment [8]. The objective of this review is to study the work carried out on the development of geopolymers, including the chemical reaction, the role and effect of the source materials, and the factors affecting mix compositions, such as curing temperature, curing time, Al2O3/SiO2 ratio in the mix, alkali concentration, pH and water/solid ratio.
Geopolymerization
Geopolymerization is a geosynthesis (reaction that chemically integrates minerals) that involves naturally occurring silico-aluminates [5]. Any pozzolanic compound or source of silica and alumina, that is readily dissolved in the alkaline solution, acts as a source of geopolymer precursor species and thus lends itself to geopolymerization [9]. The alkali component as an activator is a compound from the element of first group in the periodic table, so such material is also called as alkali activated aluminosilicate binders or alkali activated cementitious material [10]. Silicon and aluminum atoms react to form molecules that are chemically and structurally comparable to those building natural rocks [5]. The inorganic polymeric material can be considered as an amorphous equivalent of geological feldspars, but synthesized in a manner similar to thermosetting organic polymers. For this reason, these materials are termed as “geopolymers”[11].
It offers attractive option for simple industrial applications where large volume of waste materials needs to be stabilized [5]. It is named because of the similarities with the organic condensation polymers as far as their hydrothermal synthesis conditions are concerned [8]. Study of the literature and patents demonstrated, that before 1978, the idea of using this mineral chemistry for the development of a mineral polymer had been totally neglected. As a function of chemical composition of initial materials, the alkaline cements are classified into two groups.
-
(i)
Binders synthesized from materials rich in calcium such as blast furnace slag that produces calcium silicate hydrate (CSH) gel when activated with alkaline solution.
-
(ii)
Materials synthesized with raw materials low in calcium and rich in SiO2 and Al2O3 such as metakaolin. These materials when activated with alkaline solution, formation of an amorphous material (alkaline aluminosilicate) that develops high mechanical strength at early ages after a soft thermal curing [12].
These materials differ substantially from ordinary Portland cement, as they use totally different reaction pathway in order to attain structural integrity. Pozzolanic cement depends on the presence of calcium-silicate hydrate for matrix formation and strength where as geopolymers utilize the polycondensation of silica and alumina precursors (fly ash, kaolin, metakaolin) and a high alkali content to attain structural strength [13].
Chemistry of geopolymer
Geopolymerization is based on chemistry of alkali activated inorganic binders, which were accidentally discovered by Purdon [14]. He studied the sodium hydroxide on a variety of minerals and glasses containing silicon and/or aluminum and summarized it in two steps;(1) liberation of silica, alumina and lime and (2) formation of hydrated calcium silicates, aluminates as well as regeneration of caustic solution. Author proposed that the hardening mechanism of alkali activated alumino silicate binder involves dissolution of Si or Al in the presence of sodium hydroxide, and precipitation of calcium silicate or aluminum hydrate with the generation of sodium hydroxide. Similarly, Glukhovsky identified both CSH and calcium and alumino-silicate hydrate as solidification product on the alkali activation of slag binders and concluded that clay mineral reacts during alkali treatment to form aluminosilicate hydrate. Finally, Davitovits [15] developed a kind of mineral polymer material with 3-D cross-linked polysialate chain, which resulted from the hydroxylation and polycondensation reaction of natural minerals such as clay, slag, fly ash and pozzolan on alkaline activation below 160 °C (Fig. 1). This inorganic polymer was first named polysialate in 1976 and later coined as “Geopolymer”[15]. In 1980 the setting reaction of alkali activated slag cement was explained [14, 16]. Recently, Deventer [9, 13, 14, 16–29] has contributed towards the development and applications of geopolymers.
Mechanism of geopolymers involves the polycondensation reaction of geopolymeric precursors i.e. alumino-silicate oxide with alkali polysiliates yielding polymeric Si–O–Al bond [8, 16, 30, 31].
where M is the alkaline element, z is 1,2, or 3 and n is the degree of polycondensation [30].
Davitovits has suggested that certain synthesis limits existed for the formation of strong products; satisfactory compositions lay in the range M2O/SiO2, 0.2 to 0.48; SiO2/Al2O3, 3.3 to 4.5; H2O/M2O, 10–25; and M2O/Al2O3, 0.8 to 1.6 [16, 31, 32]. The geopolymeric alumino-silicate have been grouped in three families depending on the atomic ratio Si/Al that may be 1,2,or 3 [32].
Reaction Involved in Geopolymerization




Geopolymers consist of aluminum and silica tetrahedrally interlinked alternately by sharing all the oxygen atoms. A polymeric structure of Al–O–Si formed constitutes the main building blocks of geopolymeric structure. Alkali metal salts and/or hydroxide are necessary for the dissolution of silica and alumina as well as for the catalysis of the condensation reaction. The gel phase is thought to be highly reactive and produced by co-polymerization of individual alumina and silica from their source, dissolved by the alkali metal. Some cations must be present to keep the structure neutrality (since aluminum is four fold). Na, K, Ca and other metallic cations maintain this neutrality. It is still not clear whether these ions simply play a charge-balancing role or are actively bonded into the matrix. The mechanism of immobilization is expected to be the combination of chemical and physical interaction. Cation is either bonded into the matrix via Al–O or Si–O bond or present in the framework cavities to maintain electrical charge balance. A physically encapsulated cation should be substituted by another cation if its surrounding allows the diffusion process to occur [14]. Amorphous to semi-crystalline three dimensional alumino-silicate structures are of the poly(sialate) type (Si–O–Al–O–), the poly(sialate-siloxo) type (Si–O–Al–O–Si–O–) , the poly(sialate-disiloxo) type(Si–O–Al–O–Si–O–Si–O–) [8].
The coordination number of the silica and alumina in the source material is of great importance. Some of the water and NaOH are expelled out during hardening of the gel phase. It is believed that the alkali metal hydroxide acts as a catalyst and leach out from the hardened alkali activated binder in more or less the same amount as that was added during synthesis. Ions such as metal cations that are incorporated into the matrix in one way or another influence the final structure stability and therefore leaching characteristics [14].
Utilization of pozzolans results in the added technical advantages like reduction in temperature rise, improvement in durability and strength enhancement. Although in some cases strength develops slowly [33].
Geopolymers are very similar to zeolites and are formed in similar manner as zeolites do [16]. There exists a difference between zeolite formation and geopolymerization as related to the composition of the initial reaction mixtures. Zeolites usually form in closed hydrothermal systems but geopolymers do not. Geopolymers are amorphous to semi-crystalline, where as zeolites are usually crystalline in nature. When fly ash in geopolymers are mixed with the alkaline dissolution; a vitreous component is quickly dissolved. In such a situation there is not sufficient time and space for the gel to grow into a well-crystallized structure resulting in a microcrystalline, amorphous or semi-amorphous structure [34, 35] (Fig. 2). Aluminum source react with calcium hydroxide via a pozzolanic reaction to produce CSH and calcium alumino silicate, which are chemically less reactive. Zeolites usually crystallize from dilute aqueous solution, where precursor species have mobility as well as enough time to undergo proper orientation and alignment before bonding into a crystal structure.
Role of materials in geopolymer
Starting material plays an important role in the formation of geopolymer. Several studies have been conducted in order to develop various methods to improve the durability of the geopolymeric cement and concrete. Wide range of materials is presently being used for geopolymerization. These include various pozzolanic, supplementary cementitious materials, chemicals and mineral additives [36]. Materials rich in Si (like fly ash, slag and rice husk) and materials rich in Al (clays like kaolin, bentonites) are the primary requirement to undergo geopolymerization. Some other materials can also be utilized as a reactive filler material or a setting additive like ordinary Portland cement, kiln dust etc. which helps in the development of good mechanical properties. Some of the important materials used for the geopolymerization are described in the following sub-section.
Fly ash
Fly ash is one of the important source materials for geopolymer. It is available world wide yet its utilization is still limited. Fly ash consists of finely divided ashes produced by burning pulverized coal in power stations. The chemical composition depends on the mineral composition of the coal gangue (the inorganic part of the coal). Silica usually varies from 40 to 60 % and alumina from 20 to 30%. The iron content varies quite widely. Alkalis are present in an appreciable amount and potassium prevails over sodium [37]. When CaO is greater than 20% then it can be categorized as cementitious material. When CaO varies between 10% and 20% categorized as cementitious and pozzolanic material. A pozzolanic material requires calcium hydroxide (CH) in order to form strength-imparting products (pozzolanic activity). Usually the CaO content in these material is not enough to react with all the quantity of the pozzolanic compounds, and exhibit pozzolanic activity (pozzolanic and cementitious material). It is used with Portland cement, which yields CH on hydration [38, 39]. For geopolymerization high alkaline solutions are used to induce the silicon and aluminum atom in the source material to dissolve and form geopolymeric paste [2].
Fernandez-Jimenez and Palomo [35] reported that finesses of the fly ashes plays an important role in the development of the mechanical strength of the material obtained after activation. They reported that, when the particle fraction sized higher than 45 μm. is removed, mechanical strength increased remarkably, reaching 70 MPa in one day. van Jaarsveld et al. [18] reported that the surface charge on the fly ash particle affects the initial setting properties of a geopolymeric mix. This is because the mechanism of dissolution and subsequent geopolymerization involves the initial transportation of hydroxyl ions to the surface of the fly ash particles. This is followed by hydrolysis, thereby forming aluminate and silicate species. Subsequent polymerization of these monomers forms oligomers of varying geometries that causes the formation of the geopolymeric gel phase.
Burned clay and OPC
The burning of certain clays (kaolin, bentonites, montmorillonite etc) and oil shales produces ashes, that harden when mixed with lime and water. Clay mineral, such as Kaolin, gains a distinct pozzolanic activity when burned at temperature between 600 °C and 900 °C. These artificial pozzolanas are mostly composed of silica and alumina. The loss of combined water due to the thermal treatment causes the crystalline network of the clay mineral to be destroyed, while silica and alumina remains in a messy, unstable amorphous state, that reacts with CH [33]. The pozzolanic activity depends on the clay mineral content and thermal treatment conditions. Hardening results from the presence of cementitious compounds such as C2S and CS.
The highest strength obtained with mixes made of burned kaolin (metakaolin) was 27 MPa. The mechanical properties of the calcined clay can be improved by incorporating admixture, such as 0.01% ZnO to the mix [37]. Some times cement is also used as a calcium source, for the formation of strong geopolymer, which contributes tricalcium silicate (C3S) and di-calcium silicate (C2S) with the small amount of tricalcium aluminate (C3A) and calcium aluminoferrite (C4AF) [40, 41].
Kiln dust
Lime kiln dust and cement kiln dust act as absorbents and bulking agent. Lime kiln dust, also acts as a neutralizing agent in acidic condition. Kiln dusts are effective due to their calcium oxide content. This gives them high alkalinity and ability to remove free water by the hydration of CaO to Ca(OH)2 . The actual setting reaction of kiln dusts are pozzolanic and resembles to those of portland cement in many ways. Kiln dust contains silica and silicates from their natural rock genesis, with cement kiln dust generally having much higher silica content than the lime kiln dust [40].
Alkali activators
Strong alkalis are required to activate the silicon and aluminum present in the fly ash and setting additives, that allows transforming glassy structure partially or totally into a very compacted composite [2, 42]. The common activators are NaOH, Na2SO4, waterglass, Na2CO3, K2CO3, KOH, K2SO4 or a little amount of cement clinker [10]. Sodium silicate has been used for more than a century for the production of commercial products such as special cements, coatings, molded articles and catalysts. Some times silica fume is used as an alternative to the sodium silicate, which normally forms part of the reactant solution [43]. The soluble silicate is mixed with fly ash, cement, lime, slag or other source of multivalent metal ions that promotes the gelation and precipitation of silicates. More the NaOH get in contact with the reactive solid material the more silicate and aluminate monomers are released. Below a ratio of solution to solid of about 50 the concentration of the released monomers reaches saturation [44].
In Fig. 3, bars represents release of silicate and aluminate monomers in 10% NaOH solution and symbols (triangle and circle) represents concentration of released monomers at different L/S ratios.
During the pozzolanic reaction alkali cation might get incorporated in the hydration product. It is believed that the alkalis are incorporated within the interlayer of the CSH phase mostly by neutralizing SiOH group. The amount of alkali hydroxide incorporated increases with decrease in the CaO/SiO2 mole ratio in the mix [45]. Soluble silicate reduces the leachability of toxic metal ions by forming low-soluble metal oxide/silicates and by encapsulation of metal ions in the silicate-or silicate-gel matrix [40].
Superplasticizers
Addition of the superplasticizers improves the workability of the geopolymeric paste by improving the plastic and hardening properties, leading to higher compressive strength. Compressive strength value depends on the pore structure of the hardened superplastizer paste, which consists mainly of micropores leading to a denser structure [46].
Mechanical property of geopolymer: unconfined compressive strength
In order to produce a geopolymer with a high compressive strength, source materials with a high reactivity are required [23]. The interaction among the source materials and between the source material and the gel phase should also be considered. Kaolinite, with a comparatively lower reactivity allows sufficient time for such interactions to occur and ultimately increases the extent of geopolymerization. Not only a significant improvement in the compressive strength, but also a substantial reduction in reaction time can be achieved when a calcined source material (Fly ash) is added to the geopolymerization of non-calcined materials (Kaolinite). A higher strength geopolymer is associated with a more desirable internal microstructure [17].
Factor affecting unconfined compressive strength
Curing temperature
The curing temperature is an important factor in the setting of the concrete [47]. Pozzolanic reactions are accelerated by temperature increase (Table 1) [30, 52, 53]. At ambient temperature, the reaction of fly ash is extremely slow [54]. Initial curing at elevated temperature catalyzes formation in appropriate system chemistry [55]. Brooks [47] reported that, for Type I cement and fly ash concrete, setting time was decreased by a factor of six when pozzolanic reactions were accelerated by temperature increase from 6 °C to 80 °C. Increase in the curing temperature in range of 30 °C to 90 °C increased the compressive strength [2, 52]. Curing at 70°C improved the strength compared to the curing at 30 °C for the same period of time. Curing at higher temperature, for more than a couple of hours, possibly affects the development of the compressive strength (Fig. 4 and 5) [4, 38].
Investigations of Kirschner et al. [56] demonstrated that, processing at ambient temperature was unfeasible due to a delayed beginning of setting. However this could be avoided by thermal treatment. He also reported that curing at 75 °C for 4 h completed a major part of geopolymerization process and resulted in satisfactory properties of the material. No secondary treatment is required thereafter.
Similarly, Swanepoel and Strydom [52] investigated utilization of fly ash and kaolinite clay in the geopolymeric material. The compressive strength after 7 and 28 days was highest (6 and 7 MPa respectively) for the sample heated at 60 °C for 48 h. Increase in temperature from 45 °C to 65 °C, the viscosity of the mix increased five times however increasing temperature from 65 °C to 85 °C increased the viscosity 10 times [57]. Viscosity indirectly indicates the geopolymer’s compressive strength.
Palomo et al. [42] observed mechanical strength of 60 MPa, after curing fly ash at 85°C for 5 h, and stated that temperature is especially important for 2 h to 5 h of curing (Fig. 4). Bakharev [58] stated that heat is beneficial for the strength development (strength compared to1 month of curing at elevated temperature can develop in only 24 h). Wang et al. [53] reported that CKD-Fly ash when cured at 24 °C, give lower strength (6.9 and 13.8 MPa at 28 and 56 days respectively). But the strength doubled at elevated temperature. This was not same for the OPC. On curing at elevated temperature, OPC exhibit expansion behavior and leads to cracking, resulting in loss of strength, a decreased service life or other durability problem [59]. K-PS and K-PSS products gave higher mechanical strength of about 32.5 MPa at 25 °C and 45 °C. The mechanical strength obtained with added NaOH at this temperature, can be used for manufacturing of preformed building blocks [60].
It can be concluded that curing at elevated temperature is effective (in the rage of 30 °C to 90 °C) and has more significant contribution to geopolymeric reactions.
Curing time
Table 1 reveals that prolonged curing time improve the polymerization process resulting in higher compressive strength. However, increase in strength for curing periods beyond 48 h. was not very significant [2, 42, 52, 61]. Geopolymers developed compressive strength of 45 MPa in just 24 h. [62]. Puertas et al. [54] observed that the compressive strength for one day was higher, when the curing was carried out at 65 °C, and at rest of the age, paste cured at 25 °C developed higher compressive strength than those treated at 65 °C. The increasing temperature favors the dissolution of reactive species mainly, that of the slag and fly ash, in the same degree. Compressive strength decreased on curing at higher temperature for longer period of time, as prolonged curing at elevated temperature, breaks the granular structure of geopolymer mixture. This results in dehydration and excessive shrinkage due to contraction of gel, without transforming to a more semi-crystalline form. Crystalline part of geopolymer does not get affected by longer curing time. This indicates that, the change responsible for the difference in the strength originates within the amorphous phase of the structure [13].
Silicate and hydroxide ratio
The ratio of sodium silicate to sodium hydroxide plays an important role in the compressive strength development (Table 1). M2O/SiO2 ratio shows a positive effect on compressive strength. By the increase in concentration of alkali M2O (M represents Na/K/metallic ions) or decrease in added silicate SiO2, increase in compressive strength is expected [2, 23, 36]. This is because excess sodium silicate hinders water evaporation and structure formation [63]. The matrix activated with potassium silicate/KOH obtained the greatest compressive strength while sodium silicate/NaOH activated matrixes were generally weaker followed by potassium silicate/NaOH. Since K+ is more basic it allows higher rate of solubilized polymeric ionization and dissolution and leading to dense polycondensation reaction that provides greater overall network formation and an increase in the compressive strength of the matrix. Large size K may favor a greater degree of polycondensation [21, 60]. If SiO2/M2O in the sodium silicate solution is equal to or higher than 0.8, the low temperature reaction yields an amorphous alumino-silicate or “inorganic polymer glass” whereas for smaller values the sodium silicates are partially crystalline [64]. In the production of inorganic polymer, the amount of OH- ion in the alkaline solution contributes towards the dissolution step of Si4+ and Al3+ from fly ash, whilst the Na+ ion contributes to the crystallization of zeolites P.
Alkali concentration
Alkali concentration is the most significant factor for geopolymerization (Table 1) [54]. The solubility of aluminosilicate increased with increasing hydroxide ion concentration [65]. Higher concentration of NaOH yielded high compressive strength [2]. 10 N KOH showed the highest strength of 60 MPa, but the strength decreased on increasing the KOH concentration from 10 N to 15 N, probably due to excess K+ ions in the framework [63].
K2O/Na2O content plays an important role, with increasing alkali concentration, setting time increases and the strength and fire resistance characteristics can also be improved [63]. KOH leached substantially more Si and Al as compared to NaOH [18, 24]. Addition of K2O was found to benefit the compressive strength and also to reduce the occurrence of cracking [23]. High NaOH addition accelerated chemical dissolution but depressed ettringite and CH formation during the binder hydration [53]. Reduction in the CH content resulted in superior strength and durability performance [66]. An excess of OH− concentration in the system decreased the strength of the system [42]. Higher the alkalinity of the hydration water, slower the rate of the hydration [61]. Wang et al. [53] performed study using fly ash and CKD with 2% and 5% NaOH. They reported that, addition of 5% NaOH tends to increase the strength of the binder at early age (below 7 days) but the strength decreased at later age, may be that the excessive NaOH that resulted in undesirable morphology and non-uniformity of hydration products in the pastes, thereby reducing the binder strength. Kaps and Buchwald [44] reported that measurable strength could not be built below NaOH content of 15% whereas above 25% there was no improvement in the strength. Querol et al. [67] in the study of alkali activated ferro-aluminous fly ash reported that no reaction occurred on activating fly ash using distilled water even at high activation temperature of 150 °C because of the low content of free calcium oxide in the original fly ash and low total basicity (final pH = 6.8) which was inadequate for the formation of zeolitic phases.
Delay in the polymer formation occurred as activator concentration was increased. The ionic species concentration also increased, limiting the ion’s mobility and delaying the formation of coagulated structures [12]. When the alkali hydroxide concentration was increased from 5 M to 10 M, an amorphous alkaline alumino silicate (geopolymer) was formed as the dominant product, with a small amount of CSH gel [27, 68]. If enough calcium was added to the a geopolymeric system, some form of CSH gel would be obtained. However it is still unclear whether calcium participates in geopolymerization in a similar way to sodium or potassium [27]. Similarly, when activation of metakaolin was carried out with highly concentrated alkaline solution in the presence of calcium hydroxide, the main reaction product was aluminosilicate. Additionally the formation of CSH gel was also observed as a secondary product [12]. It was assumed that the CSH gel fills the voids and pores within the geopolymeric binder. This helps to bridge the gaps between the different hydrated phases and unreacted particles, thereby resulting in the increased mechanical strength [68]. Higher Na proportion in the solution would favor the formation of the CSH gel [17]. As the amount of sodium hydroxide increase in the system, less calcium would be available to react with the silicate and aluminate and calcium will precipitate out as calcium hydroxide with the formation of CSH gel. The CSH gel in such system will have significantly lower Ca/Si ratio than the CSH formed from hydration of ordinary Portland cement [27]. Yip et al. [27] in their study also reported that sodium concentration in the geopolymeric gel was much higher than that in calcium rich area (CSH gel). This indicates that, sodium added had a more predominant structurally determining role in the alumino-silicate gel than in the case of CSH gel. This further suggests that sodium played a charge-balancing role in the geopolymeric gel while it was not required in formation of CSH gel.
pH
The most significant factor controlling the compressive strength is pH. The setting time of cement decreased as the pH of the activating solution increased [69]. At lower pH values the geopolymeric mix remained viscous and behaves like cement while at higher pH, the mix attained a more fluid gel composition, which was less viscous and is more workable [21]. Strength at pH 14 was 50 times larger than those at pH 12(less than 10 MPa at pH 12, 50 MPa at pH 14) of geopolymeric matrix utilizing cement as setting additive. Higher solubility of monomers was expected by KOH than NaOH because of higher alkalinity (Fig. 6). With increasing pH there was a predominance of smaller chain oligomers and monomeric silicate available to react with soluble aluminum. Further with increase in pH soluble aluminum increases and reacts with calcium available for reaction. [21, 22, 29]. Lower pH-value of the solution leads to lower monomer concentration. Figure 6 reveals the pH-value of the single alkaline solution, varying in concentration and kind of alkali ions [44].
From the above observations it is clear that pH range 13–14 is most suitable for the formation of the geopolymers with better mechanical strength.
Silicate and aluminum ratio
A high soluble silicate dosage is necessary for synthesizing alumino-silicate gel that provides good interparticle bonding and physical strength of geopolymers (Table 1) [25]. High reactive silica content involved the formation of high amount of alkali alumino-silicate gel and consequently a high mechanical strength was developed in the resulting material [35].
Geopolymers with SiO2/Al2O3 in the range of 3.16–3.46 had better UCS which decreased with increasing ratio upto 3.86 [63]. Results of Bakharev [58] indicated that utilization of long precuring before heat treatment allowed to narrow the range of Si/Al ratios in the alumino-silicate gel (Si/Al = 1–4 for 2 h precuring) compared to (Si/Al = 1.8–3.6 for 24 h precuring). He also reported that an increase in curing temperature caused reduction in Si/Al ratios (Si/Al = 1.8–3.6 for 75 °C compared to Si/Al = 1.6–2.8 for 95 °C). Higher sodium silicate concentration was found to be beneficial to the geopolymerization of the Metakaolin/sand mixture [24].
Aluminum source
Geopolymer containing clays (Kaolin and metakaolin) were found to be the strongest under compressive strength testing while fly ash alone lacked considerable strength alone [19] (Table 2). Strength increased with the addition of Zirconia (3%)suggested that zirconia could efficiently be consolidated into the matrix and above 5% caused a reduction in the strength of matrix [22]. A small amount of clay content gets fully digested to take part in geopolymerization reaction where as at larger addition, clay becomes partially reactive filler and serves to weaken the structure [13]. Replacement by metakaolin induced a decrease in the mechanical strength during the first day but an equal resistance after 28 days. More than 15% replacement induced a decreased binding and compressive strength [24]. As the secondary Al–Si source, metakaolin enhanced the geopolymerization of fly ash. This was explained by the fact that metakaolin tends to dissolve a large extent than fly ash [24]. Due to the fineness of metakaolin grain, the consistency of mix design decreases. Five percent metakaolin induces a decrease of 14.6% in consistency while 15% induces decrease 34 % consistency. Hence, the optimum reactivity seems to be between 10% and 15% regarding the low workability, best mechanical performance and inhibition effect on the chloride diffusion and sulfate attack. No positive or negative effect of kaolin was observed; it may be due to the mineralogical characteristics of the material and lack of thermal treatment of the alumino-silicate in the base material [70]. Matrix with calcium source (cement) showed the highest compressive strength 50 MPa as compared to metakaolin and slag [21].
Liquid/solid ratio
Strength decreases as the ratio of water-to-geopolymer solid by mass increases. This trend is analogous to water-to-cement ratio in the compressive strength in OPC. Although chemical processes involved in the formation of binders of both are entirely different (Table 1) [2, 18, 30, 36, 71, 72]. The minimum water to cement ratio is approximately 0.4 by weight for Portland cement [40], whereas the fresh geopolymeric material is readily workable even at low liquid/solid ratio [73] (Fig. 7). Presence of excess amount of water is an important factor inducing crystallization in M2O–Al2O3–SiO2–H2O and M2O–CaO–Al2O3–SiO2–H2O [58].
Calcination
Calcined source material such as fly ash, slag and metakaolin display a higher reactivity during geopolymerization than non-calcined material. Calcination activates material by changing their crystalline structure into amorphous structure to store extra energy and increase their activity [23] and increasing compressive strength [36]. The burning or calcining temperature of the clay affects the pozzolanic reactive state when the calcining leads to loss of hydroxyls and results in a collapsed and disarranged clay structure. The production of the active state is usually in the range of 600–800 °C [33]. Calcination also affects the amount of Al and Si released from source material (Fig. 8). Metakaolin (calcined form) persists until the material is heated upto the temperature of about 950 °C [74]. CaO content increases upon calcination. High CaO content decreases the microstructural porosity and in turn strengthen the geopolymer by forming amorphous structure Ca–Al–Si gel during geopolymerization [14, 18, 23, 53]. This is also supported by the investigation [27] of ground granulated blast furnace slag which explains that calcium containing compounds such as calcium silicates, calcium aluminate hydrates, and calcium-silico-aluminates are formed during geopolymerization of fly ash, that affects the setting and workability of the mix [18]. Higher the temperature used during the calcinations process, shorter the time needed to obtain metakaolin that gives the maximum compressive strength [13]. The geopolymers manufactured from calcined material were found to have higher early strength, while those formed from non-calcined materials possessed higher increase in strength during the later stages of curing [23]. Jaarsveld et al. [14] reported anomalous result; greater strength was obtained for the geopolymers containing kaolin.
The heating of kaolinite at high temperature (750 °C) for 24 h produces the complete hydroxylation of the sample. The thermal pre-activation of the kaolinite samples has a great influence on the nature of the intermediate phases, but less in that of the final zeolite synthesized with the same Si/Al ratio. During the synthesis of the zeolites from alumino-silicates, the first formed zeolites are often metastable thermodynamically and are subsequently replaced by other more stable one [75]. The double-layered alumino-silicate material can be transformed to the sol-gel system only after complete dehydroxylation [76].
Relative humidity/curing conditions
Experiment showed that samples cured at higher humidity (in sealed bags) do not improve strength. This behavior is in contrast with what expected from the curing of cementitious products. Cement gains strength when cured under higher humidity. This behavior is also proved by the IR absorption peaks around 1033 cm−1 corresponding to the asymmetric stretching of Si–O and Al–O bonds which were affected by curing of samples in sealed bags and their wave numbers were slightly lower than for the samples cured without bags. Lower wave number is an indicative of weaker inter-tetrahedral bonding and could contribute to the lower strength for the samples cured in the sealed bags. Saturated atmosphere in the bags, results in conditions more suitable to the formation of the slightly weaker bonds [13].
Age of concrete
Geopolymer gains about 70% of its strength in first 3–4 h of the curing (Table 1) [8, 62, 77]. Strength of concrete does not vary with the age of concrete when cured for 24 h, which is in contrast to well-known behavior of ordinary Portland cement, which undergo hydration process and gains strength overtime [2].
Engineering properties of geopolymers
Wallah et al. [78] performed creep and drying shrinkage tests to evaluate the long-term performance and durability of geopolymer concrete. Results indicated that geopolymer concrete undergoes low creep and very little drying shrinkage. Test results of Hardjito et al. [30] showed that the drying shrinkage strain of fly ash based geopolymer concretes were found to be insignificant. The ratio of creep factor (strain-to-elastic stain) reached a value of 0.30 in approximately 6 weeks. Beyond this time, the creep factor increased marginally.
Hardjito et al. [79] studied the stress and strain behavior of fly ash based geopolymers and compared it with the portland cement. They reported that the Young’s modulus, Poisson’s ratio, Tensile strength of fly ash based geopolymer concretes was of same characteristics possessed by portland cement concrete. The measured stress-strain relations of geopolymer concrete also fit well with equations developed originally for portland cement concrete.
It was observed that the geopolymer concrete, after 12 to 24 weeks of sulfate exposure, showed no significant effect. Results indicated that changes in the compressive strength generally fall within + 1.0 of standard deviation of the mean compressive strength value and therefore, do not appear to be significant. The variation of length change, as a result of sulfate exposure after 24 weeks was found to be extremely small and generally less than 0.02%. The expansion of 0.5% of the original length is considered as failure of the concrete due to sulfate attack [78]. Ramlochan et al. [59] investigated that pozzolans, which were source of additional Al2O3, were effective in reducing or eliminating long-term expansion. Metakaolin based geopolymers mortars remained stable and showed negligible deterioration in the microstructure and strength after being soaked in seawater, sodium sulfate solution and sulfuric acid solution [31].
Davitovits [80] performed the acid resistance test with 5% of HCl and H2SO4 on the geopolymers and compared it with the traditional Portland cement matrix and some other binders. The portland cement and blended cement destroyed in the acidic environment (Fig. 9). Hardjito et al. [30] performed tests on the resistance of fly ash based geopolymer concrete and reported that there was no significant change in the compressive strength, the mass and the length of the geopolymer specimen. Bai et al. [81] have demonstrated relation between strength and sorptivity. Strength varied in a linear manner with sorptivity. In order to reduce the ingress of chloride-containing or sulfate-containing waste into concrete, minimization of sorptivity was found to be important.
Cheng and Chin [63] reported the fire resistance property of geopolymers. When a 10 mm thick panel of geopolymer is exposed to 1100 °C flame; the measured reverse-side temperature reached 240–283 °C after 35 min. Authors also observed that the fire characteristics could be improved by increasing the KOH or the alkali concentration and amount of metakaolin. It was concluded that geopolymers could be fabricated for construction purpose and have great potential for engineering application.
Introduction of ultrasonication into the geopolymerization system increased the compressive strength of geopolymers and strength increased with an increase of ultrasonication upto a certain time. However, a prolonged ultrasonication does not increase strength of the formed geopolymer, due to the polycondensation and hardening occurring simultaneously. Ultrasonication enhances the dissolution of Al–Si source material to release more Al and Si into the gel phases, thereby improving the extent of geopolymerization [24].
Geopolymers show high microbial stability. The surfaces of the geopolymers remained unaffected after 4 weeks in the solution of microbes. No noticeable change occurred to compressive strength and leachability [5].
Addition of salts affects the geopolymeric structures. Phosphate salts are the most effective gel solidification retarders, followed by chloride, oxalate, and than carbonate. Carbonate was found to accelerate Si dissolution initially and at the same time lowered the solubility of Ca. Oxalate and phosphate were effective in accelerating and increasing the extent of apparent Si dissolution. Chloride and oxalate may also induce crystallization within the geopolymers. It was concluded that the solubility of the calcium in the system could be due to the fact that both anions, C2O 2−4 and HPO 2−4 have strong affinity for calcium [25, 26].
Heavy metal inclusion does not influence the basic tetrahedral bonding blocks of the structure but it influences the structure in physical manner such as to alter the compressive strength and specific area. Inclusion of Pb served to strengthen the structure (Strength) where as Cu did not. Pb resulted in higher surface area than Cu. Although Pb ions influenced the structure in terms of causing increased porosity this effect was offset by its contribution to structural strength [20].
Micro-structural characterization
Microstructure of the alkali activated fly ash changes with the chemical composition [82]. After geopolymerization, all the main characteristic peaks of Al–Si minerals still remained, but decreased slightly. This suggested that Al–Si mineral did not dissolve totally into the gel phase. However there were no new peaks, which means that no new major crystalline phases were formed [9, 17]. The baseline broadened between 20° and 40° 2θ was an indicative of an increased amorphicity [13]. Palomo et al. [73] studied a series of fly ash samples activated under different experimental conditions and concluded that geopolymers are a family of materials with same basic chemical composition but potentially different microstructures.
IR absorption spectroscopy seems to be suitable tool to characterize geopolymeric material. The main feature of IR spectra was the central peak between 1010 cm−1 and 1040 cm−1 which is attributed to the Si–O–Si or Al–O–Si asymmetric stretching mode [20]. IR spectrum is characterized by the shift of the bending band of the Si–O bond (1050 cm−1) from metakaolin to lower frequency (990 cm –1) and a decrease of the 800 cm−1 band, with the formation of a new band at about 720 cm−1 which was reported as characteristic of the polymer formed [83]. IR spectra of alkaline alumino-silicate had a band associated to ν3 (SiO) at 997 cm−1. Other characteristics bands of the inorganic polymer were placed at 691 and 426 cm−1. Asymmetric stretching of Si–O of glassy silica shifts to lower frequencies, when substitution of Si by Al takes place. Absorption bands at 1207 and 1172 cm−1 were associated with the partial substitution of Si by Al in the gel structure [64].
The study of geopolymers using X-ray diffraction is difficult because of the fact, a large part of the structure is amorphous content between 20° and 40° 2θ. The degree of disorder in geopolymers can be inferred by the way it diffracts X-ray to form a diffraction pattern. In non-crystalline state, diffraction of X-ray results in a broad diffuse halo rather than sharp diffraction peaks [8, 28]. Peaks were of quartz, mullite and hematite of the crystalline component of the fly ash. Broad peak in region 20–30° 2θ arised from glassy phase of fly ash and peaks in the region 6–10°and 16° 2θ arised from alumino-silicate gel. Considerable amount of zeolites were found in cement-fly ash system blend, activated by highly alkaline multi-compound activator, around pH 14 and cured at 70 °C [58]. Querol et al. [67], Brougth [84] identified Phillipsite [KCa(Si5Al3)O166H2O], merlinoite [K5Ca2(Al9Si23O64).24H2O], analcime [Na(Al Si2O6). H2O] and Na–P zeolite (Na6Al6Si10O32 · 12H2O) by XRD after alkaline hydrothermal activation. Other reaction products such as calcite [(Ca(OH)2], bayerite [Al(OH)3] and nosean (Na8Al6Si6O24SO4), hydroxysodalite (Na6(Si6Al6O24) · 8H2O) ( a low silica zeolite) were also obtained [28]. Through XRD analysis in alkali activated system, phases identified were hydrotalcite (Mg6Al2CO3(OH)16 · 4H2O), calcite (CaCO3) and semi-crystalline calcium silicate hydrate [85]. Bakharev [58] reported that zeolitic phases chabazite and Na–P1 (gismondine) coexists with C–S–H in fly ash–NaOH and fly ash–NaOH– cement system [34]. Formation of zeolite and related phases in cement system is of considerable interest because significant quantity of water can be immobilized in the zeolite pores, and the zeolites can absorb significant amount of cation [84]. The technique of 27Al and 29Si MAS-NMR can be used for the interpreting the microstructure of geopolymer synthesized from different mixture [17]. Peak at 55 ppm in the aluminum spectrum indicated the presence of a three-dimensional spectrum of aluminosilicate polymeric units, including the presence of low molecular weight polymeric units such as dimmers or trimmers [64, 83]. Puertas et al. [85] reported that, presence of Q 3 unit in the activated paste with waterglass indicates tetrahedra of silicates forming cross-linked type structure. The analysis of Q 2(1Al) and Q 3(1Al) intensity confirmed that an important part of Al was implicated in the formation of this cross linked structure that could connect the neighboring structural blocks. Jimenez et al. [35, 57] reported that the MASNMR spectra of 24 h and one week were similar. Five components were displayed at −88.8, −93.2, −98.2, −103.5, −109.1 ppm, which corresponds to the formation of tectosilicate and peak at −87 ppm, correspond to the non-dissolved mullite. The 27Al MASNMR spectroscopy showed that the main 27Al signal located at + 61.4 ppm indicates that the aluminum is tetrahedral coordinated and peaks at −87.3 and −91.9 indicates the presence of Si(4Al) and Si(3Al) sites[20].
Immobilization of toxic metals by geopolymer
Presently, toxic and radioactive metals are stabilized with conventional Portland cement but the cost figures are not tolerable [16, 18]. Large amount of fly ash with a small amount of additive and activators can be utilized for the solidification/stabilization of heavy metals. About 90 % of the heavy metals get locked into the geopolymeric matrix (Fig. 10) [34].
Heavy metal immobilization may occur through a combination of physical encapsulation and chemical bonding into the amorphous phase of the geopolymeric matrix. Metal cations can be incorporated into geopolymeric network, potentially produces leach rate far superior to those of OPC based system [43]. Very few literature is present on the immobilization of the metals by geopolymers. Further research is required on the application of the geopolymer for metal immobilization.
Geopolymerization is an emerging technology in the field of hazardous metals immobilization. Davitovits [80] reported that the geopolymeric matrix was very effective in immobilizing uranium waste and nuclear waste. The toxic metals were locked into the three dimensional geopolymeric-zeolitic framework (Fig.10). Geopolymeric matrices greatly minimize the leaching of iron, cobalt, cadmium, nickel, zinc, lead, arsenic, radium and uranium. Metals included in each structure did not seem to make any difference to the crystalline part of the spectra and it was therefore assumed that the metals bound itself into the amorphous part of the matrix. Retention of the metal in the matrix was directly correlated with the liberation of Si and Al from fly ash based geopolymers [20]. NaOH was determined to be the most effective activator while sodium silicate determined was the least effective. All matrices were generally found to be highly efficient in retaining Pb within the matrix with the order of effectiveness: Fly ash > Kaolinite > K-feldspar > metakaoline [19]. The environment and the coordination number of aluminum source material have an effect on the ultimate immobilization efficiency of the geopolymeric matrix. It was observed that geopolymeric matrix synthesized from a six coordinated aluminum source (kaolinite) was more stable under leaching conditions than four-coordinated aluminum source (metakaolin). From was assumed that some chemical bonding of the metals occurred within the matrix [14]. Zeolitic phases showed excellent uptake characteristics for Pb2+. In presence of NaOH, sorption efficiency was considerably reduced, probably due to the changing speciation of Pb in response to pH. At near neutral pH Pb is largely in cationic form (Pb4(OH)4 [86]. There is a relative tendency that the matrix with the best immobilization efficiency has the smaller pore opening as well as the highest compressive strength, hence it was assumed that the immobilization take along physical encapsulation [14, 19].
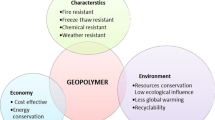
Properties and application of geopolymers
Broad properties and applications of geopolymers can be listed as follows
-
(1)
Geopolymers possess excellent mechanical strength due to high degree of Polycondensation.
-
(2)
Long-term durability: Geopolymer concrete or mortars withdraw thousands of years weathering attack without much function loss.
-
(3)
Unique high temperature properties.
-
(4)
Easily recycled, adjustable coefficient of thermal expansion.
-
(5)
Hazardous waste disposal binder for the heavy metal fixation especially for nuclear waste solidification.
-
(6)
Fire resistant: Geopolymer can withstand 1000 °C to 1200 °C without losing function.
-
(7)
It is also known as “Green material” for its low energy consumption and low waste gas emission during manufacture. Thermal processing of natural alumino-silicates at relative low temperature provides suitable geopolymeric raw material, resulting in 3/5 less energy assumption than Portland cement. In addition less CO2 is emitted [79].
-
(8)
Fast setting: Geopolymer obtain 70% of the final compressive strength in the first 4 h of setting.
-
(9)
Geopolymers are used as construction material.
Conclusions
Geopolymerization is an emerging technology for utilization of by-products like fly ash, slag, and kiln dust and also for the immobilization of toxic metal in the waste. It provides a mature and cost-effective solution to many problems where hazardous residues must be treated and stored under critical environmental conditions. Geopolymer based materials are environmentally friendly and need only moderate energy to produce. CO2 emission is reduced about 80% compared to that of ordinary Portland cement.
Any pozzolanic compound or source of silica and alumina that is readily dissolved in the alkaline solution can be used as a source of geopolymer precursor species and undergoes geopolymerization. A polymeric structure of Al–O–Si formed during geopolymerization constitutes the main building block of geopolymeric structure.
The rate of polymer formation is influenced by many parameters. Pozzolanic reactions are accelerated by curing temperature, water content, fly ash/kaolinite ratio, alkali concentration, initial solids content, silicate and aluminate ratio, pH and the type of activators used has substantial effects on the final properties of geopolymers. Certain synthesis limits existed for the formation of strong products. Compositions lay in the range M2O/SiO2, 0.2 to 0.48; SiO2/Al2O3, 3.3 to 4.5; H2O/M2O, 10–25; and M2O/Al2O3, 0.8 to 1.6 but the ratio changes while working with the waste. Research on geopolymers conforms that curing temperature and curing time significantly influence the compressive strength.
Curing temperature is an important factor in the setting of the geopolymer but curing at higher temperature for more than a couple of hours seem to possibly affect the development of compressive strength. Increase in strength for curing periods beyond 48 h was not very significant. This behavior is in contrast with the behavior of OPC. Strength decreases as the ratio of water-to-geopolymer solid by mass increases; this trend is analogous to water-to-cement ratio in the compressive strength in OPC. Fresh geopolymeric material is readily workable even at a very low liquid/solid ratio i.e. below 0.4 The sample strength is strongly dependent on both the Si:Al and Na:Al ratios of the material. High reactive silica content involves the formation of high amount of alkali alumino-silicate gel and consequently a high mechanical strength is developed in the resulting material.
pH range in 13–14 is the most suitable for the formation of the geopolymers with good mechanical strength. Alkali concentration in the range of 5–10 N plays an important role in the formation of geopolymer.
Geopolymer concrete undergo low creep and very little drying shrinkage. Young’s modulus, Poisson’s ratio and Tensile strength of fly ash based geopolymers concretes possess the characteristics similar to Portland cement concrete.
Geopolymer serves as a better alternative to OPC for immobilizing toxic metals. About 90% of the heavy metals get locked into the geopolymeric matrix.
To increase the cost effectiveness of geopolymeric binders the aluminum source like kaolin has to be replaced by some cost economic materials such as slag, CKD, builder’s waste or little amount of cement that will reduce the cost of the geopolymer as well as will strengthen the matrix. It can be concluded from the study that geopolymers are “Green materials” that gains 70% of its strength in first 3–4 h and immobilizes 90% of the toxic metals within the matrices.
Future study in the field of the application of geopolymer is required for its commercial uses.
References
Woolard CD, Petrus K, Van Der Horst M (2000) ISSN 0378-4738-Water SA 26:531
Hardjito D, Wallah SE, Sumajouw DMJ, Rangan BV (2004) ACI Mater J 101:1
Baldwin G, P.E. Rushbrook, Dent CG (1982) The testing of hazardous waste to assess their suitability for landfill disposal. Harwell report, AERE-R10737, November 1982
Wiles CC (1988) Standard handbook of hazardous waste treatment and disposal. McGraw Hills, New York, p 7.85
Hermann E, Kunze C, Gatzweiler R, Kiebig G, Davitovits J (1999) In: Proceedings of Geopolymers, p 211
Buchwald A, Schulz M (2005) Cement Concrete Res 35:968
Li Z, Ding Z, Zhang Y (2004) In: International workshop on sustainable development and concrete technology. Beijing, p 55
Davitovits J (1991) J Therm Anal 37:1633
Xu H, Van Deventer JSJ (2000) Int J Miner Process 59:247
Xiong CJ, Ban CH, Pei X, Fang Z (2004) In: International workshop on sustainable development and concrete technology. Beijing, p 299
Hos JP, Mccormick PG (2002) J Mater Sci 37:2311
Alonso S, Palomo A (2001) Mater Lett 47:55
Van Jaarsveld JGS, Van Deventer JSJ, Lukey GC (2002) Chem Eng J 89:63
Van Jaarsveld JSG, Van Deventer JSJ, Lorenzen L (1998) Metal Mater Trans B 29:283
Davidovits J, Sawyer JL (1985) US Patent, No. 4509985
Van Jaarsveld JGS, Van Deventer JSJ, Lorenzen L (1997) Miner Eng 10:659
Xu H, Van Deventer JSJ, (2002) Cement Concrete Res 32:1705
Van Jaarsveld JGS, Van Deventer JSJ, Lukey GC (2003) Mater Lett 57:1272
Phair JW, Van Deventer JSJ, Smith JD (2004) Appl Sci 19:432
Van Jaarsveld JGS, Van Deventer JSJ, Schwartzman A (1999) Miner Eng 12:75
Phair JW, Van Deventer JSJ (2001) Miner Eng 14:289
Phair JW, Van Deventer JSJ, Smith JD (2000) Eng Chem Res 39:2925
Xu H, Van Deventer JSJ (2002) Miner Eng 15:1131
Feng D, Tan H, Van Deventer JSJ (2004) J Mater Sci 39:571
Lee WKW, Van Deventer JSJ (2002) Ind Eng Chem Res 41:4550
Phair JW, Smith JD, Van Deventer JSJ (2003) Mater Lett 57:4356
Yip CK, Van Deventer JSJ (2003) J Mater Sci 38:3851
Rees C, Lukey GC, Van Deventer JSJ (2004) In: International symposium of research students on material science and engineering. December 2004, IIT Chennai India
Duxson P, Lukey GC, Van Deventer JSJ (2005) Ind Eng Chem Res 44:832
Hardjito D, Wallah SE, D.M.J Sumajouw, Rangan BV (2003) In: George Hoff Symposium, ACI, Las Vegas USA
Palomo A, Glasser FP (1992) Brit Ceram Trans J 91:107
Davitovits J, Davitovits M, Davitovits N (1994) US Patent, No. 5,342,595
Sabir BB, Wild S, Bai J (2001) Cement Concrete Res 23:441
Jimenez AF, Palomo A (2003) Fuel 82:2259
Terzano R, Spagnuolo M, Medicu L, Vekemans B, Vincze L, Janssens K, Ruggiero P (2005) Environ Sci Technol 39:6280
Sumajouw DMJ, Hardjito D, Wallah SE, Rangan BV (2004) In: Green Processing 2004. The Australian Institute of Mining and Metallurgy, Fremantle, Western Australia, p 237
Hewlette PC (ed) (1998) In: Lea’s Chemistry of Cement and Concrete, 4th ed. Butterworth Heinmann, New Delhi, p 480
Papadakis VG (2000) Cement Concrete Res 30:1647
Moropoulou A, Cakmak A, Labropoulos KC, Van Grieken R, Torfs K (2004) Cement Concrete Res 34:1
Conner JR (1990) Chemical fixation and solidification of hazardous waste. Van Nostrand Reinhold, New York, p 335
Spencer RD (ed) (1993) In: Chemistry of cement solidified waste forms. Lewis publishers, New York, p 3
Palomo A, Grutzeck MW, Blanco MT (1999) Cement Concrete Res 29:1323
Gourley JT (2003) In: CRC for sustainable resource proceeding materials conference
Kaps CH, Buchwald A (2002) In: Geopolymer 2002, Melbourne, Australia
Wu Z, Naik TR (2004) In: ACI International spring Washington, DC 2004 centennial convention Report No. CBU-2004-06
El-Hosiny F (2002) Ceram-Silikaty 46:63
Brooks JJ (2002) ACI Mater J 99:591
Lange LC, Hills CD, Poole AB (1996) Waste Manage 16:757
Yang CGC, Chen SY (1994) J Hazard Mater 39:317
Mangialardi T, Paolini AE, Polettini A, Sirini P (1999) J Hazard Mater B 70:53
Lombardi F, Mangialardi T, Piga L, Sirini P (1998) Waste Manage 18:99
Swanepoel JC, Syrtdom CA (2002) Appl Geochem 17:1143
Wang K, Shah SP, Mishulovich A (2004) Cement Concrete Res 34:299
Puertas F, Martinez-Ramirez S, Alonso S, Vazquez T (2000) Cement Concrete Res 30:1625
Atkins M, Glasser FP, Jack JJ (1995) Waste Manage 15:127
Kirschner A, Harmuth H (2004) Ceram-Silikaty 48:117
Palomo A, Alonso S, Jimenez AF (2004) J Am Soc 87:1141
Bakharev T (2005) Cement Concerete Res 35:1224
Ramlochan T, Zacarias P, Thoas MDA, Hooton RD (2003) Cement Concrete Res 33:807
Cioffi R, Maffucci L, Santoro L (2003) Resour Conserv Recy 40:27
Martinez-Ramirez S, Palomo A (2001) Cement Concrete Res 31:1581
Palomo A, Lopez de la Fuente JI (2003) Cement Concrete Res 33:281
Cheng TW, Chin JP (2003) Miner Eng 16:205
Rahier H, Simons W, Van Mele B (1997) J Mater Sci 32:2237
Gasteiger HA, Frederik WJ, Streise RC (1992) Ind Eng Chem Res 31:1183
Poon CS, Azhar S, Anson M, Wong YL (2003) Cement Concrete Comp 25:83
Querol X, Alastuey A, Turiel JLF, Soler AL (1995) Fuel 74:1226
Yip CK, Lukey GC, Van Deventer JSJ (2005) Cement Concrete Res 35:1688
Roy A, Schilling PJ, Eaton HC. US Patent, No. 5435843
Courard L, Darimont A, Schouterden M, Ferauche F, Willem X, Degeimbre R (2003) Cement Concrete Res 33:1473
Zang S, Gong K, Lu J (2004) Mater Lett 53:1292
Hardjito D, Wallah SE, Sumajouw DMJ, Rangan BV (2004) In: Seventh CANMET/ACI international conference on recent advances in concrete technology. May 2004 Las Vegas USA
Palomo A, Jimenez AF, Criado M (2004) Mater Construct 54:77
Mohammadi T, Pak A (2003) Sep Purif Technol 30:241
Madani A, Aznar A, Sanz J, Serratosa JM (1990) J Phys Chem-US 94:760
Hanzlicek T, Vondrakova MS (2002) Ceram-Silikaty 46:97
Palomo A, Palacios A (2003) Cement Concrete Res 33:289
Wallah SE, Hardjito D, Sumajouw DMJ, Rangan BV (2004) In: International conference on fiber composites, high-performance concretes and smart material. ICFRC, Chennai
Hardjito D, Wallah SE, Sumajouw DMJ, Rangan BV (2004) In: 18th Australasian conference on the mechanics of structures and materials (ACMSM)1-3. Perth Australia
Davitovits J (1994) Concrete Int 16:53
Bai J, Wild S, Sabir BB (2002) Cement Concrete Res 32:1813
Rowles M, Conner B (2003) J Mater Chem 13:1161
Alonso S, Palomo A (2001) Cement Concrete Res 31:25
Brough AR, Katz A, Sun GK, Struble LJ, Kirkpatrick RJ, Young JF (2001) Cement Concrete Res 31:1437
Puertas F, Jimenez AF, Blanco-Varela MT (2004) Cement Concrete Res 34:139
La Lglesia A, Gonzalez MV, Dufour J (2002) Environ Prog 21:105
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khale, D., Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: a review. J Mater Sci 42, 729–746 (2007). https://doi.org/10.1007/s10853-006-0401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0401-4