Abstract
Water-insoluble β-cyclodextrin polymer (β-CDP) crosslinked by citric acid was obtained with a yield of 65% through an environment friendly synthesis procedure. FT-IR spectra disclosed that the hydroxyl groups of β-CD had reacted and condensated with the carboxyl groups of citric acid, and at the same time the structural characteristics of β-CD were essentially maintained in β-CDP. The β-CDP exhibited notable adsorption capability toward phenol (q max = 13.8 mg g−1) and especially large adsorption capability toward methylene blue (q max = 105 mg g−1). The concentration of methylene blue in water could be reduced to 0.11 mg L−1 by the β-CDP, indicating the excellent adsorption sensitivity of β-CDP toward methylene blue. The adsorption results disclosed that the interior cavity and inclusion property of β-CD were maintained in the synthesized β-CDP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are torus-shaped cyclic oligosaccharides containing six to twelve glucose units [1–7]. The individual glucose units are held in a C-1 chair conformation and they are joined together by α-1,4 glycosidic linkages to form a cyclic structure. The interior cavity of CDs is relatively hydrophobic and the most characteristic feature of CDs is the ability to form inclusion complexes through host-guest interactions. The practically important, industrially produced CDs are α-, β- and γ-CDs, which are made up of six, seven and eight α-1,4-linked d-glucopyranose units respectively. β-CD is the most largely produced cyclodextrin and has been widely used in many fields including pharmaceuticals, foods, cosmetics, chemical products and technologies. The chemical structure of β-CD is as shown in Fig. 1.
Water-insoluble β-Cyclodextrin Polymer (β-CDP) is a new type of adsorbent useful for removal of organic pollutants and heavy metals in water [1, 8–24]. The general method for synthesis of water-insoluble β-CDP is by crosslinking the hydroxyl groups of β-CDs with bi- or multi-functional molecules to form a stable crosslinked network. The bi- or multi-functional molecules used are normally called crosslinking agents. Epichlorohydrin [11–15], diisocyanates [16–18], polycarboxylic acids [19–22] and anhydrides [23, 24] have been reported to be effective crosslinking agents. Epichlorohydrin is widely used in chemistry and industry because of its high reaction activity, and it is the most popularly used crosslinking reagent for β-CDP synthesis. But after all epichlorohydrin is a toxic and hazardous substance and is potentially harmful to human health and environment. At the same time, a large portion of epichlorohydrin might be wasted in the reaction, because the reaction between β-CD and epichlorohydrin required a high concentration of sodium hydroxide which will accelerate the hydrolyzation of epichlorohydrin. Diisocyanates, such as toluene diisocyanate and hexamethylene diisocyanate, are also hazardous to human and environment, and organic solvents are required in the reaction between β-CD and diisocyanates. Comparatively, polycarboxylic acids, such as citric acid, butanetetracarboxylic and polyacrylic acid, are generally low-toxic and more friendly to environment. The condensation between β-CD and polycarboxylic acids can be performed at a temperature not higher than 200 °C and without any organic solvent and harmful additive, although the reaction activities of polycarboxylic acids are generally lower than epichlorohydrin or diisocyanates.
Martel et al. has reported the synthesis and applications of β-CDP immobilized on natural or synthetic fabrics using polycarboxylic acid as crosslinking agent and sodium dihydrogen hypophosphite as catalyst [19–21]. The β-CDP immobilized on the fabrics has the ability to adsorb heavy metal ions (Pb2+, Cd2+ and Ni2+) from water because the carboxyl groups contained in β-CDP can serve as ion-exchange sites. β-CDP immobilized on the fabrics can also increase the period of release of perfumes and improve the resistance of the odor to washings with water.
In our research, water-insoluble β-CDP was synthesized using citric acid as crosslinking agent, sodium dihydrogen phosphate as catalyst and PVA-1799 as additive. The mechanism of synthesis is illustrated in Fig. 2. The β-CDP after purification was characterized by Fourier-transform infrared spectroscopy (FT-IR) and applied to adsorption experiments toward phenol and methylene blue (MB) respectively. The FT-IR spectra were studied to disclose the chemical characteristics of β-CDP. The influences of concentration, contact time and sorbent amount on the adsorption properties of β-CDP were evaluated and discussed.
Experimental
Materials
β-Cyclodextrin is a biochemical reagent (puritiy ≥ 99.0%) purchased from Tianjin Bodi Chemical Co., Ltd., China. Citric acid monohydrate, sodium dihydrogen phosphate, phenol and methylene blue, are analytical pure reagents purchased from guaranteed manufacturers in China. PVA-1799 is a commercial poly (vinyl alcohol), of which the polymerization degree is 1,700 and the degree of hydrolysis is 99.0%, purchased from Sichuan Vinylon Factory, China. Potassium bromide (KBr) used for FT-IR is a spectrum pure reagent purchased from Tianjin Guangfu Fine Chemical Research Institute, China.
Synthesis of β-CDP
About 10 g of β-CD, 5 g of citric acid monohydrate, 0.5 g of sodium dihydrogen phosphate, 1 g of PVA-1799 and 50 ml of deionized water were mixed in a round bottom flask and stirred to homogeneous in boiling water bath. The mixture was transferred into a culture dish and heated in an electric thermostatic oven (DHG-9030A, Shanghai Jinghong Laboratory Instrument Co., Ltd, China) at 140 °C for 4 h. Because the culture dish was wide open, the water was quickly driven away by heating. Then polymerization started, and the water generated during the reaction was instantly driven away, so that the equilibrium was pushed forward successively. After naturally cooling, the crude product was weighed (recorded as W c) and then grinded into fine granulae. The granulae were purified by soaking and washing with deionized water for several times, then suction filtered and dried at 50 °C to constant weight (W p). W p is the weight of the purified β-CDP, i.e. the weight of water-insoluble polymer in the product. The yield (Y) of the purified β-CDP was calculated by:
Swelling rate of β-CDP in water
About 0.2 g of the above purified β-CDP was precisely weighed (recorded as W 0) and put into a conical flask together with 50 mL of deionized water. The flask was well covered and then shaken in a water bath shaker (SHA-C, Jintan Ronghua Instrument Manufacture Co., Ltd., China) at 30 °C for 24 h. After that, the β-CDP was filtered out and precisely weighed (W 1). The swelling rate (SR) of β-CDP in water was calculated by:
FT-IR spectra of β-CDP
Small amount of the β-CDP was mixed and grinded thoroughly with KBr powder in an agate mortar and pressed into thin tablets under 16 MPa pressure using a manual hydraulic press (FW-4A, Tianjin Tuopu Instrument Co., Ltd., China). Fourier-transform Infrared (FT-IR) spectra were measured using the tablets by a FT-IR spectrometer (Thermo Nicolet IR 200, Thermo Electron Corp., USA).
Adsorption experiments
The adsorption experiments were carried out using the aqueous solution of phenol and methylene blue (MB) respectively. As has been reported, phenol is commonly encountered in aqueous effluents from various manufacturing processes and is a toxic substance that should be removed from the aquatic environment [18]. MB is a cationic dye (see Fig. 3 for its chemical structure) which may exhibit toxic effects on microbial populations and can be toxic and carcinogenic to mammalian animal [25]. Researchers have disclosed by experiments that phenol or MB in water can be adsorbed by β-cyclodextrin-containing polymers [18, 26–28]. The β-CDP synthesized by us was as well applied to adsorb phenol and MB in water to evaluate its inclusion and adsorption properties.
In order to calculate the concentration from each experiment, a calibration curve was first prepared. Different concentrations were prepared and absorbance values were measured by a UV–Vis spectrophotometer (Shimadzu UV-260, Shimadzu Corp., Japan) at λ max. The value of λ max was 269 nm for phenol and 665 nm for MB.
In each experiment, the adsorbent was precisely weighed and put into a conical flask which contains certain amount of adsorbate solution at a known concentration. The flask was well covered and then shaken in the water bath shaker for different time intervals. The concentrations of the solution at different time points were determined from the absorbance of the solution measured by the UV–Vis spectrophotometer.
The adsorption capacity of β-CDP toward the adsorbate was determined by the difference between the initial and remaining concentrations of the adsorbate solution. The equilibrium adsorption capacity, q e (mg g−1) was calculated by:
where C 0 is the initial concentration (mg L−1); C e is the equilibrium concentration (mg L−1); V is the volume of the solution used (L); and W is the weight of adsorbent used (g).
The adsorption capacity of β-CDP at time t, q t (mg g−1), was calculated as well:
where C t is the concentration at contact time t (mg L−1).
Results and discussion
Characterization of β-CDP
The synthesized β-CDP after purification was a slightly yellow powder. It could not be dissolved but was notably swelled by water. The yield (Y) of the purified β-CDP was 65% and the swelling rate (SR) of it in water was 130%, according to the calculation Eqs. 1 and 2 provided above, respectively.
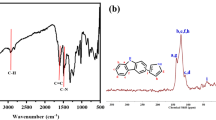
Figure 4 is the FT-IR spectra of the synthesized β-CDP (b) compared with native β-CD (a). There appeared an intensive absorption band at 1,736 cm−1 in (b) which was absent in (a), and we owed this band to the C=O stretching vibration of ester groups and carboxyl groups in β-CDP. The peak at 1,209 cm−1 in (b), which was also absent in (a), was owed to the C-O-C stretching vibration of ester groups. The absorption bands of ester groups observed in (b) indicated that the hydroxyl groups of β-CD had reacted and condensated with the carboxyl groups of citric acid and thereby a three-dimensional polymer network was formed. Because of the crosslinked network, the β-CDP was stable and insoluble in water. The strong and broad band at 3,400 cm−1 in (a) corresponded to the O-H stretching vibration of the hydroxyl groups in β-CD, and the similar band observed at 3,435 cm−1 in (b) corresponded to the integrated O–H stretching vibration of the hydroxyl groups and carboxyl groups in β-CDP. The peak at 2,925 cm−1 in (a) and the peak at 2,933 cm−1 in (b) corresponded to the CH2 asymmetric stretching vibration. The C–OH stretching vibration at 1,030 cm−1, the C–O–C stretching vibration at 1,159 cm−1, and the other absorption bands including 1,414 cm−1, 947 cm−1, 858 cm−1, 756 cm−1 and 579 cm−1 in (a) for β-CD also appeared nearly at the same wavenumbers in (b) for β-CDP, indicating that the structural characteristics of β-CD were essentially maintained in β-CDP. Because of the hydroxyl groups and carboxyl groups existing in the polymer structure, the β-CDP was hydrophilic and easily swelled by water. The FT-IR spectra of citric acid and PVA-1799 were as well compared with the spectrum of the β-CDP and the results also agreed to the above conclusion.
Adsorption of phenol on β-CDP
The calibration curve for aqueous solution of phenol was first determined, as shown in Fig. 5. It was created by running different calibration standards (10, 20, 30, 40, 50 mg L−1). The absorbance values (A) were measured for each concentration (C) at λ max = 269 nm by UV–Vis spectrometer. The concentration (C) values were plotted against the corresponding A values and the data points were linearly fitted. The resulted calibration equation was: C = 61.44 × A − 0.077, R = 0.9998. R was the correlation coefficient.
Figures 6 and 7 are the adsorption kinetics of phenol by β-CDP. Figure 6 is the concentration of phenol at contact time t (C t ) versus t and Fig. 7 is the adsorption amount (q t ) versus t. C 0 is the initial concentration of phenol. W is the weight of adsorbent used. V is the volume of the solution used. As shown in the two figures, the concentration decreased and the adsorption amount increased evidently with the increase of contact time at first and then gradually slowed down. It took about 200 min to reach adsorption equilibrium when C 0 = 85.4 mg L−1, while at least 500 min when C 0 = 393 mg L−1. The maximum adsorption amount (q max) was only 3.8 mg g−1 when C 0 = 85.4 mg L−1, while the q max increased to 9.2 mg g−1 when C 0 = 393 mg L−1.
Figure 8 is the adsorption isotherm of phenol by β-CDP. C e is the equilibrium concentration of phenol (mg L−1). q e is the corresponding equilibrium adsorption amount of phenol by β-CDP (mg g−1). q e is calculated according to Eq. 3 provided above. As shown in the figure, the isotherm ascended almost linearly when C e was lower than 200 mg L−1, but when C e was higher than 200 mg L−1, a platform was gradually formed, indicating that the adsorption capacity of β-CDP was nearly saturated. The maximum adsorption amount, q max = 13.8 mg g−1, was achieved when the equilibrium concentration was higher than 400 mg L−1.
Adsorption of MB on β-CDP
The calibration curve for MB is as shown in Fig. 9. It was created by running different calibration standards (1, 2, 3, 4, 5, 8, 10 mg L−1). The absorbance (A) values were measured for each concentration at λ max = 665 nm by UV–Vis spectrometer. The concentration (C) values were plotted against the corresponding A values and the data points were linearly fitted. The resulted calibration equation was: C = 5.514 × A − 0.059, R = 0.9996.
Figures 10 and 11 are the adsorption kinetics of MB by β-CDP. Figure 10 is the concentration of MB at contact time t (C t ) versus t and Fig. 11 is the adsorption amount (q t ) versus t. In this experiment the amount of MB in the solution was insufficient to saturate the maximum adsorption capacity of β-CDP. As shown in the two figures, the concentration of MB decreased and the adsorption amount increased quickly with the increase of contact time at first and then gradually slowed down. It took only 30 min to reach adsorption equilibrium when W = 0.1 g and 60 min when W = 0.05 g, while at least 200 min when W = 0.02 g. As shown in Fig. 10, when t = 660 min, the concentration of MB was reduced to be only 0.11 mg L−1 (W = 0.1 g), 0.17 mg L−1 (W = 0.05 g) and 0.49 mg L−1 (W = 0.02 g) respectively, which indicated the excellent adsorption sensitivity of β-CDP toward MB.
The adsorption isotherm of MB by β-CDP is as shown in Fig. 12. The isotherm ascended almost linearly when C e was lower than 20 mg L−1, then gradually slowed down when C e was between 20 and 80 mg L−1. A platform was gradually formed when C e was higher than 80 mg L−1, indicating that the adsorption capacity of β-CDP was nearly saturated. The maximum adsorption amount, q max = 105 mg g−1, was achieved when C e was higher than 100 mg L−1. Crini et al. reported an epichlorohydrin crosslinked β-CD polymer which contained carboxyl groups and had an adsorption capability of 56.5 mg g−1 toward C.I. Basic Blue 9 (i.e. MB) at the concentration of 100–140 mg L−1 [27]. They proved by experiments that the adsorption was dependent on the presence of carboxyl groups. Our result was even better than theirs, probably because β-CDP crosslinked by citric acid contained a larger number of acidic groups than the polymer crosslinked by epichlorohydrin in the presence of carboxymethylcellulose [27].
β-CD in the polymer is also considered by us very important in the adsorption toward MB. Effects of β-CD in the adsorption exist in at least two aspects. One is that the cavity of β-CD in the polymer can directly adsorb MB since the molecular size of MB is suitable to the cavity of β-CD. The other is that the rigid structure of β-CD increase the brittleness of the polymer, so that the polymer is easy to be grinded into fine granulae and the acidic groups are well exposed to outside.
Conclusions
Water-insoluble β-cyclodextrin polymer (β-CDP) was synthesized by polycondensation using citric acid as crosslinking agent, sodium dihydrogen phosphate as catalyst, PVA-1799 as additive, and in the presence of some water. The synthesis procedure was environment friendly since neither organic solvents nor harmful chemicals were used. The production yield of β-CDP after purification was 65%. The swelling rate of β-CDP in water was 130%. FT-IR spectra disclosed that the β-CDP contained ester and carboxyl groups which did not exist in β-CD, indicating that the hydroxyl groups of β-CD had reacted and condensated with the carboxyl groups of citric acid and a three-dimensional polymer network was formed. Nearly all the FT-IR absorption bands of β-CD could be found in the spectrum of β-CDP, indicating that the structural characteristics of β-CD were essentially maintained in β-CDP. The β-CDP exhibited notable adsorption toward phenol (q max = 13.8 mg g−1) and especially large adsorption capability toward MB (q max = 105 mg g−1). The concentration of MB in water could be reduced to 0.11 mg L−1 by the β-CDP, indicating the excellent adsorption sensitivity of β-CDP toward MB. The adsorption results implied that the interior cavity and inclusion property of β-CD were maintained in the β-CDP. The reason why the adsorption toward MB was much higher than phenol might exist in two aspects. One is that the molecular size of MB is more suitable than phenol for the formation of β-CD complex. The other is that the carboxyl and ester groups in β-CDP are acidic so as to contribute to the adsorption toward MB because MB is a weekly basic compound.
References
Crini, G., Morcellet, M.: Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 25(13), 789–813 (2002). doi:10.1002/1615-9314(20020901)25:13<789::AID-JSSC789>3.0.CO;2-J
Szejtli, J.: Cyclodextrin Technology, 1st edn. Kluwer, Dordrecht (1988)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98(5), 1743–1754 (1998). doi:10.1021/cr970022c
Shieh, W.J., Hedges, A.R.: Properties and applications of cyclodextrins. J. Macromol. Sci. A 33(5), 673–683 (1996). doi:10.1080/10601329608010886
Szejtli, J.: Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 7(4), 575–587 (1997). doi:10.1039/a605235e
Hedges, A.R.: Industrial applications of cyclodextrins. Chem. Rev. 98(5), 2035–2044 (1998). doi:10.1021/cr970014w
Martin Dell Valle, E.M.: Cyclodextrins and their uses: a review. Process Biochem. 39(9), 1033–1046 (2004). doi:10.1016/S0032-9592(03)00258-9
Crini, G.: Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 30(1), 38–70 (2005). doi:10.1016/j.progpolymsci.2004.11.002
Crini, G.: Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. 97(9), 1061–1085 (2006). doi:10.1016/j.biortech.2005.05.001
Vélaz, I., Isasi, J.R., Sánchez, M., et al.: Structural characteristics of some soluble and insoluble β-cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 57(1), 65–68 (2007). doi:10.1007/s10847-006-9221-z
Kitaoka, M., Hayashi, K.: Adsorption of bisphenol A by cross-linked β-cyclodextrin polymer. J. Incl. Phenom. Macrocycl. Chem. 44(1), 429–431 (2002). doi:10.1023/A:1023024004103
Shao, Y., Martel, B., Morcellet, M., et al.: Sorption of textile dyes on β-cyclodextrin-epichlorhydrin gels. J. Incl. Phenom. Macrocycl. Chem. 25(1–3), 209–212 (1996). doi:10.1007/BF01041570
Crini, G., Bertini, S., Torri, G., et al.: Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J. Appl. Polym. Sci. 68(12), 1973–1978 (1998). doi:10.1002/(SICI)1097-4628(19980620)68:12<1973::AID-APP11>3.0.CO;2-T
Gu, T., Tsai, G., Tsao, G.T.: Synthesis of rigid cyclodextrin-containing polymeric resins for adsorption. J. Incl. Phenom. Macrocycl. Chem. 56(3), 375–379 (2006). doi:10.1007/s10847-006-9119-9
Werner, T.C., Iannacone, J.L., Amoo, M.N.: The binding of pyrene and other probes to CD polymers. J. Incl. Phenom. Macrocycl. Chem. 25(1), 77–80 (1996). doi:10.1007/BF01041540
Ozmen, E.Y., Sirit, A., Yilmaz, M.: A calix [4]arene oligomer and two beta-cyclodextrin polymers: synthesis and sorption studies of azo dyes. J. Macromol. Sci. A 44, 167–173 (2007). doi:10.1080/10601320701561148
Sreenivasan, K.: Synthesis and characterization of poly (vinyl alcohol)-β-cyclodextrin copolymer. Angew. Makromol. Chem. 235, 15–20 (1996). doi:10.1002/apmc.1996.052350102
Yamasaki, H., Makihata, Y., Fukunaga, K.: Preparation of crosslinked β-cyclodextrin polymer beads and their application as a sorbent for removal of phenol from wastewater. J. Chem. Technol. Biotechnol. 83, 991–997 (2008). doi:10.1002/jctb.1904
El Ghoul, Y., Martel, B., Morcellet, M.: Mechanical and physico-chemical characterization of cyclodextrin finished polyamide fibers. J. Incl. Phenom. Macrocycl. Chem. 57, 47–52 (2007). doi:10.1007/s10847-006-9164-4
Ducoroy, L., Martel, B., Bacquet, B., et al.: Ion exchange textiles from the finishing of PET fabrics with cyclodextrins and citric acid for the sorption of metallic cations in water. J. Incl. Phenom. Macrocycl. Chem. 57(1), 271–277 (2007). doi:10.1007/s10847-006-9172-4
Martel, B., Morcellet, M., Ruffin, D., et al.: Finishing of polyester fabrics with cyclodextrins and polycarboxylic acids as crosslinking agents. J. Incl. Phenom. Macrocycl. Chem. 44(1), 443–446 (2002). doi:10.1023/A:1023080221850
Lo Nostro, P., Fratoni, L., Baglioni, P.: Modification of a cellulosic fabric with β-cyclodextrin for textile finishing applications. J. Incl. Phenom. Macrocycl. Chem. 44(1–4), 423–427 (2002). doi:10.1023/A:1023071920033
Berto, S., Bruzzoniti, M.C., Cavalli, R., et al.: Synthesis of new ionic β-cyclodextrin polymers and characterization of their heavy metals retention. J. Incl. Phenom. Macrocycl. Chem. 57, 631–636 (2007). doi:10.1007/s10847-006-9273-0
Berto, S., Bruzzoniti, M.C., Cavalli, R., et al.: Highly crosslinked ionic β-cyclodextrin polymers and their interaction with heavy metals. J. Incl. Phenom. Macrocycl. Chem. 57(1), 637–643 (2007). doi:10.1007/s10847-006-9270-3
Reife, A.: Dyes: environmental chemistry. In: Kirk-Othmer Encyclopedia of Chemical Technology, vol. 8, 4th edn, pp. 753–783. Wiley, New York (1993)
Romo, A., Pe, F.J., Isasi, J.R., et al.: Extraction of phenols from aqueous solutions by β-cyclodextrin polymers. Comparison of sorptive capacities with other sorbents. React. Funct. Polym. 68(1), 406–413 (2008). doi:10.1016/j.reactfunctpolym.2007.07.005
Crini, G., Peindy, H.N.: Adsorption of C.I. Basic Blue 9 on cyclodextrin-based material containing carboxylic groups. Dyes Pigments 70(3), 204–211 (2006). doi:10.1016/j.dyepig.2005.05.004
Crini, G.: Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 90(2), 193–198 (2003). doi:10.1016/S0960-8524(03)00111-1
Acknowledgements
We gratefully acknowledge the support of the Natural Science Foundation of Henan Province, China. We sincerely thank Mr. Zhenbang Tian, Mr. Baofeng Sha and Ms. Xiaozhuan Zhang for their valuable advices and assistances in the experiments and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, D., Zhao, L., Zhu, CS. et al. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: synthesis and adsorption properties toward phenol and methylene blue. J Incl Phenom Macrocycl Chem 63, 195–201 (2009). https://doi.org/10.1007/s10847-008-9507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9507-4