Abstract
By reacting cyclodextrin-grafted thiacalix [4]arene derivative 9 containing three alkynyl groups with bis-azido compound 6, the first example of cyclodextrin-grafted thiacalix [4]arene netty polymer 10 was designed and prepared by the click chemistry in yield of 76 %. Its structure was confirmed by elemental analysis, 1H NMR spectrum, FTIR spectrum and XRD curve. The M n of polymer 10 was 48,677, indicating average approximately 22 units of compound 9 in each polymer molecule. Its SEM image showed porous and loose morphology. Adsorption experiments of polymer 10 indicated that it possessed excellent adsorption capacities for both cationic and anionic dyes [Orange G sodium salt (OG), Brilliant ponceau 5R (BP), Victoria blue B (VB), Crystal violet (CV), Neutral red (NR) and Methylene blue (MB)]. The highest adsorption capacities for VB, CV, NR and MB were 5.821, 6.825, 6.135 and 5.381 mmol/g, respectively. Adsorption kinetics and isotherms analysis suggested that all the adsorption processes were exothermic and spontaneous. The adsorption processes obeyed pseudo second-order model and the Langmuir isotherm equation. The adsorption abilities of polymer 10 at pH values 3–11 were keep stable between 1.5–4 mmol/g. The adsorption abilities for anionic dyes decreased with the increase of pH values but opposite phenomena for cationic dyes. Polymer 10 showed good reused adsorption property after desorption in five times’ cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes and dyestuffs are widely used in various industries such as textiles, leather, dyestuffs, papers, etc. As a result, the water quality was seriously polluted by these industrial effluents containing residual dyes [1]. Therefore, the corresponding wastewaters should be treated before discharge. Many dyes are difficult to remove due to its synthetic origin and the immense volume of aromatic structures. The conventional biological wastewater treatment methods are ineffective for decolorization and degradation [2].
Up to now, many treatment processes, including chemical coagulation, fungal decolorization, precipitation, adsorption, membrane filtration, photodegradation etc., have been used to remove the dyes from wastewaters [3–7]. Among these techniques, adsorption is good choice for the removal of dissolved organic compounds from wastewater [8]. Moreover, adsorption, which can remove the complete dye molecules, is more advantage than other certain removal techniques, which destroy only the dye chromophore leaving the harmful residual moieties (like metals) in the effluent. Thus, the development of effective adsorbent is the crucial task of the dye adsorption process for treating wastewater.
Recently, supramolecular chemistries, such as cyclodextrin and calixarene, have provided a much improved solution to search for molecular structures that could serve as building blocks for the production of sophisticated molecules by anchoring functional groups oriented in such a way that they provided a suitable binding site for the dyes. Series of calixarene receptors for organic dyes were designed and synthesized by Yilmaz [9, 10], Memnon [11], Diao [12], and our group [13–15], respectively. Also, some of CDs derivatives and bis-CDs derivatives were reported as good receptors for all kinds of organic dyes [16–20]. Moreover, some cyclodextrin-calix [4]arene dyads were presented as excellent receptors for organic guests based on the cooperative complexation action of the two structural units [21–26]. On the other hand, the ideal adsorbents should not only possess excellent adsorption abilities for dyes, but also have low solubility in water and good material processing performance. One method to attain these aims is to prepare the polymers based on the effective organic receptors. For example, some calixarene polymers were reported as excellent adsorption materials for dyes [27–29]. However, although the cyclodextrin derivatives, calixarene derivatives, and cyclodextrin-calixarene dyads had been confirmed as good receptors for dyes, no cyclodextrin-calix [4]arene polymer was investigated so far.
In this paper, we designed and synthesized the first example of cyclodextrin-calix [4]arene polymer. Its structure was characterized by elemental analysis, 1H NMR, FTIR, XRD, SEM image, etc. The absorption experiments indicated it possessed excellent absorption capability for both cationic and anionic dyes. Moreover, pH influence, kinetics and equilibrium were examined to gain better comparison of the experimental results.
Experimental
Materials
Cyclodextrin derivative 3 was synthesized according to the literature [30]. Alkynylthiacalix [4] arene 8 and azido compound 6 were prepared by the reported methods [31, 32]. All dyes were purchased from Acros Organics, China. Their structures and λmax of absorbing wavelength were showed in Fig. 1. All solvents were purified by standard procedures before use. All other chemicals, except special instruction, were analytically pure and used without further purification. The obtained polymer 10 was scrunched and sieved with diameter size of 500-1000 μm.
Characterization
1H NMR spectra were recorded in CDCl3 on a Bruker-ARX 600 instrument at room temperature, using TMS as an internal standard. ESI-MS spectra were obtained from DECAX-30,000 LCQ Deca XP mass spectrometer. Elemental analyses were performed at Carlo-Erba 1106 Elemental Analyzer. Osmometric molecular weight determinations were carried out a Knauer vapour pressure osmometer (USA) at concentrations of ca. 10−3 M (based on the polymeric units) in CHCl3 at 37 °C using sucrose octaacetate for calibration. IR spectra were recorded on Perkin-Elmer 1605 FTIR spectrometer as KBr pellets. UV-VIS measurements were performed on a Varian UV-VIS instrument. Surface morphology of polymer 10 was studied by using SEM (JEOLJSM-6490LA) and AFM (JEOL JSPM-5200). X-ray diffraction (XRD) experiments were performed on SEIFERT-FPM (XRD7), using Cu Kα 1.5406 Å as the radiation source with 40 kV, 30 mA power.
Methods
Preparation of compound 9
Under N2 atmosphere, the mixture of cyclodextrin derivative 3 (0.58 g, 0.5 mmol), alkynylthiacalix [4]arene 8 (0.44 g, 0.5 mmol) and CuI (0.019 g, 0.1 mmol) were stirred in 25 mL of dry DMF at 60 °C overnight. The TLC detection indicated the disappearance of starting materials. After reaction, the DMF solvent was evaporated by rota-vapor. The residue was further purified by column chromatography (SiO2 100–200 mesh, MeOH/CH2Cl2 (3:2, V/V) as eluant). Compound 9 was obtained as grayish solid in yield of 66 %. Compound 9: 1H NMR (400 MHz, DMSO-d6) δppm: 1.04–1.24 (m, 36 H, CH3), 1.98 (s, 3 H, C ≡ CH), 2.10–3.80 (m, 40 H, H2-H6), 4.12–4.58 (m, 8 H, OH-6, NCH2), 4.62–4.93 (m, 15 H, CH, OCH2), 5.44–5.88 (m, 14 H, OH-2,3),7.33–7.58 (m, 9 H, NCH, ArH); MS m/z (%): 2056.2 (MNa+, 100). Anal. calcd for C94H125O38S4N3: C 55.51, H 6.15, N 2.07; found C 55.43, H 6.21, N 2.01.
Preparation of title polymer 10
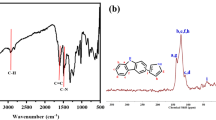
Under nitrogen atmosphere, the mixture of compound 9 (0.61 g, 0.3 mmol), compound 6 (0.072 g, 0.46 mmol), and CuI (0.019 g, 0.1 mmol) was stirred in dry DMF (10 mL) for 0.5 h. Then the mixture was heated at 90 °C for 24 h until the material of compound 9 utterly vanished under the monitor of TLC. After cooling, the reaction system was diluted by CHCl3 (30 mL) and washed by distilled water (3 × 20 mL). The organic phase was separated and dried by MgSO4. After evaporating off the solvent to dryness, the powder was washed subsequently by 10 mL of acetone and 10 mL of methanol to remove some unreacted materials and some small intramolecular-bridging molecules. The residue was then dried in vacuum. 0.52 g of polymer 10 was obtained as light yellow powder. Polymer 10: 1H NMR (400 MHz, DMSO-d6) δppm: 1.03–1.35 (m, 36 H, CH3), 3.05–3.77 (m, 40 H, H2-H6), 4.02–4.95 (m, 35 H, OH-6, CH, OCH2 and NCH2), 5.40–5.82 (m, 14 H, OH-2,3), 7.28–7.55 (m, 12 H, NCH and ArH). The obtained polymer 10 was scrunched and sieved with diameter size of 500-1000 μm. The elemental analysis, IR spectrum, SEM, and Osmometric Mn of polymer 10 were shown in Table 1, Figs. 1 and 2, respectively.
Adsorption of dyes
Referring to published methods [33], the polymer 10 was studied to determine the adsorption capacities for six cationic and anionic dyes in Fig. 1. All adsorption experiments were done in the thermostatic shaker bath with a shaking of 200 rpm at 25 °C for 24 h except the investigation of the effect of times on adsorption. In the kinetic experiments, dye solutions (10 mL, 2.0 × 10−3 M) were agitated with 5.0 mg of polymer 10 for predetermined intervals of time, respectively. For equilibrium adsorption studies, 5.0 mg of polymer 10 was added into 10 mL of dye solutions with corresponding concentration (1.0–10 × 10−3 M). These adsorption experiments were done at their original pH values (5.5 for OG, 5.3 for BP, 7.3 for VB, 7.2 for CV, 7.0 for NR, 7.5 for MB) and the pH values before and after the adsorption had only slight differences (from 0.1 to 0.2). The influences of pH values on adsorption (10 mL of dye solution with 5.0 mg of polymer 10) were studied by adjusting dye solutions (2.0 × 10−3 M) with dilute sodium hydroxide or hydrochloric acid solutions to pH range of 3–11. After filtration, the concentration of each dye solution was determined by Vis-UV measurement at corresponding wavelength shown in Fig. 1. The amount of adsorbed dyes was calculated by following Eq. (1):
Where q is the amount of absorbed dyes (mmol/g); C 0 is the known concentration of dyes before adsorption. C is the concentration of dyes after adsorption (mol/L). Average of twice-independent experiments was carried out. V is the volume of dyes aqueous solution (L); m is the dose of adsorbent (g).
Results and discussion
Synthesis and characteristic of polymer 10
Click chemistry had been used extensively in preparing the cyclodextrin derivatives and calixarene derivatives with the advantages of high yield and mild reaction condition [20, 31]. Thus, we used this reaction to synthesize the first example of cyclodextrin-grafted thiacalix [4]arene netty polymer. The synthetic route was shown in Scheme 1. Cyclodextrin derivative 3, alkynylthiacalix [4]arene 8 and azido compound 6 were prepared by the reported methods [31–33]. By reacting compound 3 with compound 8, a novel cyclodextrin-calix [4]arene dyad 9 was conveniently synthesized in yield of 66 % after column chromatography. In this reaction, the mono-substituted derivative 9 was obtained as main product by controlling the ratio of starting materials (1:1). As a result, compound 9 still possessed three alkynyl groups, which were favorable for the formation of netty polymer in the next reaction. After reaction with bis-azido compound 6, the cyclodextrin-grafted thiacalix [4]arene netty polymer based on the click chemistry were prepared smoothly in the “3 + 2” functional reaction mode. The structure and morphology of polymer 10 was characterized by FTIR, 1 H NMR, XRD, elemental analysis, and SEM image, etc.
The synthetic route of cyclodextrin-grafted thiacalix [4]arene netty polymer 10
The structure of polymer 10 was investigated preliminarily by IR spectrum. Figure 2 illustrated the IR spectra of compound 9 and polymer 10. It can be seen that, comparing with the IR spectrum of compound 9, the absorption peaks at 2100 cm−1 for alkynyl group disappeared completely in the IR spectra of polymer 10. On the other hand, the strong absorption peaks for triazole groups were appeared at 1550 ~ 1630 cm−1 approximately in the IR spectra of polymer 10. These IR spectra confirmed that almost all of the alkynyl groups of compound 9 were transferred to the triazole rings. The polymer 10 was also studied by 1H NMR spectrum. The result was shown in Fig. 3. One can see that all the protons were agreement with shifts of the signals in 1H NMR spectrum, although some complicated and overlapped peaks were observed due to the conformation flexibility of thiacalix [4]arene skeleton and the multiple similar sugary units on cyclodextrin. The integrals of signals also matched approximately the ratios of protons, which were obtained by the hypothesis of ideal polymeric reaction of compound 6 and 9 in the mol ratio of 3:2 exactly. The molecular weight of polymer 10 and elemental analysis data were illustrated in Table 1. The elemental analysis data were also approximately in accordance with the calculated data obtained by the hypothesis that ideal polymeric reaction mentioned above. The M n of polymer 10 was calculated as 48,677, indicating average approximately 22 units of compound 9 in each polymer molecule.
The surface morphology of polymer 10 was also investigated by scanning electron microscopy (SEM) as shown in Fig. 4. It exhibited loose and porous morphology, which was favorable for adsorption of guests. The X-ray diffraction analysis was further used to study the change of crystallinity of polymer 10. Figure 5 showed the XRD patterns of cyclodextrin, compound 9 and polymer 10. It can be seen that both cyclodextrin and compound 9 exhibited some sharp peaks in their XRD curves, indicating they possessed good crystallinity. However, polymer 10 showed no obvious peak suggesting the weak crystallinity. These XRD data indicated that the crystallinity decreased dramatically after polymerization. The reason could be attributed to that the polymerization destroyed the intermolecular hydrogen-bonding and reduced the symmetry of polymeric units. This kind of weak crystallinity of polymer 10, indicating the weak intermolecular force among polymeric units, was favorable for the intermolecular action between polymeric unit and dyes, and improving the adsorption [34, 35].
Adsorption kinetics
The adsorption kinetics was preliminarily investigated to study the adsorption property of polymer 10. The q t values of polymer 10 versus the contact time for six cationic and anionic dyes [Orange G sodium salt (OG), Brilliant ponceau 5R (BP), Victoria blue B (VB), Crystal violet (CV), Neutral red (NR) and Methylene blue (MB)] were tested and the results were shown in Fig. 6. From Fig. 6, it could be concluded that the adsorption process carried out rapidly in the starting stage. The adsorption equilibrium were attained gradually in 4 h. Moreover, in order to study deeply the kinetic mechanism of adsorption process, the pseudo first-order and second-order model were used to analyze the experimental data. The corresponding formulas were listed in Eqs (2) and (3) [36].
Where k 1 (min−1) and k 2 (min−1) were the adsorption rate constant, q t and q e were the amount adsorbed at time t (min) and at equilibrium, respectively, both in mmol g−1.
According to Eqs (2) and (3), the rate constants for six dyes were calculated and the results were listed in Table 2. The fitting curve for the adsorption kinetic of polymer 10 with MB by second-order model was shown in Fig. 7 as representative one. The correlation coefficients (R2) from second-order adsorption kinetics were near 1. On the other hand, the values of R2 from first-order kinetics were lower than that of second-order adsorption kinetics. Moreover, comparing with the calculated q e values from first-order kinetics, the q e of second-order model were in well agreement with the experimental data. These results suggested that the adsorption process obeyed the second-order adsorption mechanism. These results suggested that the dye adsorption was controlled by chemical process, such as hydrogen bonding, hydrophobic interactions, ion-dipole and ion-ion interactions and π-π stacking action [33, 36, 37].
Adsorption isotherms
The adsorption isotherms of polymer 10 were studied for these six dyes. The tested results were shown in Fig. 8 (a). It could be seen that, with the increase of concentration of dyes (C e), the equilibrium adsorption capacities (q e) attained stable values gradually. Furthermore, in order to study the adsorption behaviors in details, Langmuir isotherm and Freundlich isotherm, which were usually used to fit the experimental data, were applied to analyze the adsorption data. The Langmuir isotherm equation was usually used for many real monolayer adsorption processes with a finite number of identical sites. The Eq.(4) was following formula [38]:
In equation, q e and C e were the amounts of adsorption (mmol/g) and the adsorbate concentration (mmol/L) at equilibrium, respectively. K L was Langmuir constant and q m was the maximum adsorption capacity.
The heterogeneous surfaces of multilayer adsorption were normally investigated by Freundlich isotherm equation as following Eq. (5) [39]:
Freundlich constants, K F and b F , could be obtained from a linear plot of lnq e versus lnC e .
The Figure 8 (b) showed the calculation results of Langmuir isotherm. The fitting curve for the adsorption isotherm of polymer 10 with MB by Langmuir isotherm equation was shown in Fig. 9 as representative one. One can see that the plots of C e /q e against C e were a straight line, indicating that the adsorption processes of polymer 10 for six dyes obeyed the Langmuir isotherm. The corresponding parameters of the two adsorption isotherm equations for six dyes were illustrated in Tables 3 after calculation. The correlation coefficients (R 2) were near 1 for Langmuir isotherm but R 2 were only 0.9 approximately for Freundlich isotherms. Moreover, the adsorption capacities for dyes were calculated according to Langmuir isotherm. The results indicated polymer 10 had high adsorption capacities for all test dyes. The adsorption capacity for VB, CV, NR and MB were as high as 5.821, 6.825, 6.135 and 5.381 mmol/g, respectively. It is worthy of noting that these adsorption capabilities were very outstanding by comparing with the various dyes sorbents, such as other calixarene polymers or chitosan derivatives (1 ~ 5 mmol/g in most cases) [9–11, 33, 40–43].
Adsorption thermodynamics
At different temperatures (298, 208 and 318 K), the adsorption behaviors were also investigated by studying the dependence of Langmuir adsorption equilibrium constant with temperature. The standard Gibbs free energy change (ΔG o), standard enthalpy change (ΔH o) and standard entropy change (ΔS o) were calculated by van’t Hoff equation (6) and (7) [33]:
By plotting a graph of lnK L versus 1/T, the values of ΔH o, ΔS o and ΔG o were calculated. The fitting curve for the adsorption thermodynamic of polymer 10 with MB by van’t Hoff equation was shown in Fig. 10 as representative one and all results were listed in Table 4. All ΔH o, ΔS o and ΔG o were negative, suggesting that these adsorption behaviors were exothermic and spontaneous. These small values of ΔG on dye adsorption might indicate that the adsorption attained adsorption equilibrium nearly at the tested temperatures.
pH influence on adsorption capacity
The pH influences on the adsorption abilities of polymer 10 for six dyes were shown in Fig. 11. The adsorption abilities of polymer 10 for anionic dyes (OG and BP) decreased with the increase of pH values. On the contrary, the adsorption abilities for cationic dyes (VB, CV, NR and MB) increased with the increase of pH values. These results could be explained that, at low pH values, the amino groups on polymer 10 were easily converted to ammonium ion, which are favorable for electrostatic attraction of sulfonic groups in anionic dyes and not favorable for ammonium groups in cationic dyes due to the electrostatic repulsion [33, 37, 40, 44]. At high pH values, the hydroxylic groups on polymer 10 could be changed to alkoxy anions, which possess electrostatic attraction for ammonium groups but electrostatic repulsion for sulfonic groups. Nevertheless, the adsorption abilities of polymer 10 at pH values 3–11 were keep stable between 1.5–4 mmol/g, indicating it showed good adsorption abilities at various pH conditions.
The reused property of polymer 10 after desorption
In order to test the reused property of polymer 10, the adsorption property of polymer 10 reused property of polymer 10 for CV was investigated as the representative one for five times’ cycles. After adsorption for CV (the adsorption was carried out in the condition of adsorption kinetics mentioned in experimental part), the polymer 10 was desorbed by 10 % HCl and 10 % NaOH orderly. Then it was washed adequately by distilled water and dried by vacuum. The obtained polymer was reused for CV adsorption again. The five times’ adsorption percentages were detected and the adsorption capabilities were calculated as 3.61, 3.45, 3.22, 3.08, 2.91 mmol/g, respectively. These results suggested that polymer 10 exhibited excellent reused property.
Conclusion
The first example of cyclodextrin-grafted thiacalix [4]arene netty polymer 10 based on the click chemistry was prepared. Its structure and morphology were studied by elemental analysis, 1H NMR, FTIR, XRD, SEM image. Polymer 10 showed excellent adsorption abilities for six kinds of cationic and anionic dyes. The adsorption capacity for VB, CV, NR and MB were as high as 5.821, 6.825, 6.135 and 5.381 mmol/g, respectively. The adsorption processes obeyed pseudo second-order model and followed the Langmuir isotherm equation. The adsorption processes were exothermic and spontaneous. The adsorption abilities for anionic dyes decreased with the increase of pH values but increased with the increase of pH values for cationic dyes.
References
Wong Y, Yu J (1991) Laccase-catalyzed decolorization of synthetic dyes. Water Res 33:3512–3520
Bumpus J (1995) Microbial degradation of health risk compounds. In: Singh VP (ed) Inbiotransformations: Microbial degradation of azo dyes. Elsevier Science BV, Amsterdam, pp. 157–176
Peternel IT, Koprivanac N, Bozic AML, Kusic HM (2007) Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J Hazard Mater 148:477–484
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Dhodapkar R, Pande NNR, Nandy T, Devotta S (2007) Adsorption of cationic dyes on jalshakti (R), super absorbent polymer and photocatalytic regeneration of the adsorbent. React Funct Polym 67:540–548
Haider S, Binagag F, Haider A, Al-Masry WA (2014) Electrospun oxime-grafted-polyacrylonitrile nanofiber membrane and its application to the adsorption of dyes. J Polym Res 21:371–384
Ansari R, Keivani MB, Ansari R (2011) Application of polyaniline nanolayer composite for removal of tartrazine dye from aqueous solutions. J Polym Res 18:1931–1939
Dabrowski A (2001) Adsorption - from theory to practice. Adv Colloid Interf Sci 93:135–224
Akceylan E, Bahadir M, Yilmaz M (2009) Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J Hazard Mater 162:960–966
Yilmaz A, Yilmaz E, Yilmaz M (2007) Removal of azo dyes from aqueous solutions using calix[4]arene and cyclodextrin. Dyes Pigments 74:54–60
Kamboh MA, Solangi IB, Memon S (2011) Synthesis and application of p-tert-butylcalix[8]arene immobilized material for the removal of azo dyes. J Hazard Mater 186:651–656
Chen M, Shang T, Fang W, Diao G (2011) Study on adsorption and desorption properties of the starch grafted p-tert-butyl-calix[n]arene for butyl rhodamine B solution. Hazard Mater 185:914–920
Yang FF, Liu WW, Xie JW, Bai XY, Guo HY (2013) Novel deep-cavity calix[4]arene derivatives with s-triazine conjugate systems: synthesis and complexation for dyes. J Incl Phenom Macrocycl Chem 76:311–316
Yang FF, Zhang YM, Guo HY, Wei XL (2013) High efficient liquid membrane transport of dyes using calix[4]arene-linked triphenylene dimers as carriers. Sep Sci Technol 48:1565–1571
Bai XY, Yang FF, Xie JW, Guo HY (2013) Novel 1,2-3,4-bridged and 1,3-bridged calix[4]arene based on large s-triazine conjugate systems: synthesis and complexation for dyes. J Macromol Sci A 50:334–339
Shaikh M, Swamy YM, Pal H (2013) Supramolecular host–guest interaction of acridine dye with cyclodextrin macrocycles: photophysical, pKa shift and quenching study. J Photoch Photobio A 285:41–50
Liu Y, Kang SZ, Zhang HY (2001) Synthesis of cyclodextrin derivative bearing a cyclohexylamino moiety and its inclusion complexation with organic dye molecules. Microchem 70:115–121
Arun KT, Jayaram DT, Avirah RR, Ramaiah D (2011) β-cyclodextrin as a photosensitizer carrier: effect on photophysical properties and chemical reactivity of squaraine dyes. J Phys Chem B 115:7122–7128
Liu Y, Chen Y (2006) Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Accounts Chem Res 39:681–699
Liu Y, Chen Y, Li L, Huang G, You CC, Zhang HY, Wada T, Inoue Y (2001) Cooperative multiple recognition by novel calix[4]arene-tethered β-cyclodextrin and calix[4]arene-bridged bis(β-cyclodextrin). J Org Chem 66:7209–7215
Liu Y, Li B, You CC, Wada T, Inoue Y (2001) Molecular recognition studies on supramolecular systems. 32. Molecular recognition of dyes by organoselenium-bridged bis(β-cyclodextrin)s. J Org Chem 66:225–232
Zhang HC, Hao AY, Shen J (2008) Progress in cyclodextrin-calix[n]arene and prospect of its future development. Chin J Org Chem 28:954–963
Souchon V, Maisonneuve S, David O, Leray I, Xie J, Valeur B (2008) Photophysics of cyclic multichromophoric systems based on β-cyclodextrin and calix[4]arene with appended pyridin-2′-yl-1,2,3-triazole groups. Photoch Photobio Sci 7:1323–1331
Baruah U, Gogoi N, Majumdar G, Chowdhury D (2015) β-cyclodextrin and calix[4]arene-25,26,27,28-tetrol capped carbondots for selective and sensitive detection of fluoride. Carbohyd Polym 117:377–383
Mulder A, Auletta T, Sartori A, Ciotto SD, Casnati A, Ungaro R, Huskens J, Reinhoudt DN (2004) Divalent binding of a bis(adamantyl)-functionalized calix[4]arene to β-cyclodextrin-based hosts: an experimental and theoretical study on multivalent binding in solution and at self-assembled monolayers. J Am Chem Soc 126:6627–6636
Krause-Heuer AM, Wheate NJ, Tilby MJ, Pearson DG, Ottley CJ, Aldrich-Wright JR (2008) Substituted β-cyclodextrin and calix[4]arene as encapsulatory vehicles for platinum(II)-based DNA intercalators. Inorg Chem 47:6880–6888
Kamboh MA, Solangi IB, Sherazi STH (2011) A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 268:83–89
Guo HY, Yang FF, Chai XF, Jiao ZY, Li CC (2012) Synthesis of novel calix[6]-1,4-crown-based netty polymer and its excellent adsorption capabilities for aniline derivatives. Iran Polym J 21:451–456
Yang FF, Huang ZS, Zhang XY, Guo HY (2010) Thiacalix[4]amido-based netty polymers: novel sorbents for heavy metal cations and derivatives of aniline. Iran Polym J 19:309–318
Guo HY, Yang FF, Zhang YM, Di XD (2015) Facile synthesis of mono- and polytopic β-cyclodextrin aromatic aldehydes by click chemistry. Synth Commun 3:338–347
Guo HY, Yang FF, Jiao ZY, Lin JR (2013) Click synthesis and dyes extraction capabilities of novel thiacalix[4]arene derivatives with triazole groups and hydrogen bond groups. Chin Chem Lett 24:450–452
Zhan JY, Tian DM, Li HB (2009) Synthesis of calix[4]crowns containing soft and hard ion binding sites via click chemistry. New J Chem 33:725–728
Chen AH, Chen SM (2009) Biosorption of azo dyes from aqueous solution by glutaraldehyde-crosslinked chitosans. J Hazard Mater 172:1111–1121
Shaker MA, Yakout AA (2016) Optimization, isotherm, kinetic and thermodynamic studies of Pb(II) ions adsorption onto N-maleated chitosan-immobilized TiO2 nanoparticles from aqueous media. Spectrochim Acta A Mol Biomol Spectrosc 154:145–156
Yuan YC, Zhang MQ, Rong MZ (2005) Study on the adsorption behavior of crosslinked chitosan for Ni(II). Acta Chimica Sinca 63:1753–1759
Wang ZH, Xiang B, Cheng RM, Li YJ (2010) Behaviors and mechanism of acid dyes sorption onto diethylenetriamine-modified native and enzymatic hydrolysis starch. J Hazard Mater 180:224–232
Chiou MS, Li HY (2002) Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J Hazard Mater 93:233–248
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Hazard Mater 40:1361–1403
Giles CH, Smith D, Huitson A (1974) General treatment and classification of solute adsorption-isotherm. 1. Theoretical. J Colloid Interf Sci 47:755–765
Chiou MS, Ho PY, Li HY (2004) Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 60:69–84
Chiou MS, Chuang GS (2006) Competitive adsorption of dye in acid solutions on chemically metanil yellow and RB15 cross-linked chitosan beads. Chemosphere 62:731–740
Elwakeel KZ (2009) Removal of reactive black 5 from aqueous solutions using magnetic chitosan resins. J Hazard Mater 167:383–392
Yang FF, Bai XY, Xu BT, Guo HY (2013) Triphenylene-modified chitosan: novel high efficient sorbent for cationic and anionic dyes. Cellulose 20:895–906
Yoshida H, Okamoto A, Kataoka T (1993) Adsorption of acid dye on cross-linked chitosan fibers – equilibria. Chem Eng J 48:2267–2272
Acknowledgments
Financial support from the National Natural Science Foundation of China (No: 21406036), Fujian Natural Science Foundation of China (No. 2014 J01034), Key projects of science and technology of Fujian province (2014 N0025) and the Program for Innovative Research Team in Science and Technology in Fujian Province University were greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Guo, H., Yang, F. et al. Cyclodextrin-grafted thiacalix [4]arene netty polymer based on the click chemistry: preparation and efficient adsorption for organic dyes. J Polym Res 23, 28 (2016). https://doi.org/10.1007/s10965-016-0920-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0920-x