Abstract
In this work, polymers containing a large number of benzene rings and multiple functional groups were designed to remove aromatic organic pollutants. Using tetrafluoroterephthalonitrile (TFTPN) as a rigid crosslinking agent to crosslink different functionalized phenylcarbamoylated-β-cyclodextrin derivatives to prepare a series of porous multifunctional cyclodextrin (CD) polymerizations, including three preliminary polymerized adsorption materials and a mix β-cyclodextrin polymer (X-CDP) prepared via a secondary crosslinking procedure of the above three materials. The X-CDP preparation process connects the pre-formed nanoparticles and increases the presence of linkers inside the particles. At the same time, X-CDP exhibited porous structure with various functional groups such as nitro, chlorine, fluorine, and hydroxyl. Those special characteristics render this material with good adsorption ability towards various aromatic organic pollutants in water, including tetracycline, ibuprofen, dichlorophenol, norfloxacin, bisphenol A, and naphthol. Especially, the maximum adsorption capacity for tetracycline at equilibrium reached 110.56 mg·g−1, which is competitive with the adsorption capacities of other polysaccharide adsorbents. X-CDP removed organic contaminants much more quickly than other adsorbents, reaching almost ~95% of its equilibrium in only 30 s, and the rate constant reaches 2.32 g·mg−1·min−1. The main adsorption process of the pollutants by X-CDP fitted the pseudo-second-order kinetic and Langmuir isotherm well, indicating that the adsorption process is monolayer adsorption. Moreover, X-CDP possessed the good reusability where the pollutant removal rate was only reduced 8.3% after five cycles. Such advantages render the polymer great potential in the rapid treatment of organic pollutants in water bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the improvement of people’s living standard, the demand for organic compounds has increased, leading to a rapid increase in the number and variety of organic micro-pollutants in water. Since organic micro-pollutants are widely distributed in various kinds of water bodies and difficultly removed or degraded, they seriously threaten the human health and the stability of ecosystem. In particular, the molecular structure of aromatic organic pollutants containing a benzene ring structure is more stable, difficult to decompose, and highly toxic, which can cause serious pollution to the environment. Thus, the efficient water treatment technologies are attracting the increasing attentions.
In recent years, various methods have been reported to decontaminate organic pollutants and remove organic pollutants from domestic sewage or industrial effluents, such as oxidative degradation (Yuan et al. 2021; Kiejza et al. 2021; Ushani et al. 2020) and biological treatments (Ren et al. 2020; Qachach et al. 2021; Kanaujiya et al. 2019). Those methods may still suffer from low efficiency. Appropriate methodologies of removing contaminants rapidly and cleanly are required to be urgently exploited and utilized. Adsorption has attracted special interest in the treatment of organic contaminants due to its accessibility, high efficiency, and environmental friendliness. Various adsorbents, such as bentonite (Wang et al. 2021a; Shirazi et al. 2020; Kong et al. 2019), clays (Awad et al. 2019; Najafi et al. 2021), activated carbons (Jones et al. 2021; Ouyang et al. 2020), cellulose (Wang 2019; Ren et al. 2018; Somsesta et al. 2020), and biochar (Dai et al. 2019; Jang et al. 2018; Patra et al. 2021), have been synthesized to remove contaminants. Among those adsorbents, high porous adsorbents have attracted more attention for the removal of organic pollutants through non-covalent interactions. Compared with other adsorbents, the porous crosslinked polymers performed well in adsorption, due to high chemical stability, thermal stability, and surface area.
β-cyclodextrin (β-CD) is a cyclic oligosaccharide consisting of seven α-D-glucose units connected through α-(1, 4) linkages with a hydrophobic inner cavity and a hydrophilic exterior which can selectively bind various organic and inorganic species in its cavity. A stable host-guest inclusion complex polyhydroxy structure makes it possible for β-CD to form molecular complexes via interaction with other polymer network by virtue of intermolecular force. β-CD exhibited its great potential for the treatment of environmental organic pollutants. Lots of studies have been carried out on the fabrication of crosslinked β-cyclodextrin polymers for the adsorption of organic contaminants. Those crosslinkers include organic acid (citric acid or oxalic acid or terephthalic acid) (Liang and Zou 2019), epichlorohydrin (Crini 2021), and diisocyanate (Dang et al. 2020). However, polymers formed by these crosslinkers and cyclodextrins are not satisfied in terms of porosity and insolubility, which limits their performance for pollutant adsorption. Therefore, improving the insolubility of cyclodextrin and porosity of polymers has become the crucial point. Alsbaiee et al. (2016) prepared a novel copolymer via tetrafluoroterephthalonitrile (TFTPN)-crosslinked β-CD to remove organic pollutants from water rapidly due to its high surface area and permanent porosity. This material can only bind to organic pollutants through hydrogen bonding and the inclusion of cyclodextrins.
As it is known, CD hydroxyl moieties can be functionalized with phenylcarbamoyl moieties to extend the CD cavity. The introduction of different functional groups can lead to diverse pore structures and functionalities to provide abundant adsorption capabilities. In particular, the introduction of benzene ring makes the polymer easier to combine with aromatic organic pollutants through π-π interaction. Although there have appeared various CD polymer materials by far, there is still no publication to explore the potential of phenylcarbamoylated-β-CD (Ph-β-CD) polymers.
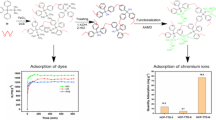
This work synthesized a series of Ph-β-CD derivatives with different electronegative groups, which were thereafter crosslinked with TFTPN to fabricate a series of porous multifunctional cyclodextrin polymers (Fig. 1a). Furthermore, TFTPN is used again to crosslink the cyclodextrin polymer twice. This process connects the nanoparticles to each other again, which changes the pore structure of the polymer and enables different electronegative groups to play a synergistic effect (Fig. 1b). This work verified the adsorption performance of the material and the interaction between the material and the adsorbed pollutants through adsorption experiments on typical organic pollutants, such as tetracycline, ibuprofen, dichlorophenol, norfloxacin, bisphenol A, and naphthol.
Materials and methods
Materials
β-CD (98% purity) was purchased from Heowns (Tianjin, China). 4-chlorophenyl isocyanate (98% purity) and 4-trifluoromethyl phenyl isocyanate (98% purity) were purchased from Macklin (Shanghai, China). 4-nitrophenyl isocyanate (98% purity) was purchased from Aladdin (Shanghai, China). Tetrafluoroterephthalonitrile (TFTPN) (98% purity) was purchased from Dibai (Shanghai, China). Whatman inorganic membrane filter (0.2 μm, PTFE) was purchased from Wishes (Shanghai, China). All other reagents were commercially available and of analytical grade.
Preparation of modified β-CD
First, 4-chlorophenyl isocyanate (0.82 g), 4-nitrophenyl isocyanate (0.88 g), and 4-trifluoromethyl phenyl isocyanate (0.99 g) were, respectively, added in three 100 mL dry twin-neck flasks equipped with magnetic stirring bar. Then, β-CD (2.00 g) and anhydrous pyridine (30 mL) were added. The above solution was kept in an oil bath pan at 80 °C for 18 h. After removing the solvent by reduced pressure distillation, the crude product was obtained. The product was washed with water and acetone repeatedly for further purification and then dried under vacuum for 24 h at 80 °C to obtain three modified β-cyclodextrins.
Crosslinking of modified β-CD using TFTPN+
A dry 20 mL three-necked flask was charged with the modified β-CD (0.20 g), TFTPN (0.10 g), and K2CO3 (0.32 g) equipped with a magnetic stir bar. The flask was flushed with N2 gas for 5 min; then, an anhydrous THF/DMF mixture (9:1 v/v, 8 mL) was added under nitrogen atmosphere. The mixture was placed on a hot stirring plate (85 °C) and stirred at 500 rpm for 48 h.
The orange suspension was cooled and then filtered. To remove unreacted K2CO3, 1-mol·L−1 HCl was slowly added until there is no bubble observed. The product was isolated and then immersed in deionized water (2 × 10 mL) for 15 min, THF (2 × 10 mL) for 30 min, and CH2Cl2 (2 × 15 mL) for 15 min. Finally, the solid was dried under liquid nitrogen conditions (77 K) for 10 min and stranded at room temperature for 2–3 days to obtain three cyclodextrin polymers (CDPs) named Cl-CDP, NO2-CDP, and F-CDP, respectively.
Preparation of mixed functionalized β-cyclodextrin
Cl-CDP, NO2-CDP, and F-CDP were mixed with the same mass (0.1g) and added in a dry 20 mL three-necked flask equipped with a magnetic stir bar. TFTPN (0.10 g) and K2CO3 (0.32 g) were also added at the same time. The flask was flushed with N2 gas for 5 min, and then an anhydrous THF/DMF mixture (9:1 v/v, 8 mL) was added under nitrogen atmosphere. The mixture was placed on a hot stirring plate (85 °C) and stirred at 500 rpm for 48 h. The post-treatment steps are the same as described in the section “Crosslinking of modified β-CD using TFTPN+” to obtain a mixed secondary crosslinked β-CD polymer (X-CDP).
Characterization
FTIR analysis of Cl-CDP, NO2-CDP, F-CDP, and X-CDP was performed at 2 cm−1 resolution on a Bio-Rad FTS3000 IR Spectrum Scanner. Pellets were prepared from the powder samples using KBr. Thermogravimetric analysis (TG) of Cl-CDP, NO2-CDP, F-CDP, and X-CDP powders was tested on a ZTY-ZP thermal analyzer. Samples were heated from room temperature to 700°C at a heating rate of 10°C/min in a nitrogen atmosphere. The external surfaces of Cl-CDP, NO2-CDP, F-CDP, and X-CDP powders were viewed using a Hitachi S-8100 scanning electron microscope. The specific surface area is measured by Autosorb-iQ2-MP. Solid-state13C NMR was performed on a Varian Infinityplus 600 NMR spectrometer (600 MHz, 7.0 T; USA). UV spectrum is detected by MAPADA UV-1800 PC (transmittance accuracy = ±0.2%τ). Stock solutions of six pollutants at 0.005mM, 0.01mM, 0.02mM, 0.05mM, and 0.1mM concentrations are prepared in water. The standard curve is measured before each measurement, and the correlation coefficient can reach more than 0.999. The six pollutants can be quantitatively detected at concentrations below 0.1 mM.
Adsorption experiment
Adsorption kinetic studies were performed in 20 mL test tube. All studies were conducted at 25°C on an incubator shaker with a shaken speed of 120 rpm. The polymers (10 mg) and 0.1-mM pollutant stock solution (10 mL) were added to a 20 mL test tube. The mixture was shaken immediately. Of the suspension, 2 mL aliquots were taken at certain intervals via syringe and immediately filtered by a Whatman 0.2-μm inorganic membrane filter. The residual concentration of the pollutant in each sample was determined by UV-vis spectroscopy. Prepare a 0.5-mM pollutant stock solution, and dilute to obtain a series of pollutant solutions with a concentration of 0.4mM, 0.3mM, 0.2mM, 0.1mM, and 0.05mM, which are used for adsorption isotherm experiments. In the regeneration experiment, 10-mg X-CDP after adsorbing organic pollutants was washed with 5 mL methanol and then dried for the next reuse. The cycle was repeated five times. Each experiment was repeated six times. The mean values and standard deviations were presented.
Results and discussion
Characterization of porous CD and derivatives
Figure 2 shows the SEM micrographs of the surface of composites under the magnification. It was observed that the composites exhibit rough surface and porosity. The surfaces of the four adsorbents were dense and porous with a large number of channels, which is conducive to the interaction between the adsorbent and organic pollutants to improve the adsorption efficiency. Figure 2d further reveals that, through the two-step crosslinking reaction, X-CDP obtains a larger pore size and a better pore structure than the other three adsorbents, which makes the material have better adsorption performance.
The TGA curves for the pyrolysis of β-CD polymer were shown in Fig. 3a. The mass loss of 3–5% at 150 °C was assigned to the evaporation of the water. At 150–260 °C, the TGA curve kept steady, indicating that the β-CD polymer was thermostable at this temperature range. At temperature above 260 °C, there was a mass loss of 30–40%, ascribed to the decomposition of β-CD monomer and TFTPN. Compared with the other three adsorbents, X-CDP lost the least quality at this stage. The re-crosslinking of TFTPN enhanced the thermal stability of the material. At temperature higher than 370 °C, there was still a mass loss of 15–20%, which was caused by the decomposition of the carbon chain. These indicated that the as-synthesized β-CD polymer was thermostable enough for organic pollutant adsorption.
The specific surface area of several different composite materials was measured. SBET of Cl-CDP, NO2-CDP, F-CDP, and X-CDP was 16.863 m2·g−1, 2.125 m2·g−1, 16.532 m2·g−1, and 200.974 m2·g−1, respectively. The obvious increase in the specific surface area of X-CDP indicates that X-CDP is not simply obtained by physical mixing of the first three polymers. During the preparation of X-CDP, the large pores of the polymer were broken, and more small pores appeared. The higher specific surface area makes X-CDP have better adsorption capacity for micro-pollutants.
The FT-IR spectra of TFTPN, β-CD, Cl-CDP, NO2-CDP, F-CDP, and X-CDP are shown in Fig. 3b. For the CDPs, the broad bands and peaks show that the composites contain functional groups of isocyanate and β-CD, which is similar to the previous studies (Li et al. 2018). Particularly, the absorption peak at 3423 cm−1 was ascribed to the –OH groups in β-CD. The absorption at about 1157 cm−1 was from C-O bond stretching in the C-O-H group, and the peaks at 1105 and 1035 cm−1 were ascribed to C-O bond stretching in the C-O-C group of the anhydroglucose ring. The peak at 2924 cm−1 was associated to the stretching vibrations of aliphatic C–H. The peak at 1730 cm−1 is attributed to the vibrational absorption of C=O, which is a characteristic peak of isocyanate modification.
The peaks at 2242 cm−1 and 1626 cm−1, respectively, correspond to the stretching vibration of C≡N and the C-C aromatic extension. Both the TFTPN and the final product composites spectra contained C-F stretching vibration peak at 1477 cm−1. After the cyclodextrins were modified with isocyanate and crosslinked with tetrafluoroterephthalonitrile, the peak intensity was weaker than the reactant material. However, the C-F bond peak can still be clearly observed in the spectrum.
The 13C solid-state NMR spectra of X-CDP are shown in Fig. 3c. The resonance associated with β-CD is exhibited at δ=74 ppm. Resonances at δ=98 and 142 ppm correspond to the newly formed alkoxy groups and aromatic carbons, respectively. Resonances at δ=111 and 124 ppm are related to the two unsubstituted carbons on the benzene ring introduced by isocyanate substitution. This reveals that the cyclodextrin was successfully phenylcarbamoylated and crosslinked by tetrafluoroterephthalonitrile.
The swelling and water retention properties of X-CDP are measured. In many experiments, the polymer can absorb 198% of its own mass of water after soaking for 2 h. Afterwards, the polymer can completely lose water by placing it in an environment of 25°C under normal pressure for 30 min. This shows that X-CDP is a rigid material with good swelling resistance.
The adsorption of organic pollutants
Cl-CDP, NO2-CDP, F-CDP, and X-CDP are used to adsorb the common and commercialized organic pollutants in the field of water purification (Fig. 4). The negative inductive effect of −Cl, −NO2, and −CF3 reduces the electron cloud density on the benzene ring, making it easier for the adsorbent to form hydrogen bonds with pollutants. The pollutants containing benzene ring can be adsorbed by π-π interaction with the benzene ring on the adsorbent. Combined with the inherent inclusion effect of the cyclodextrin cavity, especially non-planar compounds are more easily captured by the cavities of cyclodextrin; the adsorbent exhibits good performance in adsorbing pollutants.
Furthermore, X-CDP has a high adsorption capacity for almost all pollutants due to its porous structure and effect synergy of various functional groups which can form various intermolecular forces and hydrogen bonds. As shown in Fig. 5b, the adsorption effect of X-CDP on a variety of pollutants is stronger than that of the other three polymers. Especially for tetracycline (Fig. 5a), the effect is significantly stronger than that for others, which means that the X-CDP obtained by mixing and crosslinking the three polymers is not only the sum of the effects of the three polymers.
Good performance of compound X-CDP for adsorption of organic micro-pollutants. a Adsorption efficiency of tetracycline by four adsorbents in 30 s. b Percentage removal efficiency of each pollutant obtained by the four adsorbents of NO2-CDP (yellow), Cl-CDP (red), F-CDP (gray), and X-CDP (navy). The error bars represent the standard deviation
Batch adsorption kinetic modeling
As shown in Fig. 6, when equilibrium is reached, different adsorbents have different adsorption effects for the same compound. The characteristic peaks of ibuprofen, dichlorophenol, naphthol, norfloxacin, bisphenol A, and tetracycline are located at 225 nm, 287 nm, 287 nm, 275 nm, 275 nm, and 355 nm, respectively. Obviously, after adsorption, the characteristic peak intensity decreased with different degrees. Those adsorbents have obvious absorption for the above organic substances. X-CDP has the most obvious adsorption effect on various pollutions. The removal rate of norfloxacin and tetracycline by X-CDP in 30 s is over 95%, and the adsorption equilibrium is basically reached within 2 min. It took 10 min for other adsorbents to reach equilibrium and adsorbed only 46% of its equilibrium value in 30 s. The rapid removal rate of pollutants has never been reported before.
The effect of contact time on adsorption of organic contaminants by X-CDP and other adsorbents is shown in Fig. 7a and b. The adsorption of contaminants by X-CDP was high and quick in the initial 2 min due to its porosity. Especially for polycyclic and high molecular weight pollutants, such as tetracycline and norfloxacin, these pollutants are more likely to be quickly included due to the three-dimensional cavity structure of cyclodextrin.
The pseudo-first-order kinetic model and pseudo-second-order model were used to investigate adsorption kinetics. Using the nonlinear form of pseudo-first-order kinetic model (Eq. (1)) and the pseudo-second-order kinetic model (Eq. (2)), the adsorption process was expressed as:
where qt (mg·g−1) is the amount of adsorbed organic contaminants at any time t(min);k1 (min−1) is the first-order rate constant; k2 (mg·g−1·min−1) is the second-order rate constant; and qe (mg·g−1) represents the amount of adsorbed organic contaminants at equilibrium. Kinetic constants of the kinetic models are estimated by the experimental data in Tables 1 and 2. The R2 of the pseudo-first-order kinetic model is higher than that of the pseudo-second-order kinetic model, which shows that the adsorption experiment is more in line with the pseudo-second-order kinetic model. The apparent pseudo-second-order rate constant (k2) of the adsorption of tetracycline by X-CDP is 2.02 g·mg−1·min−1, which is higher than that of F-CDP, Cl-CDP, NO2-CDP, and the other studied adsorbents for tetracycline. The k2 values reported in the literature are listed in Table 3. X-CDP’s superior k2 for adsorbing organic contaminants indicates that nearly all of its β-CD binding sites are readily accessible, and the mount of binding sites is higher than many adsorbents.
Batch adsorption isotherm modeling
Adsorption isotherm is important for determining the adsorption behavior of an adsorbent. In order to better investigate the adsorption mechanisms, the Langmuir model (Eq. (3)) and the Freundlich model (Eq. (4)) were applied to the experimental data using the following equations:
where qe (mg·g−1) is the adsorption capacity at equilibrium; qmax (mg·g−1) is the maximum adsorption capacity; ce (mg·L−1) is the equilibrium concentration in the solution; and kL (L·mg−1) is a constant related to the affinity between an adsorbent and adsorbate. kF mg·g−1·(mg·L−1)−n is the Freundlich constant, and n(dimensionless) is the Freundlich intensity parameter, which indicates the magnitude of the adsorption driving force or the surface heterogeneity. The data of qe−ce, the Langmuir model data, and the Freundlich model data of the four adsorbents for tetracycline were shown in Fig. 8 and Table 4.
According to the equilibrium adsorption value and the concentration of tetracycline after adsorption, the Langmuir model was fitter than the Freundlich model, which means the adsorption process accorded with Langmuir model. The adsorption process is monolayer adsorption, and there is no interaction between adsorbates. Furthermore, the maximum adsorption capacity of X-CDP at equilibrium was found to be 110.56 mg·g−1; the qmax value for X-CDP was competitive with the adsorption capacities of tetracycline of other adsorbents, such as chitosan powder (23.92 mg/g) (Huang et al. 2011), Fe-HAP (45 mg/g) (Li et al. 2017), and BM-biochar (85 mg/g) (Xiang et al. 2020).
The adsorption-desorption cycles of X-CDP
An ideal adsorbent should have high adsorption ability as well as excellent desorption performance, which reduce the operating cost for the adsorbent application. As is shown in Fig. 9, the efficiency (%) was the ratio of the weight of absorbed (or desorbed) tetracycline at any time. Thus, the adsorption capacity of the desorbed X-CDP was examined by using methanol as eluent, and the regenerated X-CDP was reused in tetracycline solution with known concentration. From Fig. 9, the removal rate of tetracycline is still 86.7%, which is only 8.3% lower than the first adsorption after five cycles. This reduction might be ascribed to the loss of binding sites after each desorption procedure. These results demonstrated that the recovery efficiency of X-CDP was relatively high with slightly affected by the five consecutive regeneration cycles and X-CDP showed excellent re-adsorption effect.
Conclusions
In summary, four new adsorbent materials were prepared by crosslinking a series of modified beta-cyclodextrin with the rigid aromatic compound TFTPN. Both the crosslinking agent TFTPN and the polymerized monomer phenylcarbamoylated-β-cyclodextrin contain a large amount of benzene rings, which makes the polymer easy to combine with aromatic organic pollutants through π-π interaction. The electron-withdrawing effects of chlorine, trifluoromethyl, and nitro further strengthen the interaction between the two. The large number of hydroxyl groups in cyclodextrin and the carbonyl group brought about by isocyanate modification enables the polymer to bond with pollutants through hydrogen bonds. Its three-dimensional cavity is easier to adsorb non-planar compounds. The adsorbent has the superior performance for adsorption of micro-pollutants. Each adsorbent can quickly reach the adsorption equilibrium, especially for X-CDP; it reached almost 95% of its equilibrium in 30 s. In the experiment of adsorption of tetracycline, the rate constant reached 2.02 g·mg−1·min−1, and the maximum adsorption capacity of the mixed adsorbent was more significant, reaching 110.56 mg·g−1 at equilibrium. The characteristics of rapid adsorption, large adsorption capacity, easy separation, and ability to adsorb a variety of pollutants make X-CDP have great potentials to be used in the removal of a broad-spectrum of organic micro-pollutants from water.
Data availability
The data used in this research are available by the corresponding author upon reasonable request.
References
Alsbaiee A, Smith BJ, Xiao LL, Ling YH, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 529(7585):190–U146. https://doi.org/10.1038/nature16185
Alvarez-Torrellas S, Rodriguez A, Ovejero G, Garcia J (2016) Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem Eng J 283:936–947. https://doi.org/10.1016/j.cej.2015.08.023
Awad AM, Shaikh SMR, Jalab R, Gulied MH, Nasser MS, Benamor A, Adham S (2019) Adsorption of organic pollutants by natural and modified clays: a comprehensive review. Sep Purif Technol 228:115719. https://doi.org/10.1016/j.seppur.2019.115719
Crini G (2021)Cyclodextrin-epichlorohydrin polymers synthesis, characterization and applications to wastewater treatment: a review. Environ Chem Lett 19(3):2383–2403. https://doi.org/10.1007/s10311-021-01204-z
Dai YJ, Zhang NX, Xing CM, Cui QX, Sun QY (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223:12–27. https://doi.org/10.1016/j.chemosphere.2019.01.161
Dang C, Jiranek V, Taylor DK, Wilkinson KL (2020) Removal of volatile phenols from wine using crosslinked cyclodextrin polymers. Molecules 25(4):910. https://doi.org/10.3390/molecules25040910
de Lima HHC, Llop MEG, Maniezzo RD, Moises MP, Janeiro V, Arroyo PA, Guilherme MR, Rinaldi AW (2021) Enhanced removal of bisphenol A using pine-fruit shell-derived hydrochars: adsorption mechanisms and reusability. J Hazard Mater 416:126167. https://doi.org/10.1016/j.jhazmat.2021.126167
Essandoh M, Kunwar B, Pittman CU, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 365:219–227. https://doi.org/10.1016/j.cej.2014.12.006
Fang X, Wu SB, Wu YH, Yang W, Li YL, He JH, Hong PD, Nie MX, Chao X, Wu ZJ, Zhang KS, Kong LT, Liu JH (2020)High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: dynamics, thermodynamics, and mechanisms. Appl Surf Sci 518:146226. https://doi.org/10.1016/j.apsusc.2020.146226
Guo ZP, Yang FJ, Yang RG, Sun L, Li Y, Xu JZ (2021) Preparation of novel ZnO-NP@Zn-MOF-74 composites for simultaneous removal of copper and tetracycline from aqueous solution. Sep Purif Technol 274:118949. https://doi.org/10.1016/j.seppur.2021.118949
Huang LH, Sun YY, Wang WL, Yue QY, Yang T (2011) Comparative study on characterization of activated carbons prepared by microwave and conventional heating methods and application in removal of oxytetracycline (OTC). Chemical Engineering Journal 171(3):1446–1453. https://doi.org/10.1016/j.cej.2011.05.041
Jang HM, Yoo S, Choi YK, Park S, Kan E (2018) Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresource Technol 259:24–31. https://doi.org/10.1016/j.biortech.2018.03.013
Jones I, Zhu MM, Zhang J, Zhang ZZ, Preciado-Hernandez J, Gao J, Zhang DK (2021) The application of spent tyre activated carbons as low-cost environmental pollution adsorbents: a technical review. J Clean Prod 312:127566. https://doi.org/10.1016/j.jclepro.2021.127566
Kanaujiya DK, Paul T, Sinharoy A, Pakshirajan K (2019) Biological treatment processes for the removal of organic micropollutants from wastewater: a review. Curr Pollut Rep 5(3):112–128. https://doi.org/10.1007/s40726-019-00110-x
Kiejza D, Kotowska U, Polinska W, Karpinska J (2021) Peracids - new oxidants in advanced oxidation processes: the use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern - a review. Sci Total Environ 790:148195. https://doi.org/10.1016/j.scitotenv.2021.148195
Kong Y, Wang L, Ge YY, Su HY, Li ZL (2019) Lignin xanthate resin-bentonite clay composite as a highly effective and low-cost adsorbent for the removal of doxycycline hydrochloride antibiotic and mercury ions in water. J Hazard Mater 368:33–41. https://doi.org/10.1016/j.jhazmat.2019.01.026
Li L, Wang S, Zhang Y, Han R, Wei W (2017) Enhanced tetracycline adsorption onto hydroxyapatite by Fe (III) incorporation. J Mol Liq 247:171–181. https://doi.org/10.1016/j.molliq.2017.09.110
Li XX, Li J, Kang Q, Wang Y (2018) Polarity tuned perphenylcarbamoylated cyclodextrin separation materials for achiral and chiral differentiation. Talanta 185:328–334. https://doi.org/10.1016/j.talanta.2018.03.065
Liang H, Zou CJ (2019) Adsorption of naphthenic acids from oil sand process-affected water with water-insoluble poly(beta-cyclodextrin-citric acid). Can J Chem Eng 97(6):1894–1902. https://doi.org/10.1002/cjce.23452
Najafi H, Farajfaed S, Zolgharnian S, Mirak SHM, Asasian-Kolur N, Sharifian S (2021) A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf Environ 147:8–36. https://doi.org/10.1016/j.psep.2021.09.028
Ouyang JB, Zhou LM, Liu ZR, Heng JYY, Chen WQ (2020)Biomass-derived activated carbons for the removal of pharmaceutical micropollutants from wastewater: a review. Sep Purif Technol 253:117536. https://doi.org/10.1016/j.seppur.2020.117536
Patra BR, Mukherjee A, Nanda S, Dalai AK (2021) Biochar production, activation and adsorptive applications: a review. Environ Chem Lett 19(3):2237–2259. https://doi.org/10.1007/s10311-020-01165-9
Qachach H, Abriak N, El Mahrad B, Souabi S, Tahiri M (2021) Biological treatment of fuel wastewater generated from a thermal power plant by continuous and discontinuous aeration. Desalin Water Treat 222:145–155. https://doi.org/10.5004/dwt.2021.27120
Ren WJ, Gao JK, Lei C, Xie YB, Cai YR, Ni QQ, Yao JM (2018) Recyclable metal-organicframework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants. Chem Eng J 349:766–774. https://doi.org/10.1016/j.cej.2018.05.143
Ren RY, Yang LH, Han JL, Cheng HY, Ajibade FO, Guadie A, Wang HC, Liu B, Wang AJ (2020) Perylene pigment wastewater treatment by fenton-enhanced biological process. Environ Res 186:109522. https://doi.org/10.1016/j.envres.2020.109522
Saghir S, Xiao ZG (2021) Facile preparation of metal-organic frameworks-8 (ZIF-8) and its simultaneous adsorption of tetracycline (TC) and minocycline (MC) from aqueous solutions. Mater Res Bull 141:111372. https://doi.org/10.1016/j.materresbull.2021.111372
Shah J, Jan MR, Zeeshan M, Iqbal M (2016) Solid phase extraction and removal of 2,4-dichlorophenol from aqueous samples using magnetic graphene nanocomposite. Sep Sci Technol 51(9):1480–1489. https://doi.org/10.1016/j.materresbull.2021.111372
Shirazi EK, Metzger JW, Fischer K, Hassani AH (2020) Removal of textile dyes from single and binary component systems by Persian bentonite and a mixed adsorbent of bentonite/charred dolomite. Colloid Surface A 598:124807. https://doi.org/10.1016/j.colsurfa.2020.124807
Somsesta N, Sricharoenchaikul V, Aht-Ong D (2020) Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: equilibrium and kinetic studies. Mater Chem Phys 240:122221. https://doi.org/10.1016/j.matchemphys.2019.122221
Thaveemas P, Chuenchom L, Kaowphong S, Techasakul S, Saparpakorn P, Dechtrirat D (2021) Magnetic carbon nanofiber composite adsorbent through green in-situ conversion of bacterial cellulose for highly efficient removal of bisphenol A. Bioresource Technol 333:125184. https://doi.org/10.1016/j.biortech.2021.125184
Ushani U, Lu XQ, Wang JH, Zhang ZY, Dai JJ, Tan YJ, Wang SS, Li WJ, Niu CX, Cai T, Wang N, Zhen GY (2020) Sulfate radicals-based advanced oxidation technology in various environmental remediation: a state-of-the-art review. Chem Eng J 402:126232. https://doi.org/10.1016/j.cej.2020.126232
Wang D (2019) A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 26(2):687–701. https://doi.org/10.1007/s10570-018-2143-2
Wang JH, Lei SL, Liang LQ (2020) Preparation of porous activated carbon from semi-coke by high temperature activation with KOH for the high-efficiency adsorption of aqueous tetracycline. Appl Surf Sci 530:147187. https://doi.org/10.1016/j.apsusc.2020.147187
Wang ZK, Muhammad Y, Tang R, Lu CM, Yu SS, Song RR, Tong ZF, Han BA, Zhang HB (2021a) Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacin. Sep Purif Technol 274:119059. https://doi.org/10.1016/j.seppur.2021.119059
Wang ZB, Ren DJ, Yu HY, Jiang S, Cheng YH, Zhang SQ, Zhang XQ (2021b) Adsorption kinetic and isothermal studies of 2,4-dichlorophenol from aqueous solutions with zeolitic imidazolate framework-8 (ZIF-8). Environ Eng Sci 38(6):537–546. https://doi.org/10.1089/ees.2020.0286
Xiang W, Wan YS, Zhang XY, Tan ZZ, Xia TT, Zheng YL, Gao B (2020) Adsorption of tetracycline hydrochloride onto ball-milled biochar: governing factors and mechanisms. Chemosphere 255:127057. https://doi.org/10.1016/j.chemosphere.2020.127057
Yan XL, Hu XY, Chen T, Zhang SY, Zhou M (2017) Adsorptive removal of 1-naphthol from water with zeolitic imidazolate framework-67. J Phys Chem Solids 107:50–54. https://doi.org/10.1016/j.jpcs.2017.03.024
Yang WB, Lu YP, Zheng FF, Xue XX, Li N, Liu DM (2012) Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem Eng J 179:112–118. https://doi.org/10.1016/j.cej.2011.10.068
Yuan DL, Yang K, Pan SY, Xiang Y, Tang SF, Huang LT, Sun MT, Zhang XY, Jiao TF, Zhang QR (2021) Peracetic acid enhanced electrochemical advanced oxidation for organic pollutant elimination. Sep Purif Technol 276:119317. https://doi.org/10.1016/j.seppur.2021.119317
Zuo LM, Yu SM, Cheng LL, Du EL (2013) Adsorption of phenol and 1-naphthol onto XC-72 carbon. Korean J Chem Eng 30(3):714–723. https://doi.org/10.1007/s11814-012-0194-x
Funding
This work was financially funded by the National Natural Science Foundation of China (No. 21922409) and Tianjin Research Program of Application Foundation and Advanced Technology (17JCYBJC20500).
Author information
Authors and Affiliations
Contributions
Yong Wang received support from the funding. Yong Wang and Xiaofei Ma conceived and designed the study. The participation of He Wang and Congzhi Liu includes data collection and analyzing the results. Congzhi Liu, He Wang, Xiaofei Ma, and Yong Wang interpreted and wrote the manuscript.
Corresponding authors
Ethics declarations
The authors confirm that this article is original research and has not been published or presented previously in any journal or conference in any language (in whole or in part).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 700 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Liu, C., Ma, X. et al. Porous multifunctional phenylcarbamoylated-β-cyclodextrin polymers for rapid removal of aromatic organic pollutants. Environ Sci Pollut Res 29, 13893–13904 (2022). https://doi.org/10.1007/s11356-021-16656-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16656-7