Abstract
Isolation and climate have protected Southern Ocean Islands from non-native species. Relatively recent introductions have had wide-ranging, sometimes devastating, impacts across a range of species and ecosystems, including invertebrates, which are the main terrestrial fauna. In our comprehensive review, we found that despite the high abundance of non-native plants across the region, their impacts on native invertebrates are not well-studied and remain largely unknown. We highlight that non-native invertebrates are numerous and continue to arrive. Their impacts are multi-directional, including changing nutrient cycling regimes, establishing new functional guilds, out-competing native species, and mutually assisting spread of other non-native species. Non-native herbivorous and omnivorous vertebrates have caused declines in invertebrate habitat, but data that quantifies implications for invertebrates are rare. Predatory mammals not only indirectly effect invertebrates through predation of ecosystem engineers such as seabirds, but also directly shape community assemblages through invertebrate diet preferences and size-selective feeding. We found that research bias is not only skewed towards investigating impacts of mice, but is also focused more intensely on some islands, such as Marion Island, and towards some taxa, such as beetles and moths. The results of our review support and build on previous assessments of non-native species in the Antarctic region—that the responses of invertebrate fauna on these islands are under-reported and often poorly understood. Given the importance of invertebrates as indicators of environmental change, and their potential utility in quantifying change associated with island restoration projects (such as eradications), these knowledge gaps need to be urgently addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species are the greatest driver of global biodiversity loss and ecosystem disruption on islands (Mack et al. 2000; McCreless et al. 2016). Although islands comprise only 5% of the world’s landmass, their level of endemism is a magnitude higher than for continents, and many global biodiversity hotspots are islands and archipelagos (Kier et al. 2009; Bellard et al. 2014; Courchamp et al. 2014). Thus, island ecosystems have proportionally high biodiversity, and often host näive indigenous species that lack competitive traits and are highly adapted (Bowen and van Vuren 1997; Convey et al. 2006a; Smith 2007).
Sub-Antarctic and cool-temperate islands of the Southern Ocean are some of the most remote environments in the world, yet their ecosystems are susceptible to invasion by non-native species (Frenot et al. 2005; Convey et al. 2006b; Shaw 2013; McGeoch et al. 2015). Low temperature, remoteness and associated low human visitation to these islands have historically been an important limiting factor in the establishment of non-native species (Smith and Steenkamp 1990; Chown et al. 1998, 2005; Gabriel et al. 2001). However, increasing human visitation has intensified opportunities for new introductions (Whinam et al. 2005; Convey et al. 2006b; Chown et al. 2012), and relatively recent climatic changes are likely to further reduce physical barriers to invasion and enhance colonisation success (Walther et al. 2009; Janion et al. 2010; Chown and Convey 2016; Laparie and Renault 2016). The relatively simple ecosystems found on Southern Ocean Islands (SOI) provide a unique opportunity to understand the processes of colonisation, establishment and impacts of non-native species.
Most of the 560 non-native species documented for the Antarctic and Southern Ocean region are established on SOI (McGeoch et al. 2015). The majority are plants and invertebrates inadvertently introduced through human activity (Frenot et al. 2005). Although some non-native invertebrates and plant species have been studied individually (e.g. Convey et al. 2010; Laparie et al. 2010; Williams et al. 2016), their broader ecosystem impacts remain largely unknown. Vertebrates such as house mice (Mus musculus), black rats (Rattus rattus) and brown rats (Rattus norvegicus) were introduced unintentionally, but others such as rabbits (Oryctolagus cuniculus), cats (Felis catus), sheep (Ovis aries), mouflon (Ovis ammon musiman), cattle (Bos Taurus), goats (Capra hircus), pigs (Sus Scrofa), reindeer (Rangifer tarandus), weka (Gallirallus australis), and trout (Salmo trutta) were purposefully released as companions, food or game (Headland 2012; McGeoch et al. 2015). As has occurred globally, non-native mammals have transformed SOI ecosystems through habitat destruction, causing extinctions and altering ecosystem processes (e.g. Courchamp et al.2003; Campbell and Donlan 2005; Frenot et al. 2005; Wanless et al. 2007; Jones et al. 2008; Nogales et al. 2013; McGeoch et al. 2015; McCreless et al. 2016).
The impact of species invasions on islands has typically been determined through monitoring the responses of iconic or charismatic species, like albatrosses (Towns et al. 2006; Towns 2009; Angel et al. 2009; St. Clair 2011; Jones et al. 2016). On SOI, this translates to extensive research on the impacts of non-native vertebrates on seabirds and vegetation (e.g. Copson and Whinam 1998; Cuthbert and Hilton 2004; Scott and Kirkpatrick 2008). Impacts on invertebrates have been less comprehensively studied. This is despite the fact that invertebrates comprise most of the terrestrial fauna on SOI and perform a variety of critical ecosystem functions such as soil nutrient cycling (Convey et al. 2006a; Smith 2008; Chown and Convey 2016). In consequence, their suppression or extinction by invasive species has a range of important implications (Fukami et al. 2006; St Clair 2011; Collen et al. 2012). Furthermore, because of their diversity, invertebrates are important biological indicators of environmental change, and can be useful for conservation planning and monitoring (Kremen et al. 1993; Gerlach et al. 2013). Comprehensive understanding of the interactions between invasive species and invertebrates is therefore critical for future SOI conservation and management (Chown et al. 2008). Non-native vertebrates, invertebrates and plants threaten native SOI invertebrates through predation, competition, and loss of habitat, but our understanding of these interactions is limited compared to other threatened taxa (McGeoch et al. 2015). Here we review the current state of knowledge of non-native species interactions with, and impacts on, native invertebrates on SOI and discuss the consequences and ramifications for these ecosystems.
Terminology
Greenslade and Convey (2012) outline terminology around invasive species that we follow here, including for the terms ‘invasive’, ‘introduced’, ‘exotic’, ‘naturalised’, ‘native’, and ‘endemic’. Taxa that we refer to as ‘non-native’ are synonymous with ‘introduced’ in Greenslade and Convey (2012), and are those which have clearly been transported to a novel locality, directly or indirectly, by human activities. Taxa that we refer to as ‘invasive’ are those that have followed the invasion pathway, i.e. they been introduced to a new location, colonised, reproduced and spread, causing disruption to the pre-existing ecosystem (Williamson and Fitter 1996).
The study area
Southern Ocean Islands
Southern Ocean Islands (SOI) comprise a series of relatively small, isolated, oceanic islands and archipelagos located on either side of the Antarctic Polar Frontal Zone (APFZ) between 37°S and 55°S (Fig. 1) (Shaw 2013). They are known for their cool, wet, windy but equable climates (Bergstrom and Chown 1999; Huiskes et al. 2006; Pendlebury and Barnes-Keoghan 2007). Almost all are designated as protected areas (Shaw 2013). Despite similarities in their contemporary climate, SOI have significantly different climatic histories, glaciations, geological origins and ages that have influenced natural (ie not mediated by humans) colonisation by terrestrial biota, speciation, and species turnover (Bergstrom and Chown 1999; Shaw et al. 2010; Shaw 2013). Most SOI have no resident human settlements (except for the Falkland Islands and Tristan da Cunha), but some island groups are visited by tourists, and most are either regularly visited by research expeditions or have permanent research stations (de Villiers et al. 2006).
Ecosystems
A significant component of the fauna of Southern Ocean Islands are the millions of marine mammals and seabirds, across numerous species, which live and breed on them (Shirahai 2007). There are no native amphibians, reptiles or terrestrial mammals, and very few land-based birds (Bergstrom and Chown 1999; Convey 2007). Compared to continental ecosystems, terrestrial ecosystems on SOI are relatively simple, characterised by non-utilized ecological resources and unrepresented functional groups (Convey et al. 2006a; Whinam et al. 2005). Although levels of endemism are high (Chown and Convey 2016), species diversity is generally low, and many taxonomic and functional groups typically found at lower latitudes are absent (Block 1984; Convey and Lebouvier 2009). A lack of functional redundancy is linked to a higher likelihood of establishment by exotic species, given that new arrivals may experience little competitive resistance (Frenot et al. 2005; Convey et al. 2006b). Low temperatures and numerous, but relatively inefficient, invertebrate detritivores mean that organic decay is generally slow (Tréhen et al. 1990; Smith 2008), resulting in ‘bottlenecks’ in available nutrients (Slabber and Chown 2002). Across the region, vegetation communities are dominated by grasslands, herbfields (including megaherbs) and are characteristically devoid of trees. High altitude areas support feldmark communities populated by cushion plants and bryophytes (Greenslade 2006; Convey 2007; Bergstrom et al. 2006). The absence of native herbivores means that vegetation is generally vulnerable to grazing by non-native herbivorous mammals due to a lack of defences and high palatability (Bowen and van Vuren 1997; Courchamp et al. 1999; Chapuis et al. 2004; Hullé 2012).
Invertebrate assemblages are characterised by few herbivores or predators and a high number of decomposers (Smith and Steenkamp 1990; Vernon et al. 1998). Typically, assemblages feature adversity or stress selection traits (Crafford et al. 1986; Convey 1996a, b, 1997; Chown 2001) such as low reproductive investment, limited competitive and dispersal abilities, investment in stress tolerance (Chown and Convey 2016) and unusually long life cycles (Haupt et al. 2014). The most abundant native invertebrate groups are mites (Acarina) and springtails (Collembola) (Chown and Convey 2016). Flies (Diptera) and beetles (Coleoptera) are the most common insects in the region (Chown and Convey 2016). Other abundant native groups are Araneae (spiders), Lepidoptera (moths), enchytraeids, earthworms, tardigrades, and nematodes (Convey 2007; Chown and Convey 2016). Flightlessness is unusually common (Roff 1990). Given there are very few large, native predators of invertebrates on SOI, the ecology of native invertebrate communities is unlikely to include adaptations to predation pressure (Convey and Lebouvier 2009).
Biogeography
Consistent low levels of immigration from nearby continents have shaped the terrestrial environments of older SOI with continental origins (Chown et al. 1998; Bergstrom and Chown 1999), as per classic island biogeography theory (MacArthur and Wilson 1967). Informative examples are the Falkland Islands and Auckland Island group. For the majority of SOI, which are typically more isolated, younger, volcanic islands, the origin of most biota remains largely unknown (Bergstrom and Chown 1999). Natural colonisation and dispersal in the region occurs broadly via wind, air, water or with assistance from vectors such as animals, birds or marine debris (Gressitt 1970; Barnes et al. 2006; Hughes et al. 2006; Moon et al. 2017). Native insect and vascular plant species richness are linked, but principally explained by island isolation and temperature (Chown et al. 1998; Leihy et al. 2018). Native vascular plant species richness varies widely across SOI, ranging from 180 species on Auckland Island and one species on Bounty Island, but some inter-regional similarities are apparent (Leihy et al. 2018). Native insect assemblages are more similar between island groups close to each other (Greve et al. 2005; Shaw et al. 2010; Leihy et al. 2018), but richness is highly variable across the region—with as few as six species on MacDonald Island to over 230 on Auckland Island (Chown et al. 1998; Bergstrom and Chown 1999; Chown and Convey 2016). For SOI invertebrates other than insects (e.g. springtails, mites, and spiders), drivers of diversity and distribution are less well known, largely due to imbalanced survey effort and lack of data for many island groups (Chown et al. 1998, 2008). Repeated surveys of some islands reveal incremental increases in diversity over time, likely due to increased search effort in new habitats and documentation of more cryptic species (e.g. Jones et al. 2003c; Green and Mound 1994; Grobler et al. 2011a, b). Non-native species invertebrate richness on SOI is strongly correlated with native species richness, energy availability, island temperature and area, and the frequency of human visitation (Chown et al. 1998, 2005).
Climate and invasive species
Climate change is occurring across the region (Pendlebury and Barnes-Keoghan 2007; Le Roux 2008; Bergstrom et al. 2015). At some locations, warming is occurring rapidly, at more than twice the mean global rate (Le Roux 2008). Warming increases the likelihood of non-native species establishment (Gabriel et al. 2001; Janion et al. 2010; Chown and Convey 2016; Laparie and Renault 2016), while also putting pressure on native species (van der Merwe et al. 1997). Duffy et al. (2017) modelled the future climatic suitability for some of the world’s most invasive species to SOI and the Antarctic region. They found all SOI suitable and at invasion risk under future climate scenarios, particularly Macquarie Island and the New Zealand sub-Antarctic islands. Many non-native species are generally more adaptable (Duffy et al. 2017) and have broader climatic tolerance (Chown et al. 2002; Janion et al. 2010), than native species that are cool-climate adapted and vulnerable to increasing thermal conditions (Convey 1996a, b, 1997; van der Merwe et al. 1997). Even if invertebrates that are non-native to SOI originate from a cool region, their competitive advantage in a warming climate is amplified (Lebouvier et al. 2011; Laparie and Renault 2016). Thus, native invertebrate species on SOI are likely to be disadvantaged and outcompeted with rapid climate change (Chown et al. 2004; McGeoch et al. 2006; Duffy et al. 2017).
Concurrently, accidental transport of non-native species has increased as human activity in the region has escalated (Frenot et al. 2005; Convey et al. 2006b; Hughes et al. 2006; Chown et al. 2012; McGeoch et al. 2015; Duffy et al. 2017). Already for some SOI, the rate of natural colonisations has been surpassed by human-facilitated introductions. On Gough Island, the rate of non-native invertebrate establishment is 2–3 orders of magnitude in excess of the natural rate (Gaston et al. 2003), and for Iles Crozet and Kerguelen 3–4 orders of magnitude for plant and invertebrate species respectively (Frenot et al. 2001, 2008; Lebouvier and Frenot 2007).
Literature search
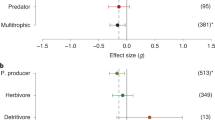
Our primary objective was to determine how invasions on SOI impact native invertebrates. Therefore, we conducted a literature search in Web of Science using the terms “invertebrate*”, “insect*”, “non-native”, “impact” and “invasive” and three groups of invertebrates widespread on SOI “Diptera”, “Coleoptera” and “Lepidoptera”, searching each term individually but paired with each of one of the 32 named Southern Ocean Islands (between 37°S and 55°S) (e.g. “Campbell Island”, “Macquarie Island”, “Prince Edward Island” etc), adding “Antarctic” to focus the search where necessary (e.g. where multiple islands of the same name exist). The reference lists of all relevant publications were further examined to identify other relevant publications. The titles and abstracts of these papers were viewed and 45 publications were identified that measure, through experiment or observation, impacts of invasive species on invertebrates on SOI. By island group, the break down was: Marion Island (18—including three also investigating Prince Edward Islands), South Georgia (8), the Kerguelen archipelago (6), Antipodes Island and Bollons Islands (4), Auckland Island (3), Macquarie Island (3), Gough Island (2), and Falkland Islands (1). Impacts were identified for taxa from 11 higher groups of invertebrates. Of the papers examined the most studied taxa were: Coleoptera (21 times), Lepidoptera (19), and Annelidae (10), followed by Araneae (9), Diptera (7), Amphipoda (4), Collembola (4), Hemiptera (3), Orthoptera (1), Mollusca (1) and Chilopoda (1). Many studies did not specifically identify which invertebrate groups were impacted, rather discussing indirect effects through changes to vegetation or soil habitat. In these cases, the impact group was identified as ‘Unspecified’. Twenty studies identified impacts on invertebrates from predators (14 of these relating to mice alone, one on mice and rats, two on mice and cats, and four focussing on either, or both, brown and black rats), 17 invasive invertebrate papers, three invasive plant papers, two invasive omnivore papers, and one study which explicitly tested the impacts of herbivores on invertebrate communities. A summary of these papers is presented in Table 1 in Supplementary Material. This table underpins our review of invasive species impacts on native invertebrates on SOI.
Non-native species impacts on native invertebrates
Non-native plants
More than 250 non-native plant species, mostly grasses and small herbs, have established across the SOI (Shaw 2013). Despite the relatively high number and diversity of non-native plants across the region, making up a substantial component of vascular plant species and altering habitat (Frenot et al. 2001; Jones et al. 2003b; Le Roux et al. 2013), their impacts are generally considered to be relatively minor (e.g. Frenot et al. 2005). This assumption may well be a product of limited investigation (Le Roux et al. 2013) as very few studies have investigated SOI invertebrate and non-native plant interactions (Table 1 in Supplementary Material).
There is compelling evidence that habitat quality and composition strongly influence invertebrate assemblages and species richness on SOI (Crafford and Scholtz 1987; Davies and Melbourne 1999; Barendse et al. 2002; Terauds et al. 2011; Errington et al. 2018). Often, specific plants or communities provide habitat and food for native SOI invertebrates (e.g. Hugo et al. 2004, 2006; Nyakatya and McGeoch 2008; Phiri et al. 2009; Greenslade et al. 2011). Some endemic plants are particularly important—for example Azorella selago cushions act as climatically benign, resource-rich refuges and support diverse invertebrate communities (Barendse and Chown 2001; Hugo et al. 2004). Habitat specificity by invertebrate fauna on SOI is identified by some studies (e.g. earthworms and flies—Tréhen et al. 1985; weevils and spiders—Davies 1973; Davies et al. 2011). Others show that many arthropod taxa demonstrate broad habitat tolerances (Burger 1985; Gressitt 1971; Convey et al. 1999; Hänel and Chown 1998; Greenslade 2006).
The effect of non-native plants on insect assemblages varies among taxa (Gremmen et al. 1998 and references therein), but they can greatly reduce local invertebrate diversity (Gremmen et al. 1998, 2001). Gremmen et al. (1998) described impacts on both overall insect species composition and individual species population densities of micro-invertebrates and soil macroinvertebrates due to the increasingly dominant non-native grass Agrostis stolonifera on Marion Island. They showed that up to 30% of native invertebrate species were absent from drainage areas dominated by A. stolonifera, and that enchytraeid worm biomass declined in these areas. Only one other study (Chown and Block 1997) tested and demonstrated detrimental effects of non-native plant species on invertebrates on SOI. This study demonstrated that the poorer nutrition absorption potential of the invasive Poa annua compared to native grasses affected the foraging dynamics of the native herbivorous beetle Hydromedion sparsutum on South Georgia, with implications for its body size and fitness. P. annua is the most widespread weed in the region (McGeoch et al. 2015). Despite the paucity of published data on the effect of P. annua on invertebrates from other SOI, detrimental effects on invertebrate taxa or communities are expected, especially where disturbance and grazing pressure by mammalian herbivores has encouraged spread of the grass (eg Macquarie Island, Scott and Kirkpatrick 2008, 2013).
The extent of impacts due to non-native plants on SOI may take some time to be realised due to complicated interactions with native and non-native plants and invertebrates, and associated lag times. For example, mutually beneficial relationships can form between non-native plants and non-native invertebrates, including mutually-assisted dispersal (Barnes et al. 2006). Very few native plants on SOI are insect-pollinated—a consequence of the lack of native pollinating insect fauna (Convey et al. 2006a) and reflected in plant floral structures (Shrestha et al. 2016). Thus, the establishment of pollinating insects such as the hoverfly Eristalis croceimaculata and the blowfly Calliphora vicina on South Georgia, represent a novel ecological guild, aiding the seed set and dispersal of currently-localised non-native plants such as dandelions (Taraxacum officinale) that require pollination (Convey et al. 2010). Moreover, the dandelions themselves are likely to have facilitated the spread of these pollinating flies (Convey et al. 2010). Several other non-native plants on South Georgia have, until recently, also lacked suitable pollinators (Barnes et al. 2006). In time, native plants on SOI may be outcompeted by non-native insect-pollinated plants (Frenot et al. 2005, 2008) as new species of insect pollinators become established (Convey et al. 2010). Invertebrate communities on SOI reliant on native vegetation could be impacted, but this is yet to be tested.
Non-native invertebrates
Non-native invertebrates on the SOI include flatworms, earthworms, moths, terrestrial crustaceans, predatory carabid beetles, parasitic wasps, slugs, isopods, spiders, booklouse, flies, aphids, springtails, mites and more (Frenot et al. 2005). The non-native insects total more than 180 species across the region (McGeoch et al. 2015). Most species are from families that include well-documented pest species worldwide—e.g. Thripidae, Aphididae, Noctuidae and Calliphoridae (Annecke and Moran 1982; Roques et al. 2009). However, the composition of non-native species can vary between islands—for example, non-native springtails comprise 38% of the introduced fauna on Marion, compared to 10% on South Georgia (Frenot et al. 2005). On some islands, non-native invertebrates represent the majority of terrestrial species—60% on the Kerguelen archipelago (Frenot et al. 2005), and 70% on Gough Island (Jones et al. 2003b).
A small number of introduced invertebrates are considered invasive on SOI, with the majority regarded as ‘persistent’ (i.e. not expanding their range) and/or synanthropic (i.e. occurring in and/around research stations alongside humans) (Frenot et al. 2005; Greenslade 2006; Convey 2011). Some have naturalised with little apparent impact (Frenot et al. 2005), although this may change over time due to potential changes in temperature, nutrient availability and water availability (Crooks et al. 1999; Nielsen and Wall 2013). Non-native invertebrates can change status from transient, persistent or synanthropic to invasive if environmental circumstances change in their favour (Chown and Avenant 1992; Chown and Language 1994; Bergstrom and Chown 1999; Frenot et al. 2005; Lebouvier et al. 2011). For example on Marion Island, the establishment of the diamond-back moth, Plutella xylostella, a globally significant crucifer crop pest (Crafford and Chown 1990), is thought due to recent warming in the region (Chown and Avenant 1992). Similarly, the blowfly Calliphora vicina, repeatedly arrived but did not establish at Kerguelen Island until a temperature threshold was reached in the early 1970s, facilitating the completion of its life cycle, followed by a rapid increase in range (Lebouvier et al. 2011). C. vicina and the hoverfly Eristalis croceimaculata larvae are macrodetrivores. Their activity intensifies nutrient cycling naturally performed by microarthropod soil fauna and native insects, thereby altering soil nutrient availability and decomposition dynamics (Convey et al. 2010). Native flies on South Georgia such as Paractora trichosterna are likely outcompeted (Convey et al. 2010), as has occurred to native flies on Kerguelen due to C. vicina invasion (Laparie et al. 2010).
Some invertebrate species introduced to SOI may impact terrestrial ecosystem structure and function (Chown et al. 2008; Convey and Lebouvier 2009; Convey et al. 2010; Lebouvier et al. 2011). Not only do non-native invertebrates often outcompete native species (Bergstrom et al. 2006; Janion et al. 2010), but by exploiting previously unutilised ecological resources or dominating unsaturated niches, new arrivals can experience little to no competition (Ernsting et al. 1999; Convey 2007; Smith 2007). Their establishment can significantly alter trophic complexity, nutrient turnover, native prey, resource availability and may assist the spread of other non-native species (Lee et al. 2007; Greenslade et al. 2007, 2008; Convey and Lebouvier 2009; Convey et al. 2010). Examples include a new guild of pollinating flies (E. croceimaculata and C. vicina), which have established on South Georgia Island and two species of invasive, terrestrial crustaceans on Macquarie Island, which as macro-detritivores occupy an unsaturated trophic level. While the long-term effects of the crustaceans are largely unknown, it is likely that they will alter rates of soil nutrient turnover (Greenslade et al. 2008). There are relatively few naturally occuring invertebrate herbivores on SOI (Vernon et al. 1998). Thus, non-native aphids found at the Kerguelen archipelago that are sap-feeders, a guild nearly vacant on these islands, capitalise on plant resources previously unutilized (Hullé et al. 2010). At least one species, Myzus ascalonicus, occurs in colones 2-7 larger on native plants than on non-native host plants (Hullé 2012). The parasitic wasp Aphidius matricariae (an aphid parasite), is the only kind of species of this guild in the Marion Island ecosystem, and has rapidly expanded its range since its introduction in 2001 (likely by a single gravid female—Lee et al. 2007). Its colonisation and spread is facilitated by an established non-native aphid (Lee and Chown 2016). Non-native, predatory carabid ground beetles on South Georgia Island and Kerguelen Island (Trechisibus antarcticus, Oopterus soledadinus and Merizodus soledadinus), occupy a novel guild as arthropod predators (Ernsting et al. 1995, 1999; Chevrier et al. 1997; Laparie et al. 2010; Lebouvier et al. 2011). Through predation, T. antarcticus and O. soledadinus have reduced the abundance and increased the adult body size of the endemic herbivorous beetle Hydromedion sparsutum on South Georgia Island (Ernsting et al. 1995). Larger adult body sizes in H. sparsutum increase as predation of juveniles by the invasive carabids increase, a direct response of both selection by the predator in favour of larvae with rapid growth rate and reduced competition for high-quality food for the survivors (Ernsting et al. 1995). Across the Kerguelen archipelago, M. soledadinus has steadily increased its dominance in arthropod communities. It lacks competitors as it is the only predatory insect species (Laparie et al. 2010; Frenot et al. 2005). Since the 1990s, it has expanded its range from the introduction site to remote locations (including other islands in the archipelago) at an accelerating rate of colonisation (Chevrier et al. 1997), and dramatically increased in abundance (Laparie et al. 2010). M. soledadinus invasion has led to the near disappearance of its preferred prey, the native wingless flies Anatalanta aptera (Diptera: Sphaeroceridae) and Calycopteryz moseleyi (Diptera: Micropezidae) in some areas (Laparie et al. 2010; Lebouvier et al. 2011). In contrast, M. soledadinus on South Georgia only colonises a limited area (Ernsting 1993), is found in lower abundance, and has a much reduced rate of expansion (Brandjes et al. 1999), probably due to cooler annual temperatures than the Kerguelen Archipelago (Lebouvier et al. 2011).
Non-native soil invertebrates compete directly with native species and alter nutrient turnover in soils (Hänel and Chown 1998; Smith 2007; Smith and Steenkamp 1990, 1992a, b; Greenslade et al. 2008). Through competition and/or predation, invasive species can eventually lead to a decline in abundance or local extinction of native species that may play major roles in organic material decomposition (Greenslade et al. 2007; Convey et al. 2011). For example, on Gough Island, the only indigenous terrestrial isopod, Styloniscus australis, is abundant only in upland sites where the non-native terrestrial isopod Porcellio scaber is rare, and is rare where P. scaber is abundant in the lowlands (Jones et al. 2003b). Some of the non-native fauna on Gough Island, including worms (Ogliochaeta), P. scaber and a millipede, are the most abundant on the island (Jones et al. 2003b). The long-term effect of such a large biomass of macro-detritivores on Gough Island, in a system naturally lacking such species, is likely to considerably speed up organic nutrient cycling, affecting peat formation, and substantially changing floral and faunal assemblages (Jones et al. 2002, 2003b; Reynolds et al. 2002; Smith 2007, 2008). On Tristan da Cunha, introduced millipedes and earthworms may similarly be impacting soil types, as native litter-decomposing invertebrates are relatively few (Holdgate 1966). Detritivores on Marion Island, including the European slug Deroceras panormitanum (also herbivorous), the chironomid midge Limnophyes minimus and P. scaber, also process considerable quantities of litter in competition with native species, and substantially alter nutrient turnover (Smith and Steenkamp 1992b; Hänel and Chown 1998; Slabber and Chown 2002; Smith 2008). L. minimus is estimated to ingest litter at a rate that is an order of magnitude more than that consumed by the endemic flightless moth larvae Pringleophaga marioni, the primary native detritivore on the island (Hänel and Chown 1998). The relatively slow rate of litter processing by this native species is thought to represent a nutrient-cycling bottleneck that once released by non-native macro-detritivores will have implications for primary productivity and peat formation in the island’s ecosystem (Smith and Steenkamp 1990; Hänel and Chown 1998; Jones et al. 2003c). The non-native slugs greatly exacerbate rates of nutrient mineralisation from litter and ratios of C:N and N:P released are different than for the native caterpillars on Marion Island (Smith and Steenkamp 1992a, b). This also ultimately affects peat nutrient quality, decomposition rates and primary production, which are important drivers of ecological succession (Smith 2007, 2008). If the nutrient-cycling caterpillars become replaced by non-native slugs (which, unlike the indigenous caterpillar, are not palatable to mice), consequences for ecosystem structure and function are inevitable (Smith 2007, 2008). Though it may be difficult to predict cascading impacts of the non-native detritivores, altered vegetation and soil properties will undoubtedly have implications for native invertebrate life.
Non-native vertebrate impacts
Herbivores and Omnivores
Rabbits, cattle, sheep, corsican mouflon, pigs, goats and reindeer were introduced to SOI in the nineteenth century to either provide food for camps of seal-hunters or for farming (Headland 2012). Typically, herbivore introductions on SOI have led to major declines in vegetation cover, particularly of endemic megaherbs and large tussock grasses that are important habitat for invertebrates (Micol and Jouventin 1995; Chapuis et al. 2004; Scott and Kirkpatrick 2008). With the reduction of some plant species through grazing, short grasses and herbs can thrive, associated with the expansion of grazing-tolerant non-native plants (Frenot et al. 2005), such as P. annua (Chown and Block 1997; Williams et al. 2013). The largely detritus-based food webs of SOI, which are composed of weakly efficient detritivores (Tréhen et al. 1990; Smith 2008), are further affected by the conversion of plant matter to herbivore dung rather than accumulated litter (Burger 1985; Tréhen et al. 1990).
Rabbits established on many SOI; however, most have been eradicated in recent times or died out (Headland 2012). Rabbits can reach plague proportions on these islands (e.g. Macquarie Island—Terauds et al. 2014) leading to drastic changes in vegetation (Convey and Lebouvier 2009; Scott and Kirkpatrick 2013; Whinam et al. 2014). The flow-on effects of vegetation loss can lead to soil exposure and erosion (Scott 1988; Chapuis et al. 1994; Scott and Kirkpatrick 2008, 2013), degradation of waterways and associated freshwater invertebrate life (Marchant et al. 2011), as well as reduction of seabird nesting habitat (Chapuis et al. 1994; Copson and Whinam 1998). Reduction in seabird nesting habitat ultimately leads to fewer nesting seabirds. In this way, grazing-mediated declines in seabird densities alter the dynamics of terrestrial communities by reducing marine nutrient inputs that underpin vegetation growth, affecting nutrient turnover and soil integrity, with consequences for food webs and invertebrates (Anderson and Polis 1999; Maron et al. 2006; Smith 2008; Pisanu et al. 2011). Such drastic ecosystem changes occurred on Macquarie Island before rabbits were eradicated, where they caused considerable loss of biomass of vegetation such as tall tussock grasslands (dominated by Poa foliosa) and herbfields (dominated by Stilbocarpa polaris and Pleurophyllum hookeri). This led to degradation of seabird habitat, extreme habitat and edaphic modification, landscape denudation, and increased land-slipping and erosion (Copson and Whinam 1998, 2001; Scott and Kirkpatrick 2008; Stevens et al. 2010), and resulted in substantial changes to invertebrate populations (Copson and Whinam 2001). This is especially the case as invertebrate richness is highest in vegetation communities that were impacted by rabbits (Davies and Melbourne 1999; Terauds et al. 2011; Whinam et al. 2014; Errington et al. 2018). Rabbits have had similar impacts on the Kerguelen archipelago, causing erosion and rapid declines in some native plant species, which are often replaced by monospecific communities of the less palatable, and increasingly dominant, Acaena magellanica (Holdgate 1966; Burger 1985; Chapuis et al. 1994). Some studies have suggested that the reduced range and abundance of unique SOI invertebrate fauna is related to herbivore-induced disappearance of natural vegetation communities dominated by Pringlea antiscorbutica and Azorella selago (Holdgate and Wace 1961; Holdgate 1966; Chapuis et al. 1994), but there are no empirical data to support these claims.
Livestock on SOI have caused severe damage to native vegetation (Holdgate 1966; Taylor 1971; Chapuis et al. 1994; Seddon and Maloney 2003), leading to erosion, compaction, elimination of deep organic soils, the spread of non-native plants and presumably, commensurate reductions in associated invertebrates dependant on intact habitat (Holdgate 1966; van Vuren 1992; Chapuis et al. 1994). Many herbivores prefer particular endemic plant species, causing large-scale alteration of plant community composition across a range of islands (e.g. Holdgate 1966; Taylor 1971; Campbell and Rudge 1984; Convey and Lebouvier 2009). Feral cattle, recently eradicated from Iles Amsterdam (Váňa et al. 2014), had major environmental impacts (Micol and Jouventin 1995; Convey and Lebouvier 2009). Monitoring following initial control fencing and cattle removal from half of the island showed some vegetation regeneration (Micol and Jouventin 1995), but there are no published data available that quantify either the initial impacts of livestock on invertebrates, or the benefits of recent livestock control on invertebrates. Pigs remain on Auckland Island where their effects have been described as ‘severe’ (Headland 2012). They eat large amounts of native vegetative matter, particularly megaherbs, and prey directly on annelids (annelids—26% dry weight of stomach content), insect larvae, and amphipods (Challies 1975; Chimera et al. 1995). Eight of the ten species of annelids found on Auckland Island are endemic (Lee 1959). Whether any of these species are threatened with extinction as a result of pigs is unknown (Chimera et al. 1995).
Reindeer are extant on Iles Kerguelen and South Georgia (Courchamp et al. 2003), although they are near eradicated from South Georgia Island at the time of this publication. While feeding on Iles Kerguelen they turn over A. selago cushions (Chapuis et al. 1994), key invertebrate habitat (Phiri et al. 2009; Barendse and Chown 2001). Reindeer grazing and trampling impacts on South Georgia alter soil integrity and destroy large tracts of the dominant vegetation, the grass P. flabellata and herb A. magellanica, which are succeeded by the grazing resistant native grass Festuca contracta, and the grazing tolerant non-native grass P. annua (Vogel et al. 1984; Leader-Williams et al. 1987; Chown and Block 1997). Although the impacts of reindeer-mediated vegetation change have not been quantified at an invertebrate community level, some species-specific impacts have been documented (Vogel et al. 1984; Chown and Block 1997). One example is the increased frequency of sciarid flies in grazed areas (which are possibly non-native), likely due to the ability of their larvae to establish larger populations in deep soil and hardened substrates (a result of trampling—Vogel et al. 1984). Another is reduced abundance of the primary decomposer perimylopid beetle (Hydromedion sparsutum), and increased frequency of their egg parasite Notomymar aptenosoma (Hymenoptera, Mymaridae) (Vogel et al. 1984). Furthermore, trampling may have facilitated a shift in the proportions of Collembola (major prey invertebrates) and spiders (predators) found in pitfall traps—in ungrazed areas the ratio of invertebrates to spiders was 1: 1.3, compared to 1: 0.82 in grazed areas (Vogel et al. 1984).
In general, suppression or extinction of vegetation that invertebrates rely on for food or shelter, strongly influences native invertebrate extinctions (Dunn et al. 2009). Although rodents are omnivorous, in our review we treat them as predators, given their severe direct impacts on invertebrates as prey. However, rodents on SOI can also affect seedling recruitment and vegetation communities through consumption of seeds and plant material (Shaw et al. 2005; Copson 1986), nesting (Barendse and Chown 2001; Phiri et al. 2009), burrowing, sediment removal and erosion (Gremmen and Smith 1981; Eriksson and Eldridge 2014). These activities have consequences for invertebrates dependant on preferred plants or plant communities (Hugo et al. 2004; Phiri et al. 2009; St Clair 2011).
Predators
Native terrestrial mammalian predators are absent from SOI, therefore the introduction of non-native predators has led to severe impacts on a suite of native taxa, predominantly seabirds, that have evolved few defences (Courchamp et al. 2003; Frenot et al. 2005; Convey 2011). These effects have provided the impetus for several eradication programs, both completed and planned (e.g. Angel and Cooper 2006; Russell 2012; Robinson and Copson 2014; Springer 2016). For invertebrates, the impacts of predatory mammals on SOI (such as rodents and cats) are both indirect and direct (Copson 1986; Courchamp et al. 2003; Angel et al. 2009; St Clair 2011; McGeoch et al. 2015). Rats and mice have caused extensive damage to SOI ecosystems, including impacts on invertebrate richness and diversity, exemplified by comparative studies of invaded and uninvaded islands, such as those in the Falkland Islands (St Clair et al. 2011), Marion Island and neighbouring Prince Edward Islands (Crafford and Scholtz 1987; Chown and Smith 1993; Treasure and Chown 2014; McClelland et al. 2018) and uninvaded Bollons and Archway islands, which are offshore from Antipodes Island (Marris 2000; McIntosh 2001; Russell 2012). Globally, there are well-documented impacts by rats on terrestrial communities, ecosystem properties, and other native taxa, particularly seabirds (Fukami et al. 2006; Towns et al. 2006; Jones et al. 2008; Drake and Hunt 2009; Mulder et al. 2009; Wardle et al. 2009; Pisanu et al. 2011). However, rat impacts on invertebrates are less frequently quantified (St Clair et al. 2011). Towns et al. (2006) cite only nine examples of direct rat-invertebrate interactions in their global review of rat impacts, and only a few published studies directly measure these interactions for SOI (e.g. St. Clair et al. 2011; Pisanu et al. 2011). In general, rats on SOI are found to augment their largely plant-based diet, with large-bodied invertebrates such as caterpillars, annelids, beetles and weevils (Pye and Bonner 1980; Copson 1986; Pisanu et al. 2011; St Clair et al. 2011). St Clair et al. (2011) investigated the effects of rats on large-bodied invertebrates in the Falkland Islands (particularly the endemic Falkland camel cricket) across 37 invaded, uninvaded and recently cleared islands. They found uninvaded islands had up to an order of magnitude more camel crickets than invaded or recently rat-free islands, but also that camel cricket populations recover quickly following rat eradication. Alongside their plant diet preferences, the preferences of rats to predate on the most abundant and large bodied terrestrial invertebrates on SOI implicate them in species suppression and ecosystem transformation.
By contrast, the predation effects of mice on SOI invertebrates are better documented. Eleven SOI have been invaded by mice and their predatory impacts are considerable (Angel et al. 2009), especially for invertebrate populations on Gough Island (Jones et al. 2003a, b, c), Guillou Island (in the Kerguelen archipelago) (Le Roux et al. 2002), Antipodes Island (Marris 2000), Macquarie Island (Copson 1986) and Marion Island (Gleeson and van Rensburg 1982; Crafford and Scholtz 1987; Smith et al. 2002; McClelland et al. 2018). Where mice are the sole predator, their effects on invertebrates are typically greater, such as on Antipodes, Marion, and Gough islands (Angel et al. 2009; Russell 2012; McClelland et al. 2018). Furthermore, climate warming in the region underpins increases in mice biomass and further exploitation of invertebrate prey (McClelland et al. 2018). A good example is the 197.6-fold decrease in invertebrate biomass on Marion Island between 1976 and 2006 due to mice, or around 90% loss of biomass each year, linked to climate-driven mice population increases (McClelland et al. 2018). Mice predation is implicated in the extinction of island endemic invertebrate species on Antipodes Island (Marris 2000) and increases the extinction threat for several species on Gough Island (Jones et al. 2003a) and Marion Island (Crafford and Scholtz 1987; Rowe-Rowe et al. 1989; Angel et al. 2009; McClelland et al. 2018). Where mice are abundant they are responsible for localised extinction of invertebrates in lowland and coastal areas on SOI, restricting once abundant species to upland areas (e.g. Gough Island—Jones et al. 2003b; Antipodes Island—Marris 2000). Large-bodied invertebrates are more at risk to suppression or local extinction through rodent predation than smaller-bodied taxa (Chown and Smith 1993; Pisanu et al. 2011; St Clair 2011). Pronounced impacts of size-selective feeding are evident in changes to the size distribution of weevils on Marion (Treasure et al. 2014), and is potentially responsible for the hybridization of two weevil species that may previously have been differentiated by size (Chown 1990; Grobler et al. 2006). Depending on the season and availability of preferred prey, mice can switch preferred food items (Copson 1986; Smith et al. 2002; James C Russell et al. (in review)). For example, on Antipodes Island, mice seem to exhaust their preferred invertebrate prey to the point of severe suppression or local extinction, before moving on to the next preference, thus systematically eating their way through the ecosystem (James C Russell et al. (in review)). Such extirpation of preferred invertebrate prey, particularly during winter on islands with elevated populations of mice as the sole predator, may be a driver behind mice switching diets to seabirds (James C Russell et al. (in review)), such as on Gough Island (Wanless et al. 2012; Dilley et al. 2015) and Marion Island (Jones and Ryan 2010). Through their dynamic dietary preferences, the above studies have shown that mice can drastically modify the structure of ecosystems on SOI.
Mice predation on invertebrates also alters overall ecosystem processes. Suppression of invertebrates influencing nutrient cycling and mineralisation, has profound implications for SOI ecosystems that are already nutrient poor (McClelland et al. 2018). Alteration of ecosystem processes by mice on Marion Island occurs through predation of a keystone nutrient-cycling species, the caterpillar of a flightless moth, P. marioni (Smith and Steenkamp 1992a; Klok and Chown 1998), as well as depletion of other invertebrate prey such as spiders, weevil larvae and weevil adults (Crafford and Scholtz 1987; Rowe-Rowe et al. 1989; Chown et al. 2002). The substantial reduction in the flightless moth caterpillars, which are vital to nutrient supply for primary producers, threatens to slow plant growth, reduce litter quality, potentially affect the formation of peat on the island, and alter vegetation success (Smith and Steenkamp 1990; Smith 2008; Haupt et al. 2014).
Caterpillar declines are also linked to a declining population of lesser-sheathbills (Chionis minor), whose overwintering diet is dependent on them (Huyser et al. 2000). This is a clear example of the cascading ecosystem effects of invertebrate impacts. Potentially similar effects have been documented on Gough Island, where two endemic brachtyperous moths (Dimorphinoctua goughensis and Peridroma goughi), which are key nutrient cycling species, are preferentially eaten by mice (Jones et al. 2002, 2003a). On Macquarie Island, before a successful rodent eradication, amongst a wide variety of invertebrate prey detected in 108 mouse stomachs, spiders were recorded in 67% of them (Copson 1986). Given that all three spider species on Macquarie Island are major predators of small invertebrates (Greenslade 2006), depletion of spiders by rodents likely had flow on-effects in the ecosystem and the invertebrate community as small invertebrates prey were released from spider predation.
Indirect effects on invertebrates can occur through reduction of seabird populations due to rodent predation. Mice can prey-switch to seabirds as invert biomass falls in winter (Jones and Ryan 2010; Dilley et al. 2015), over time reducing seabird-driven marine inputs to the ecosystem and leading to soil impoverishment. Guano deposition by seabirds leads to increased cover, vitality and growth in plant communities (Smith 1976, 1978; Erskine et al. 1998), which in turn support a disproportionately high biomass of invertebrate detritivores and herbivores compared with areas free of seabirds (Burger 1978; Crafford and Scholtz 1987). In this respect, seabirds act as ‘ecosystem engineers’, influencing the base of food webs (Sanchez-Pinero and Polis 2000; Jones 2010; Russell 2012; Buxton et al. 2014). Thus, predation of seabirds on islands can reduce soil fertility (Fukami et al. 2006; Wardle et al. 2009), affect vegetation recruitment and growth, litter composition and decomposition (Wardle et al. 2007, 2009), and exert considerable multi-trophic, cascading effects in the terrestrial ecosystem (Croll et al. 2005; Fukami et al. 2006; Maron et al. 2006; Wardle et al. 2009; Mulder et al. 2009; Towns 2009). Rodent predation on seabirds can indirectly lead to reduced abundance of major orders of soil and above-ground invertebrates (Fukami et al. 2006; Towns 2009). Furthermore, invertebrate food webs are smaller and less complex on islands not dominated by seabirds, leading to lower trophic-level redundancy and therefore lower ecosystem resistance (Thoresen et al. 2017). These cumulative predator impacts have contributed to severe, sometimes permanent ecosystem alteration (Fukami et al. 2006; Mulder et al. 2009; Towns 2009; Wardle et al. 2009; Jones 2010). In light of these trends, cat eradication success on Macquarie and Marion Islands is predicted to have implications for their ecosystems beyond seabird population increase, including for invertebrates (van Aarde et al. 1996; Raymond et al. 2011). Unexpected consequences of ecosystem change following predator eradication has also been observed, such as the expansion of a non-native springtail on Marion Island, as its preferred habitat, a native grass, responds favourably to recovery of seabirds and associated nutrients since cat removal (Treasure and Chown 2013).
Discussion
Southern Ocean Islands have been visited by scientists for over 100 years. Despite this, some islands and some taxa have received more attention than others (Chown et al. 2008). The significance of such extensive studies in the region is valuable for invertebrate ecology. However, Table 1 in Supplementary Material highlights that research gaps remain for some ecological processes, some taxa and some islands. For example, non-native species on SOI have wide-ranging, sometimes severe, impacts on native invertebrates, either through direct mechanisms (e.g. predation and competition) or indirectly, through habitat modification, changes in soil integrity and reduced nutrient subsides by seabirds. Yet there is still limited understanding of these impacts for invertebrates and empirical data remain scarce. Similarly, non-native plant species are common on most SOI, and in some instances widespread, yet to date, only three studies have investigated the response of invertebrates to non-native plant expansion. This is despite the fact that we know native invertebrates rely on native plant species for food, habitat and cover (e.g. Hänel 1999; Hugo et al. 2004, 2006) and flow on effects are likely for native invertebrates when non-native plants flourish. Furthermore, whilst numerous studies have referred to the likelihood of considerably altered invertebrate populations associated with extensive damage to soils and native vegetation by grazing mammals (e.g. Chapuis et al. 1994, 2004; Copson and Whinam 2001; Courchamp et al. 2003), few have supported their claims with empirical data. Island-wide modification of habitat and soil through grazing and trampling have had wide-ranging ecosystem impacts (e.g. Courchamp et al. 2003; Chapuis et al. 2004; Frenot et al. 2005; Scott and Kirkpatrick 2008). The impacts on lower trophic levels, such as among invertebrates, are often implied (e.g. Chapuis et al. 1994; Micol and Jouventin 2002) but rarely quantified. Only two publications explicitly test the indirect effects of herbivore-induced plant community changes and activity on local associated invertebrates—Vogel et al. (1984) through reindeer trampling effects, and Chown and Block (1997), via the effects of grazing-mediated, non-native P. annua grass on native invertebrate fitness (Table 1 in Supplementary Material). More than 50 years have passed since Holdgate and Wace (1961) noted that elimination of native vegetation by rabbits on Kerguelen is accompanied by ‘the loss of the remarkable invertebrate fauna associated with it’, but there has been few, if any, comprehensive studies measuring invertebrate responses to non-native mammal grazing and vegetation damage on SOI. Even at a global scale, a lack of meaningful reporting on vegetation responses to herbivore eradication persists, which underpins a gap in our understanding for whole-ecosystem conservation benefits (Schweizer et al. 2016), including the fate of invertebrates. As a result, although eradications of mammalian herbivores have occurred on SOI for conservation benefits, to date we can only assume there are associated benefits to invertebrate communities.
While the 17 studies on invasive invertebrates in Table 1 in Supplementary Material include a broad suite of 26 invasive invertebrate species impacting on native fauna and ecosystems, the 20 studies researching the impact of mammalian predators on native invertebrates on SOI is overwhelmingly skewed towards studies of mouse-invertebrate interactions (17 of the 20 predator-related studies). Not only do mice studies dominate predator research for invertebrates, but the native invertebrate taxa studied is skewed heavily towards Coleoptera (beetles—21 times), and Lepidoptera (moths—19 times). Within the Coleoptera studies, the weevils Ectemnorrhinus spp. dominated (7 studies) and within the Lepidoptera studies flightless moths, Pringleophaga spp., were the primary focus (14 studies). Some of this research bias is due to the profusion of work on certain islands where these species exist. Marion Island, for example, is the site of 18 of our 45 studies incorporating invertebrate and non-native species interactions, with the second most-studied island, South Georgia, producing only 8 studies. The Kerguelen archipelago, which has a suite of non-native mammal, plant and invertebrate species across numerous islands, is the subject of only six studies that investigate their impacts on native invertebrates. This summary highlights gaps in our knowledge for unknown or under-surveyed invertebrate species on SOI that are impacted by invasive species, and also emphasises the lack of survey effort that persists for some SOI.
While it has been widely shown that non-native mammals drastically alter SOI, our review also highlights the considerable impacts of non-native invertebrates on native invertebrates and island ecosystems more broadly. To date these impacts have been underestimated, and their long-term extent is still largely unknown (McGeoch et al. 2015). Moreover, variation remains in the level of survey effort and knowledge among taxonomic groups (Convey et al. 2006b). Despite these impacts and improved biosecurity, non-native invertebrates continue to arrive and establish on SOI (Hughes et al. 2011; Houghton et al. 2016; Phillips et al. 2017). Invasions are expected to increase as the climate warms (Chown et al. 2008; Chown and Convey 2016). Eradications of invertebrates are rare, although recently demonstrated to be possible—for example, a butterfly in the South Island of New Zealand (Department of Conservation 2018); and ants on Tiritiri Matangi, New Zealand (Green (2019), and Lord Howe Island (Boland et al. 2011; Hoffmann et al. 2017). Nevertheless, the most cost-effective management strategy to reduce the impacts of invasive invertebrates on SOI is through improved biosecurity. For this reason, extending our knowledge of native and non-native invertebrate interactions in SOI ecosystems is critical, particularly in identifying high-risk taxa and developing targeted biosecurity procedures. Such knowledge increases the likelihood that island managers can reduce or even avoid completely, incursions of taxa likely to establish, invade and have impact. Similarly, improving our understanding of how established invasive invertebrates interact with flora and other invertebrate in SOI ecosystems, and their influence on food webs and nutrient cycling, is critical to our future management of present non-native species and developing responses to future incursions.
We have a good understanding of rodent impacts on SOI invertebrates from a suite of studies (Table 1 in Supplementary Material). However, the indirect consequences of seabird predation by rodents on above- and below-ground invertebrate communities and food webs can only be assumed from comprehensive studies of the seabird impacts on New Zealand islands (e.g. Fukami et al. 2006; Towns 2009; Thoresen et al. 2017). To date, no published study has measured the consequences of predator-mediated seabird declines for native SOI invertebrates, although the topic is often discussed (e.g. by Crafford and Scholtz 1987 in relation to cat impacts on Marion Island and by Huyser et al. 2000 for cats, mice, seabird invertebrate interactions). Furthermore, although some comparative island studies document rodent impacts on invertebrates on SOI (e.g. Crafford and Scholtz 1987; Marris 2000; McClelland et al. 2018), we still know little about how invertebrate communities respond once the target invasive species is eradicated. Furthermore, we have very little understanding of how these invertebrate responses to such management may influence recovery (or otherwise) of the whole island ecosystem. The study by St Clair et al. (2011) on the Falkland Islands showing camel cricket recovery following rat removal is an exception. How interactions between plants and native invertebrates may change in response to mammal eradications are also not well described (Angel and Cooper 2006).
There are several underlying reasons behind the limited monitoring of SOI invertebrate response to eradications to date. A key contributing factor is that conservation efforts have traditionally focused on large, charismatic megafauna (Samways 2007; Angel et al. 2009; Collen et al. 2012). Invertebrate surveys are time-consuming, and often considered too difficult, yielding enormous abundance and diversity of species for which few specialists are available to process and even less to identify (Ward and Larivière 2004). Finally, the dramatic speed of invertebrate responses (e.g. St Clair et al. 2011; Watts et al. 2011) mean that unless surveys are planned and undertaken before and soon after eradications, nuanced changes can be difficult to identify. The consistent lack of invertebrate baseline data on SOI prior to non-native species introductions means that it is rarely clear which (or if any) species have experienced population declines or been lost altogether. In order to quantify change with confidence, invertebrate monitoring must begin with meaningful pre-treatment baseline surveys, accompanied by comprehensive and timely post-treatment monitoring.
The results of our review support and build on the assessments and findings of McGeogh et al. (2015), and Jones et al. (2016) who suggest that the responses of invertebrate fauna on islands are under-reported and poorly understood. Although critical to ecosystem functioning and high in diversity, invertebrates rarely generate interest in conservation funding (Angel et al. 2009). Furthermore, being inconspicuous, with cryptic habits, invertebrates are often overlooked in restoration programmes to date, even though they comprise the base of trophic pyramids and changes in their abundance and distribution can affect the whole ecosystem (Courchamp et al. 2003; Angel et al. 2009; Shaw et al. 2011). More recently, the importance of invertebrates has been recognised in SOI mammal eradication feasibility studies and planning processes, For example, invertebrate recovery was one of the objectives of the Macquarie Island Pest Eradication program (rodents and rabbits; Parks and Wildlife Service 2008), the Antipodes Island mice eradication (Elliot et al. 2015) and the proposed mice eradication on Gough Island (Parkes 2008). However, given the recent completion or near implementation of these programs (post-eradication monitoring is currently underway on Antipodes and Macquarie Islands, pre-eradication monitoring is underway on Gough), meaningful data and reporting on species interactions and the responses of invertebrates to mammalian predator and herbivore removal remain elusive. While these studies are yet to be completed (or published), they represent an important and necessary shift in thinking by scientists, funders and land managers. As we have highlighted here, the impacts of non-native species on invertebrates are far too wide-ranging to be ignored any longer.
References
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118:324–332
Angel A, Cooper J (2006) A review of the impacts of introduced rodents on the islands of Tristan da Cunha and Gough. RSPC Conservation Science Department, Royal Society for the Protection of Birds, Sandy
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biol Invasions 11:1743–1754. https://doi.org/10.1007/s10530-008-9401-4
Annecke DP, Moran VC (1982) Insects and mites of cultivated plants in South Africa. Butterworths, Durban
Barendse J, Chown SL (2001) Abundance and seasonality of mid-altitude fellfield arthropods from Marion Island. Polar Biol 24:73–82. https://doi.org/10.1007/s003000000172
Barendse J, Mercer RD, Marshall DJ, Chown SL (2002) Habitat specificity of mites on sub-Antarctic Marion Island. Environ Entomol 31:612–625
Barnes DKA, Hodgson DA, Convey P, Allen CS, Clarke A (2006) Incursion and excursion of Antarctic biota: past, present and future. Glob Ecol Biogeogr 15:121–142
Bellard C, Leclerc C, Courchamp F (2014) Impact of sea level rise on the 10 insular biodiversity hotspots. Global Ecology Biogeography 23:203–212
Bergstrom DM, Chown SL (1999) Life at the front: history, ecology and change on Southern Ocean Islands. Tree 14:472–477
Bergstrom DM, Hodgson DA, Convey P (2006) The physical setting of the Antarctic. In: Bergstrom D, Convey P, Huiskes AHL (eds) Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a global indicator. Springer, Dordrecht, pp 15–33
Bergstrom DM, Bricher PK, Raymond B, Terauds A, Doley D, McGeoch MA, Whinam J, Glen M, Yuan Z, Kiefer K, Shaw JD, Bramely-Alves J, Rudman T, Mohammed C, Lucieer A, Visoiu M, van Vuuren BJ, Marilyn CB (2015) Rapid collapse of a sub-Antarctic alpine ecosystem: the role of climate and pathogens. J Appl Ecol 52(3):774–783
Block W (1984) Terrestrial microbiology, invertebrates and ecosystems. In: Laws RM (ed) Antarctic Ecology. Academic Press, London, pp 163–236
Boland C, Smith M, Maple D, Tiernan B, Barr R, Reeves R, Napier F (2011) Heli-baiting using low concentration fipronil to control invasive yellow crazy ant supercolonies on Christmas Island, Indian Ocean Island invasives: eradication and management. IUCN, Gland, pp 152–156
Bowen L, Vuren DV (1997) Insular endemic plants lack defenses against herbivores. Conserv Biol 11:1249–1254
Brandjes GJ, Block W, Ernsting G (1999) Spatial dynamics of two introduced species of carabid beetles on the sub-Antarctic island of South Georgia. Polar Biol 21:326–334
Burger A (1978) Terrestrial invertebrates: a food resource for birds at Marion Island. S Afr J Antarct Res 8:87–99
Burger A (1985) Terrestrial food webs in the sub-Antarctic: island effects. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 582–591
Buxton RT, Jones C, Moller H, Towns DR (2014) Drivers of seabird population recovery on New Zealand Islands after predator eradication. Conserv Biol 28:333–344. https://doi.org/10.1111/cobi.12228
Campbell K, Donlan CJ (2005) Feral goat eradications on islands. Conserv Biol 19:1362–1374 doi. https://doi.org/10.1111/j.1523-1739.2005.00228.x
Campbell D, Rudge M (1984) Vegetation changes induced over ten years by goats and pigs at Port Ross, Auckland Islands (Subantarctic). N Z J Ecol 7:103–118
Challies CN (1975) Feral pigs (Sus scrofa) on Auckland Island: status, and effects on vegetation and nesting sea birds. N Z J Zool 2:479–490
Chapuis JL, Boussés P, Barnaud G (1994) Alien mammals, impact and management in the French sub-Antarctic Islands. Biol Conserv 67:97–104
Chapuis J-L, Frenot Y, Lebouvier M (2004) Recovery of native plant communities after eradication of rabbits from the subantarctic Kerguelen Islands, and influence of climate change. Biol Conserv 117:167–179. https://doi.org/10.1016/S0006-3207(03)00290-8
Chevrier M, Vernon P, Frenot Y (1997) Potential effects of two alien insects on a subantarctic wingless fly in the Kerguelen Islands. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 424–431
Chimera C, Coleman M, Parkes J (1995) Diet of feral goats and feral pigs on Auckland Island, New Zealand. N Z J Ecol 19:203–207
Chown S (1990) Speciation in the sub-Antarctic weevil genus Dusmoecetes Jeannel (Coleoptera: Curculionidae). Syst Entomol 15:283–296
Chown SL (2001) Physiological variation in insects: hierarchical levels and implications for ecological diversity. J Insect Physiol 47:649–660
Chown S, Avenant N (1992) Status of Plutella xylostella at Marion Island six years after its colonisation. S Afr J Antarct Res 22:37–41
Chown SL, Block W (1997) Comparative nutritional ecology of grass-feeding in a sub-Antarctic beetle: the impact of introduced species on Hydromedion sparsutum from South Georgia. Oecologia 111:216–224
Chown SL, Convey P (2016) Antarctic entomology. Annu Rev Entomol 61:119–137
Chown SL, Language K (1994) Recently established Diptera and Lepidoptera on sub-Antarctic Marion Island. Afr Entomol 2:57–76
Chown SL, Smith VR (1993) Climate change and the short-term impact of feral house mice at the sub-Antarctic Prince Edward Islands. Oecologia 96:508–516. https://doi.org/10.1007/BF00320508
Chown SL, Gremmen NJM, Gaston KJ (1998) Ecological biogeography of Southern islands: species-area relationships, human impacts and conservation. Am Nat 152:562–575
Chown S, McGeoch M, Marshall D (2002) Diversity and conservation of invertebrates on the sub-Antarctic Prince Edward Islands. Afr Entomol 10:67–82
Chown SL, Klok CJ, McGeoch MA (2004) Weather to go out: activity of Bothrometopus brevis (Curculionidae) at Heard Island. Polar Biol 27:217–221. https://doi.org/10.1007/s00300-003-0579-8
Chown SL, Hull B, Gaston KJ (2005) Human impacts, energy availability and invasion across Southern Ocean Islands. Glob Ecol Biogeogr 14:521–528
Chown SL, Lee JE, Shaw JD (2008) Conservation of Southern Ocean Islands: invertebrates as exemplars. J Insect Conserv 12:277–291. https://doi.org/10.1007/s10841-008-9151-8
Chown SL, Huiskes AHL, Gremmen NJM, Lee JE, Terauds A, Crosbie K, Frenot Y, Hughes KA, Imura S, Kiefer K, Lebouvier M, Raymond B, Tsujimotoi M, Ware C, Van de Vijver B, Bergstrom DM (2012) Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proc Natl Acad Sci 109(13):4938–4943
Collen B, Böhm M, Kemp R, Baillie JE (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, London
Convey P (1996a) The influence of environmental characteristics on the life history attributes of Antarctic terrestrial biota. Biol Rev 71:191–225
Convey P (1996b) Overwintering strategies of terrestrial invertebrates from Antarctica—the significance of flexibility in extremely seasonal environments. Eur J Entomol 93:489–505
Convey P (1997) How are the life history strategies of Antarctic terrestrial invertebrates influenced by extreme environmental conditions? J Therm Biol 22(6):429–440
Convey P (2007) Influences on and the origins of terrestrial biodiversity of the Sub-Antarctic Islands. Pap Proc R Soc Tasman 141:83–93
Convey P (2011) Antarctic terrestrial biodiversity in a changing world. Polar Biol 34:1629–1641
Convey P, Lebouvier M (2009) Environmental change and human impacts on terrestrial ecosystems of the sub-Antarctic islands between their discovery and the mid-twentieth century. Pap Proc R Soc Tasman 143:33–44
Convey P, Greenslade P, Arnold RJ, Block W (1999) Collembola of sub-Antarctic South Georgia. Polar Biol 22:1–6
Convey P, Chown SL, Wasley J, Bergstrom DM (2006a) Life history traits. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in antarctic terrestrial and limnetic ecosystems: antarctica as a global indicator. Springer, Dordrecht, pp 101–127. https://doi.org/10.1007/1-4020-5277-4_6
Convey P, Frenot Y, Gremmen N, Bergstrom DM (2006b) Biological invasions. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic terrestrial and limnetic ecosystems: antarctica as a global indicator. Springer, Dordrecht, pp 191–218. https://doi.org/10.1007/1-4020-5277-4_6
Convey P, Key RS, Key RJD (2010) The establishment of a new ecological guild of pollinating insects on sub-antarctic South Georgia. Antarct Sci 22:508–512
Convey P, Key RS, Key RJD, Belchier M, Waller CL (2011) Recent range expansions in non-native predatory beetles on sub-Antarctic South Georgia. Polar Biol 34(4):597–602
Copson G (1986) The diet of the introduced rodents Mus-Musculus L and Rattus-Rattus L on sub-antarctic Macquarie island. Wildl Res 13:441–445. https://doi.org/10.1071/WR9860441
Copson G, Whinam J (1998) Response of vegetation on subantarctic Macquarie Island to reduced rabbit grazing. Aust J Bot 46:15–24
Copson G, Whinam J (2001) Review of ecological restoration programme on subantarctic Macquarie Island: pest management progress and future directions. Ecol Manag Restor 2:129–138
Courchamp F, Langlais M, Sugihara G (1999) Control of rabbits to protect island birds from cat predation. Biol Conserv 89:219–225
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Courchamp F, Hoffmann BD, Russell JC, Leclerc C, Bellard C (2014) Climate change, sea-level rise, and conservation: keeping island biodiversity afloat. Trends Ecol Evol 29:127–130
Crafford JE, Chown SL (1990) The introduction and establishment of the diamondback moth (Plutella xylostella L., Plutellidae) on Marion Island. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Springer, Berlin, pp 354–358
Crafford JE, Scholtz CH (1987) Quantitative differences between the insect faunas of sub-antarctic Marion and Prince Edward islands: a result of human intervention? Biol Conserv 40:255–262. https://doi.org/10.1016/0006-3207(87)90119-4
Crafford J, Scholtz C, Chown S (1986) The insects of sub-Antarctic Marion and Prince Edward Islands; with a bibliography of entomology of the Kerguelen Biogeographical Province. S Afr J Antarct Res 16:41–84
Croll DA, Marion JA, Estes EM, Danner EM, Byrd GV (2005) Introduced predators transform subarctic islands from grassland to tundra. Science 307:1959–1961. https://doi.org/10.1126/science.1108485
Crooks J, Soulé ME (1999) Lag times in population explosions of invasive species: causes and implications. In: Sandlund OT, Schei SJ, Vikens A (eds) Invasive Species and Biodiversity Management. Kluwer Academic Publishers, The Netherlands, pp 103–125
Cuthbert R, Hilton G (2004) Introduced house mice Mus musculus: a significant predator of threatened and endemic birds on Gough Island, South Atlantic Ocean? Biol Conserv 117:483–489. https://doi.org/10.1016/j.biocon.2003.08.007
Davies L (1973) Observations on the distribution of surface-living land arthropods on the sub-Antarctic Ile de la Possession, Iles Crozet. J Nat Hist 7:241–253
Davies KF, Melbourne BA (1999) Statistical models of invertebrate distribution on Macquarie Island: a tool to assess climate change and local human impacts. Polar Biol 21:240–250
Davies KF, Melbourne BA, McClenahan JL, Tuff T (2011) Statistical models for monitoring and predicting effects of climate change and invasion on the free-living insects and a spider from sub-Antarctic Heard Island. Polar Biol 34:119–125
de Villiers MS et al (2006) Conservation management at Southern Ocean Islands: towards the development of Best-Practise Guidelines. Polarforshung 75:113–131
Department of Conservation NZ (2018) Great white butterfly. Department of Conservation, Wellington. Accessed 3 April 2018
Dilley BJ, Davies D, Bond AL, Ryan PG (2015) Effects of mouse predation on burrowing petrel chicks at Gough Island. Antarct Sci 27:543–553. https://doi.org/10.1017/S0954102015000279
Drake DR, Hunt TL (2009) Invasive rodents on islands: integrating historical and contemporary ecology. Biol Invasions 11:1483–1487. https://doi.org/10.1007/s10530-008-9392-1
Duffy GA, Coetzee BWT, Latombe G, Akerman AH, McGeoch MA, Chown SL (2017) Barriers to globally invasive species are weakening across the Antarctic. Divers Distrib. https://doi.org/10.1111/ddi.12593
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc Lond B 276:3037–3045
Elliott GP, Greene TC, Nathan HW, Russell JC (2015) Winter bait uptake trials and related field work on Antipodes Island in preparation for mouse (Mus musculus) eradication, vol. 345. DOC Research and Development Series, Wellington
Eriksson B, Eldridge DJ (2014) Surface destabilisation by the invasive burrowing engineer Mus musculus on a sub-Antarctic island. Geomorphology 223:61–66
Ernsting G (1993) Observations on life cycle and feeding ecology of two recently introduced predatory beetle species at South Georgia, sub-Antarctic. Polar Biol 13:423–428
Ernsting G, Block W, MacAlister H, Todd C (1995) The invasion of the carnivorous carabid beetle Trechisibus antarcticus on South Georgia (Sub-Antarctic) and its effect on the endemic herbivorous beetle Hydromedion sparsutum. Oecologia 103:34–42
Ernsting G, Brandjes G, Block W, Isaaks J (1999) Life-history consequences of predation for a subantarctic beetle: evaluating the contribution of direct and indirect effects. J Anim Ecol 68:741–752
Errington I, King CK, Houlahan S, George SC, Michie A, Hose GC (2018) The influence of vegetation and soil properties on springtail communities in a diesel-contaminated soil. Sci Total Environ 619–620:1098–1104
Erskine PD, Bergstrom DM, Schmidt S, Stewart GR, Tweedie CE, Shaw JD (1998) Subantarctic Macquarie Island—a model ecosystem for studying animal-derived nitrogen sources using 15 N natural abundance. Oecologia 117:187–193
Frenot Y, Gloaguen JC, Massé L, Lebouvier M (2001) Human activities, ecosystem disturbance and plant invasions in subantarctic Crozet, Kerguelen Amsterdam Islands. Biol Conserv 101:33–50. https://doi.org/10.1016/S0006-3207(01)00052-0
Frenot Y, Chown SL, Whinam J, Selkrik PM, Convey P, Skotnicki M, Bergstrom DM (2005) Biological invasions in the Antarctic: extent, impacts and implications. Biol Rev 80:45–72
Frenot Y et al (2008) Antarctic and subantarctic biological invasions: sources, extents, impacts and implications non-native species in the Antarctic. Proceedings, pp 53–96
Fukami T et al (2006) Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol Lett 9:1299–1307. https://doi.org/10.1111/j.1461-0248.2006.00983.x
Gabriel AGA, Chown SL, Barendse J, Marshall DJ, Mercer RD, Pugh PJA, Smith VR (2001) Biological invasions of Southern Ocean Islands: the Collembola of Marion Island as a test of generalities. Ecography 24:421–430. https://doi.org/10.1034/j.1600-0587.2001.d01-198.x
Gaston KJ, Jones AG, Hänel C, Chown SL (2003) Rates of species introduction to a remote oceanic island. Proc R Soc Lond Ser B 270:1091–1098. https://doi.org/10.1098/rspb.2003.2332
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Gleeson J, Van Rensburg P (1982) Feeding ecology of the house mouse Mus musculus on Marion Island. S Afr J Antarct Res 12:34–39
Green, C. (2019). Effort required to confirm eradication of an Argentine ant invasion: Tiritiri Matangi Island, New Zealand. Island invasives: scaling up to meet the challenge, (62), 370
Green K, Mound L (1994) An extension to the insect fauna of Heard Island. Polar Rec 30:131–132
Greenslade P (2006) The invertebrates of Macquarie Island. Australian Antarctic Division, Kingston
Greenslade P, Convey P (2012) Exotic Collembola on subantarctic islands: pathways, origins and biology. Biol Invasions 14:405–417 doi. https://doi.org/10.1007/s10530-011-0086-8
Greenslade P, Stevens MI, Edwards R (2007) Invasion of two exotic terrestrial flatworms to subantarctic Macquarie Island. Polar Biol 30:961–967
Greenslade P, Melbourne BA, Stevens MI (2008) The status of two exotic terrestrial Crustacea on sub-Antarctic Macquarie Island. Polar Record 44:15–23
Greenslade P, Vernon P, Smith D (2011) Ecology of heard island diptera. Polar Biol 35(6):841–850
Gremmen N, Smith V (1981) Agrostis stolonifera on Marion Island (sub-Antarctic). S Afr J Antarct Res:33–34
Gremmen NJM, Chown SL, Marshall DJ (1998) Impact of the introduced grass Agrostis stolonifera on vegetation and soil fauna communities at Marion Island, sub-Antarctic. Biol Conserv 85:223–231. https://doi.org/10.1016/S0006-3207(97)00178-X
Gremmen N, Barendse J, Orr I (2001) Invasion and eradication of Sagina procumbens L. (Procumbent pearlwort) on Gough Island. Aliens 14:19–20
Gressitt JL (1970) Subantarctic entomology and biogeography. Pacific Insects Monograph 23:295–374
Gressitt JL (1971) Antarctic entomology with emphasis on biogeographical aspects. Pac Insects Monogr 25:167–178
Greve M, Gremmen NJ, Gaston KJ, Chown SL (2005) Nestedness of Southern Ocean island biotas: ecological perspectives on a biogeographical conundrum. J Biogeogr 32:155–168
Grobler GC, Bastos ADS, Chimimba CT, Chown SL (2011a) Inter-island dispersal of flightless Bothrometopus huntleyi (Coleoptera: Curculionidae) from the sub-Antarctic Prince Edward Island archipelago. Antarct Sci 23:1–10
Grobler GC, Bastos ADS, Treasure AM, Chown SL (2011b) Cryptic species, biogeographic complexity and the evolutionary history of the Ectemnorhinus group in the sub-Antarctic, including a description of Bothrometopus huntleyi, n. sp. Antarct Sci 23:211–224. https://doi.org/10.1017/S0954102011000101
Hanel C (1999) The distribution and abundance of macro-invertebrates in the major vegetation communities of Marion Island and the impact of alien species. University of Pretoria, Pretoria
Hänel C, Chown SL (1998) The impact of a small, alien invertebrate on a sub-Antarctic terrestrial ecosystem: Limnophyes minimus (Diptera, Chironomidae) at Marion Island. Polar Biol 20:99–106. https://doi.org/10.1007/s003000050282
Haupt TM, Crafford J, Chown SL (2014) Solving the puzzle of Pringleophaga–threatened, keystone detritivores in the sub-Antarctic. Insect Conserv Divers 7:308–313
Headland RK (2012) History of exotic terrestrial mammals in Antarctic regions. Polar Rec 48:123–144
Hoffmann BD, Graham R, Smith D (2017) Ant species accumulation on Lord Howe Island highlights the increasing need for effective biosecurity on islands. NeoBiota 34:41
Holdgate MW (1966) The influence of introduced species on the ecosystems of temperate oceanic islands. In Proceedings of the 10th technical meeting of the international union for the conservation of nature and natural resources [IUCN], Lucerne, vol 9, pp 151–176
Holdgate MW, Wace NM (1961) The influence of man on the floras and faunas of southern islands. Polar Rec 10(68):475
Houghton M, McQuillan PB, Bergstrom DM, Frost L, van den Hoff J, Shaw J (2016) Pathways of alien invertebrate transfer to the Antarctic region. Polar Biol:1–11 https://doi.org/10.1007/s00300-014-1599-2
Hughes KA, Ott S, Bölter M, Convey P (2006) Colonisation processes. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in antarctic terrestrial and limnetic ecosystems: antarctica as a global indicator. Springer, Dordrecht, pp 35–54. https://doi.org/10.1007/1-4020-5277-4_3
Hughes KA et al (2011) Food for thought: risks of non-native species transfer to the Antarctic region with fresh produce. Biol Conserv 144:1682–1689
Hugo EA, McGeoch MA, Marshall DJ, Chown SL (2004) Fine scale variation in microarthropod communities inhabiting the keystone species Azorella selago on Marion Island. Polar Biol 27:466–473. https://doi.org/10.1007/s00300-004-0614-4
Hugo EA, Chown SL, McGeoch MA (2006) The microarthropods of sub-Antarctic Prince Edward Island: a quantitative assessment. Polar Biol 30:109–119. https://doi.org/10.1007/s00300-006-0166-x
Huiskes AHL, Convey P, Bergstrom DM (2006) Trends in Antarctic terrestrial and limnetic ecosystems. In: Bergstrom D, Convey P, Huiskes AHL (eds) Trends in Antarctic terrestrial and limnetic ecosystems: Antarctica as a global indicator. Springer, Dordrecht, pp 1–13
Hullé M (2012) Myzus ascalonicus, an aphid recently introduced to sub-antarctic islands, prefers native to exotic host-plants. Environ Entomol 41:1398–1404
Hullé M, Pannetier D, Simon JC, Vernon P, Frenot Y (2003) Aphids of sub-Antarctic iles crozet and kerguelen: species diversity, host range and spatial distribution. Antarct Sci 15(2):203–209
Hullé M, Turpeau E, Hudaverdian S, Chaubet B, Outreman Y, Lebouvier M (2010) Aphids and associated natural enemies on ile amsterdam and ile saint-paul, southern indian ocean. Antarct Sci 22(4):379–385
Huyser O, Ryan PG, Cooper J (2000) Changes in population size, habitat use and breeding biology of lesser sheathbills (Chionis minor) at Marion Island: impacts of cats, mice and climate change? Biol Conserv 92:299–310. https://doi.org/10.1016/S0006-3207(99)00096-8
Janion C, Leinaas HP, Terblanche JS, Chown SL (2010) Trait means and reaction norms: the consequences of climate change/invasion interactions at the organism level. Evol Ecol 24:1365–1380. https://doi.org/10.1007/s10682-010-9405-2
Jones HP (2010) Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago. Ecol Appl 20:1204–1216. https://doi.org/10.1890/09-1172.1
Jones MGW, Ryan PG (2010) Evidence of mouse attacks on albatross chicks on sub-Antarctic Marion Island. Antarct Sci 22:39–42. https://doi.org/10.1017/S0954102009990459
Jones A, Chown S, Gaston K (2002) Terrestrial invertebrates of Gough Island: an assemblage under threat? Afr Entomol 10:83–91
Jones AG, Chown SL, Gaston KJ (2003a) Introduced house mice as a conservation concern on Gough Island. Biodivers Conserv 12:2107–2119. https://doi.org/10.1023/A:1024190331384
Jones AG, Chown SL, Ryan PG, Gremmen NJM, Gaston KJ (2003b) A review of conservation threats on Gough Island: a case study for terrestrial conservation in the Southern Oceans. Biol Conserv 113:75–87. https://doi.org/10.1016/S0006-3207(02)00351-8
Jones AG, Chown SL, Webb TJ, Gaston KJ (2003c) The free-living pterygote insects of Gough Island, South Atlantic Ocean. Syst Biodivers 1:213–273. https://doi.org/10.1017/S1477200003001142
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26. https://doi.org/10.1111/j.1523-1739.2007.00859.x
Jones HP et al (2016) Invasive mammal eradication on islands results in substantial conservation gains. Proc Natl Acad Sci USA 113:4033–4038
Kier G et al (2009) A global assessment of endemism and species richness across island and mainland regions. Proc Natl Acad Sci USA 106:9322–9327. https://doi.org/10.1073/pnas.0810306106
Klok CJ, Chown SL (1998) Interactions between desiccation resistance, host-plant contact and the thermal biology of a leaf-dwelling sub-antarctic caterpillar, Embryonopsis halticella (Lepidoptera: Yponomeutidae). J Insect Physiol 44:615–628. https://doi.org/10.1016/S0022-1910(98)00052-3
Kremen C, Colwell R, Erwin T, Murphy D, Noss R, Sanjayan M (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conserv Biol 7:796–808
Kuschel G, Worthy TH (1996) Past distribution of large weevils (Coleoptera: curculionidae) in the South Island, New Zealand, based on Holocene fossil remains. NZ Entomol 19(1):15–22
Laparie M, Renault D (2016) Physiological responses to temperature in Merizodus soledadinus (Col., Carabidae), a subpolar carabid beetle invading sub-Antarctic islands. Polar Biol 39:35–45
Laparie M, Lebouvier M, Lalouette L, Renault D (2010) Variation of morphometric traits in populations of an invasive carabid predator (Merizodus soledadinus) within a sub-Antarctic island. Biol Invasions 12:3405–3417
Le Roux P (2008) Climate and climate change. In: Chown SL, Froneman PW (eds) The Prince Edward Islands: land-sea interactions in a changing ecosystem. SUN Press, Stellenbosch
Le Roux V, Chapuis J-L, Frenot Y, Vernon P (2002) Diet of the house mouse (Mus musculus) on Guillou Island, Kerguelen archipelago, Subantarctic. Polar Biol 25:49–57. https://doi.org/10.1007/s003000100310
Le Roux PC et al (2013) Human activities, propagule pressure and alien plants in the sub-Antarctic: tests of generalities and evidence in support of management. Biol Conserv 161:18. https://doi.org/10.1016/j.biocon.2013.02.005
Leader-Williams N, Smith RL, Rothery P (1987) Influence of introduced reindeer on the vegetation of South Georgia: results from a long-term exclusion experiment. J Appl Ecol 24:801–822
Lebouvier M, Frenot Y (2007) Conservation and management in the French sub-Antarctic islands and surrounding seas. Pap Proc R S Tasman 141:23–28
Lebouvier M et al (2011) The significance of the sub-Antarctic Kerguelen Islands for the assessment of the vulnerability of native communities to climate change, alien insect invasions and plant virus. Biol Invasions 13:1195–1208
Lee KE (1959) The earthworm fauna of New Zealand. New Zealand Department of Scientific and Industrial Research Bulletin 130, Wellington
Lee JE, Chown SL (2016) Range expansion and increasing impact of the introduced wasp Aphidius matricariae Haliday on sub-Antarctic Marion Island. Biol Invasions:1–12 doi:https://doi.org/10.1007/s10530-015-0967-3
Lee JE, Slabber S, van Vuuren BJ, van Noort S, Chown SL (2007) Colonisation of sub-Antarctic Marion Island by a non-indigenous aphid parasitoid Aphidius matricariae (Hymenoptera, Braconidae). Polar Biol 30:1195–1201
Leihy RI, Duffy GA, Chown SL (2018) Species richness and turnover among indigenous and introduced plants and insects of the Southern Ocean Islands. Ecosphere 9:1–15
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz A F (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Marchant R, Kefford B, Wasley J, King C, Doube J, Nugegoda D (2011) Response of stream invertebrate communities to vegetation damage from overgrazing by exotic rabbits on subantarctic Macquarie Island. Mar Freshw Res 62:404–413
Maron JL, Estes JA, Croll DA, Danner EM, Elmendorf SC, Buckelew SL (2006) An introduced predator alters Aluetian island plant communities by thwarting nutrient subsidies. Ecol Monogr 76:3–24
Marris JWM (2000) The beetle (Coleoptera) fauna of the Antipodes Islands, with comments on the impact of mice; and an annotated checklist of the insect and arachnid fauna. J R Soc N Z 30:169–195. https://doi.org/10.1080/03014223.2000.9517616
McClelland GT, Altwegg R, Aarde RJ, Ferreira S, Burger AE, Chown SL (2018) Climate change leads to increasing population density and impacts of a key island invader. Ecol Appl 28:212–224
McCreless EE et al (2016) Past and estimated future impact of invasive alien mammals on insular threatened vertebrate populations. Nat Commun 7:1–11
McGeoch MA, Le Roux PC, Hugo EA, Chown SL (2006) Species and community responses to short-term climate manipulation: microarthropods in the sub-Antarctic. Austral Ecol 31:719–731
McGeoch MA, Shaw JD, Terauds A, Lee JE, Chown SL (2015) Monitoring biological invasion across the broader Antarctic: A baseline and indicator framework. Glob Environ Chang 32:108–125. https://doi.org/10.1016/j.gloenvcha.2014.12.012
McIntosh A (2001) The impact of mice on the Antipodes Islands. Antipodes Island Expedition. Department of Conservation, New Zealand
Micol T, Jouventin P (1995) Restoration of Amsterdam Island, South Indian Ocean, following control of feral cattle. Biol Conserv 73:199–206
Micol T, Jouventin P (2002) Eradication of rats and rabbits from Saint-Paul Island, French Southern territories. In: Turning the tide: the eradication of invasive species, pp 199–205
Moon KL, Chown SL, Fraser CI (2017) Reconsidering connectivity in the sub-Antarctic. Biol Rev:1–18 doi:https://doi.org/10.1111/brv.12327
Mulder CPH et al (2009) Direct and indirect effects of rats: does rat eradication restore ecosystem functioning of New Zealand seabird islands? Biol Invasions 11:1671–1688. https://doi.org/10.1007/s10530-008-9396-x
Nielsen UN, Wall DH (2013) The future of soil invertebrate communities in polar regions: different climate change responses in the Arctic and Antarctic? Ecol Lett 16:409–419
Nogales M, Vidal E, Medina FM, Bonnaud E, Tershy BR, Campbell KJ, Zavaleta ES (2013) Feral cats and biodiversity conservation: the urgent prioritization of island management. Bioscience 63:804–810. https://doi.org/10.1525/bio.2013.63.10.7
Nyakatya M, McGeoch M (2008) Temperature variation across Marion Island associated with a keystone plant species (Azorella selago Hook (Apiaceae)). Polar Biol 31:139–151
Parkes J (2008) A feasibility study for the eradication of house mice from Gough Island. RSPB Conservation Science Department, Royal Society for the Protection of Birds, Sandy
Parks and Wildlife (2008) Macquarie Island pest eradication plan. Part A: overview. Department of the Environment, Parks, Heritage and the Arts, Hobart, Tasmania
Pendlebury S, Barnes-Keoghan I (2007) Climate and climate change in the sub-Antarctic. Pap Proc R Soc Tasman 141:67–81
Phillips L, Janion-Scheepers C, Houghton M, Terauds A, Potapov M, Chown SL (2017) Range expansion of two invasive springtails on sub-Antarctic Macquarie Island. Polar Biol:1–6 doi:https://doi.org/10.1007/s00300-017-2129-9
Phiri EE, McGeoch MA, Chown SL (2009) Spatial variation in structural damage to a keystone plant species in the sub-Antarctic: interactions between Azorella selago and invasive house mice. Antarct Sci 21:189–196. https://doi.org/10.1017/S0954102008001569
Pisanu B, Caut S, Gutjahr S, Vernon P, Chapuis J-L (2011) Introduced black rats Rattus rattus on Ile de la Possession (Iles Crozet, Subantarctic): diet and trophic position in food webs. Polar Biol 34:169–180. https://doi.org/10.1007/s00300-010-0867-z
Pye T, Bonner W (1980) Feral brown rats, Rattus norvegicus, in South Georgia (South Atlantic Ocean). J Zool 192:237–255
Raymond B, McInnes J, Dambacher JM, Way S, Bergstrom DM (2011) Qualitative modelling of invasive species eradication on subantarctic Macquarie Island. J Appl Ecol 48:181–191
Reynolds, JW, Jones AG, Gaston KJ, Chown SL (2002) The earthworms (Oligochaeta: Lumbricidae) of gough island, south atlantic ocean. Megadrilogica 9(2):5–15
Robinson SA, Copson GR (2014) Eradication of cats (Felis catus) from subantarctic Macquarie Island. Ecol Manag Restor 15:34–40. https://doi.org/10.1111/emr.12073
Roff DA (1990) The evolution of flightlessness in insects. Ecol Monogr 60:389–421
Roques A, Rabitsch W, Rasplus J-Y, Lopez-Vaamonde C, Nentwig W, Kenis M (2009) Alien terrestrial invertebrates of Europe. In: Drake JA (eds) Handbook of alien species in Europe. Springer, Berlin, pp 63–79
Rowe-Rowe D, Green B, Crafford J (1989) Estimated impact of feral house mice on sub-Antarctic invertebrates at Marion Island. Polar Biol 9:457–460
Russell JC (2012) Spatio-temporal patterns of introduced mice and invertebrates on Antipodes Island. Polar Biol 35:1187–1195. https://doi.org/10.1007/s00300-012-1165-8
Samways MJ (2007) Insect conservation: a synthetic management approach. Annu Rev Entomol 52:465–487
Sanchez-Pinero F, Polis GA (2000) Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81:3117–3132
Schweizer D, Jones HP, Holmes ND (2016) Literature review and meta-analysis of vegetation responses to goat and European rabbit eradications on islands. Pac Sci 70:55–71. https://doi.org/10.2984/70.1.5
Scott J (1988) Rabbit distribution history and related land disturbance, Macquarie Island. Pap Proc R Soc Tasman 122:255–266
Scott J, Kirkpatrick J (2008) Rabbits, landslips and vegetation change on the coastal slopes of subantarctic Macquarie Island, 1980–2007: implications for management. Polar Biol 31:409–419
Scott JJ, Kirkpatrick JB (2013) Changes in the cover of plant species associated with climate change and grazing pressure on the Macquarie Island coastal slopes, 1980–2009. Polar Biol 36:127–136. https://doi.org/10.1007/s00300-012-1243-y
Seddon PJ, Maloney R (2003) Campbell Island teal re-introduction plan. Department of Conservation, Wellington
Shaw JD (2013) Southern Ocean Islands invaded: conserving biodiversity in the world’s last wilderness. In: Plant invasions in protected areas. Springer, Dordrecht, pp 449–470
Shaw JD, Hovenden MJ, Bergstrom DM (2005) The impact of introduced ship rats (Rattus rattus) on seedling recruitment and distribution of a subantarctic megaherb (Pleurophyllum hookeri). Austral Ecol 30:118–125. https://doi.org/10.1111/j.1442-9993.2005.01430.x
Shaw JD, Spear D, Greve M, Chown SL (2010) Taxonomic homogenization and differentiation across Southern Ocean Islands differ among insects and vascular plants. J Biogeogr 37:217–228
Shaw J, Terauds A, Bergstrom D (2011) Rapid commencement of ecosystem recovery following aerial baiting on sub-Antarctic Macquarie Island. Ecol Manag Restor 12:241–244. https://doi.org/10.1111/j.1442-8903.2011.00611.x
Shirahai H (2007) A Complete guide to antarctic wildlife: the birds and marine mammals of the antarctic continent and the southern ocean. A&C Black, London
Shrestha M, Lunau K, Dorin A, Schulze B, Bischoff M, Burd M, Dyer AG (2016) Floral colours in a world without birds and bees: the plants of Macquarie Island. Plant Biol 18:842–850
Slabber S, Chown SL (2002) The first record of a terrestrial crustacean, Porcellio scaber (Isopoda: Porcellionidae), from sub-Antarctic Marion Island. Polar Biol 25:855–858
Smith V (1976) The effect of burrowing species of Procellariidae on the nutrient status of inland tussock grasslands on Marion Island. J South Afr Bot 42:265–272
Smith V (1978) Animal-plant-soil nutrient relationships on Marion Island (Subantarctic). Oecologia 32:239–253
Smith VR (2007) Terrrestrial ecological processes and problems on sub-Antarctic islands. Pap Proc R Soc Tasman 141:99–110
Smith VR (2008) Energy flow and nutrient cycling in the Marion Island terrestrial ecosystem: 30 years on. Polar Rec 44:211–226 https://doi.org/10.1017/S0032247407007218
Smith V, Steenkamp M (1990) Climatic change and its ecological implications at a subantarctic island. Oecologia 85:14–24
Smith VR, Steenkamp M (1992a) Soil nitrogen transformations on a subantarctic island. Antarct Sci 4:41–50
Smith VR, Steenkamp M (1992b) Soil macrofauna and nitrogen on a sub-antarctic island. Oecologia 92:201–206. https://doi.org/10.1007/BF00317365
Smith V, Avenant N, Chown S (2002) The diet and impact of house mice on a sub-Antarctic island. Polar Biol 25:703. https://doi.org/10.1007/s00300-002-0405-8
Springer K (2016) Methodology and challenges of a complex multi-species eradication in the sub-Antarctic and immediate effects of invasive species removal. N Z J Ecol 40:273
St Clair JJH (2011) The impacts of invasive rodents on island invertebrates. Biol Conserv 144:68–81. https://doi.org/10.1016/j.biocon.2010.10.006
St Clair JJH, Poncet S, Sheehan DK, Székely T, Hilton GM (2011) Responses of an island endemic invertebrate to rodent invasion and eradication. Anim Conserv 14:66–73. https://doi.org/10.1111/j.1469-1795.2010.00391.x
Stevens M, Hudson P, Greenslade P, Potter M (2010) Report on invertebrate monitoring of long-term field sites on Macquarie Island 2009/2010. South Australia Museum, South Australia
Taylor RH (1971) Influence of man on vegetation and wildlife of Enderby and Rose Islands, Auckland Islands. N Z J Bot 9:225–268
Terauds A, Chown SL, Bergstrom DM (2011) Spatial scale and species identity influence the indigenous—alien diversity relationship in springtails. Ecology 92:1436–1447. https://doi.org/10.1890/10-2216.1
Terauds A, Doube J, McKinlay J, Springer K (2014) Using long-term population trends of an invasive herbivore to quantify the impact of management actions in the sub-Antarctic. Polar Biol 37(6):833–843
Thoresen JJ, Towns D, Leuzinger S, Durrett M, Mulder CP, Wardle DA (2017) Invasive rodents have multiple indirect effects on seabird island invertebrate food web structure. Ecol Appl 27:1190–1198
Towns DR (2009) Eradications as reverse invasions: lessons from Pacific rat (Rattus exulans) removals on New Zealand islands. Biol Invasions 11:1719–1733. https://doi.org/10.1007/s10530-008-9399-7
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions 8:863–891. https://doi.org/10.1007/s10530-005-0421-z
Treasure AM, Chown SL (2013) Contingent absences account for range limits but not the local abundance structure of an invasive springtail. Ecography 35:001–011
Treasure AM, Chown SL, Diez J (2014) Antagonistic effects of biological invasion and temperature change on body size of island ectotherms. Divers Distrib 20:202–213. https://doi.org/10.1111/ddi.12153
Tréhen P, Frenot Y, Lebouvier M, Vernon P (1990) Invertebrate fauna and their role in the degradation of cattle dung at Amsterdam Island. In: Antarctic ecosystems, Springer, Berlin, Heidelberg, pp 337–346
Tréhen P, Bouche M, Vernon P, Frenot Y (1985) Organization and dynamics of the Oligochaeta and Diptera populations on Possession Island. In: Siegfried W, Condy P, Laws R (eds) Antarctic nutrient cycles and food webs (Proceedings of the 4th SCAR symposium on Antarctic biology). Springer
Váňa J, Ochyra R, Lebouvier M, Cykowska-Marzencka B (2014) Bryophytes of Île Amsterdam in the South Indian Ocean: 1. Liverworts. Cryptogam Bryol 35(4):335–372
van Vuren D (1992) Eradication of feral goats and sheep from island ecosystems. In: Proceedings of the fifteenth vertebrate pest conference 1992
van Aarde R, Ferreira S, Wassenaar T, Erasmus D (1996) With the cats away the mice may play. South African J Sci 92:357–358
Van Aarde RJ, Ferreira SM, Wassenaar TD (2004) Do feral house mice have an impact on invertebrate communities on sub-Antarctic Marion Island? Aust Ecol 29(2):215–224
van der Merwe M, Chown SL, Smith VR (1997) Thermal tolerance limits in six weevil species (Coleoptera, Curculionidae) from sub-Antarctic Marion Island. Polar Biol 18:331–336. https://doi.org/10.1007/s003000050196
Vernon P, Vannier G, Trehen P (1998) A comparative approach to the entomological diversity of polar regions. Acta Oecol 19:303–308. https://doi.org/10.1016/S1146-609X(98)80034-9
Vogel M, Remmert H, Smith RIL (1984) Introduced reindeer and their effects on the vegetation and the epigeic invertebrate fauna of South Georgia (subantarctic). Oecologia 62(1):102–109
Walther G-R et al (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Wanless RM, Angel A, Cuthbert RJ, Hilton GM, Ryan PG (2007) Can predation by invasive mice drive seabird extinctions? Biol Lett 3:241–244. https://doi.org/10.1098/rsbl.2007.0120
Wanless RM et al (2012) Predation of Atlantic Petrel chicks by house mice on Gough Island. Anim Conserv 15:472–479. https://doi.org/10.1111/j.1469-1795.2012.00534.x
Ward DF, Larivière MC (2004) Terrestrial invertebrate surveys and rapid biodiversity assessment in New Zealand: lessons from Australia. NZ J Ecol 151–159
Wardle DA, Bellingham PJ, Fukami T, Mulder CP (2007) Promotion of ecosystem carbon sequestration by invasive predators. Biol Lett 3(5):479–482
Wardle DA, Bellingham PJ, Bonner KI, Mulder CPH (2009) Indirect effects of invasive predators on litter decomposition and nutrient resorption on seabird-dominated islands. Ecology 90:452–464
Watts CH, Armstrong DP, Innes J, Thornburrow D (2011) Dramatic increases in weta (Orthoptera) following mammal eradication on Maungatautari-evidence from pitfalls and tracking tunnels. NZ J Ecol 35(3):261
Whinam J, Chilcot N, Bergstrom DM (2005) Subantarctic hitchhikers: expeditioners as vectors for the introduction of alien organisms. Biol Conserv 121:207–219
Whinam J, Fitzgerald N, Visoiu M, Copson G (2014) Thirty years of vegetation dynamics in response to a fluctuating rabbit population on sub-Antarctic Macquarie Island. Ecol Manag Restor 15:41–51
Williams L, Kristiansen P, Shaw J, Sindel B, Wilson SC (2013) Weeds down under: invasion of the sub-Antarctic wilderness of Macquarie Island. Plant Prot Q 28(3):71
Williams LK, Kristiansen P, Sindel BM, Wilson SC, Shaw JD (2016) Quantifying the seed bank of an invasive grass in the sub-Antarctic: seed density, depth, persistence and viability. Biol Invasions 18:2093–2106
Williamson MH, Fitter A (1996) The characters of successful invaders. Biol Conserv 78:163–170
Acknowledgements
We thank Paulo A. V. Borges and an anonymous reviewer for their valuable comments on the manuscript. We thank the Australian Academy of Science for their support to MH through the Max Day Environmental Fellowship. This research is also supported by funding from the Australian Government’s National Environmental Science Programme through the Threatened Species Recovery Hub and the Australian Antarctic Science program (AAS 4305).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicting or competing interests.
Research involving human participants and/or animals
This research did not involve human participants or animals requiring ethics approval.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Houghton, M., Terauds, A., Merritt, D. et al. The impacts of non-native species on the invertebrates of Southern Ocean Islands. J Insect Conserv 23, 435–452 (2019). https://doi.org/10.1007/s10841-019-00147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-019-00147-9