Abstract
Invasive predators may change their own trophic conditions by progressively displacing or reducing diversity and abundance of native prey. As food quality and quantity are two main factors determining adult body size in arthropods, alteration of the available resources may thus affect predators’ morphology. The flightless carabid beetle Merizodus soledadinus was accidentally introduced to Iles Kerguelen in a single site in 1913. Its successful spreading process has been monitored over the long term, providing an exceptional research opportunity with multiple snapshots of similar colonized sites mostly differing by the residence time of M. soledadinus. To test if M. soledadinus’ morphology is correlated with its residence time in each habitat, we measured nine morphometric traits in five populations. We detected significant morphological differences: individuals from the first colonized site were the smallest, whereas individuals from the most recently colonized site were the largest. Our study also highlighted among-site variation in sexual dimorphism of the last abdominal sternite: its length differed between sites for females, but not for males. We discuss this diminution of M. soledadinus’ size in the light of both a priori (development under diet restriction, survival) and a posteriori (intrapopulation competition, cannibalism) effects on growth and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several parameters, from physiological processes to environmental pressures, play a role in determining the body size and related morphological parameters in insects. Among a wide range of factors, ontogenesis, biomechanical constraints, sexual selection, fecundity, size-specific predation, resource quality and availability, overcrowding, competition and temperature have often been reported as the most prominent ones (Angilletta and Dunham 2003; Berven and Gill 1983; Juliano 1986; Wheeler 1996). Most of these factors may vary from one habitat to another and geographic variation in body size has thus been studied extensively (Boggs and Freeman 2005; Chown and Klok 2003; Schmidt-Nielsen 1984).
At a large geographic scale, clinal variation of morphological parameters within species from different taxa has been found (Blanckenhorn and Demont 2004; Hallas et al. 2002). The nature of such variation has been addressed frequently along altitudinal and/or latitudinal climatic gradients (Arthur et al. 2008; Blanckenhorn et al. 2006). However, the mechanisms driving a differential expression of the genotype over a large range of thermal environments are not fully understood (Angilletta and Dunham 2003; Cabanita and Atkinson 2006). Fewer studies have examined divergence in the expression of morphological traits at local geographical scales, where trophic resources and trophic competition appear as prime determinants of adult size and morphology. When nutritional resources are limiting, metabolic trade-offs constrain the allocation of energy inputs to growth, somatic maintenance and reproduction [as shown for example in Allomyrina dichotoma (Coleoptera, Scarabaeidae) by Moczek (1998), and in Onthophagus taurus (Coleoptera, Scarabaeidae) by Karino et al. (2004)]. In holometabolous insects, restriction in the quality and quantity of the larval diet may result in altered adult morphology and fitness (Boggs and Freeman 2005), with imagos exhibiting specific allometric relationships among various body parts such as wings, flight muscles, ovaries and head.
Within insect species, the size of each organ, appendage or body region bears a specific relationship to overall body size (Shingleton et al. 2007). Positive correlations have been found between wings and body size (Stern and Emlen 1999), fore-femur length and body size (Stern et al. 1996), and body size and morphological traits associated with feeding (mandibles, head) (Thompson 1992). Besides these correlations, head width or mandible length can be related to an ability to consume larger food items (Pearson and Stemberger 1980). Such a positive relationship between feeding morphology and body size might also differ among populations because of distinct resource availability or foraging strategies. This is particularly significant in many predatory arthropods, often food-limited in natural situations (Bommarco 1998; Pearson and Knisley 1985), and for which food intake provides a major part of the resources used for reproduction (Juliano 1986; Sota 1985; Wise 1979).
Invasive predators appear even more affected by availability of resources. They often change their own trophic conditions by displacing or reducing diversity and abundance of native prey (Kenis et al. 2008; Snyder and Evans 2006), as found in the ground beetle Merizodus soledadinus Guérin-Méneville 1832 (Coleoptera, Carabidae) (Chevrier 1996). This insect was introduced from the Falkland Islands to a single site (Port Couvreux) on Iles Kerguelen in 1913 (Jeannel 1940), where it has no efficient competitor [the only native predator species on Iles Kerguelen are one staphylinid, Antarctophytosus atriceps (Coleoptera, Staphylinidae) and two spiders, Myro kerguelensis (Araneae, Desidae) and Neomaso antarcticus (Araneae, Linyphiidae)]. During the past century, this flightless ground beetle has spread over the eastern part of the archipelago and colonized several habitats far from Port Couvreux. This spread has resulted in the formation of geographically distant populations characterized by distinct residence times in each habitat. Because the temporal evolution of the distribution of this alien predator was monitored, we have a set of snapshots ranging from formerly to recently colonized habitats. In addition, this insect can rapidly become a dominant species once established in a new site (Chevrier et al. 1997). The quality and quantity of available resources may be quickly altered and differ among colonized habitats according to the residence time of M. soledadinus, impacting individuals’ development and morphometry. Also, most animals exhibit a sexual dimorphism mainly related to different exploitation of food resources by females for reserve storage (fat, proteins) for egg maturation, and to reduced longevity in males (Atchley 1971; Butler 1986; Fairbairn 1997). M. soledadinus exhibits a sexual size-related dimorphism (Chevrier 1996), hence its growth ability may be differentially affected in males and females by short-term variation in food abundance.
Using a morphometric analysis, we asked if long-term colonization process on Iles Kerguelen induced differences between M. soledadinus individuals. To address this question, we measured nine quantitative parameters in adults sampled in five distinct sites colonized at distinct periods. We hypothesized that (i) individuals of M. soledadinus are characterized by reduced sizes and morphological traits in habitats colonized early because this predator altered the amount of available trophic resources, (ii) food limitation would lead to divergence in food-gathering characters and (iii) the selection pressure resulting from the availability of food resources affect males and females differentially.
Materials and methods
Collection sites and insect sampling
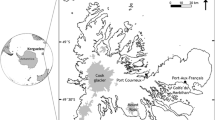
Wild specimens of M. soledadinus were sampled in December 2006 in five distinct sites (Fig. 1): (1) Port Couvreux (49°16′35.3′′S, 69°41′5.1′′E) where the species was first introduced to Iles Kerguelen in 1913 (Jeannel 1940), (2) Port Elizabeth (49°12′58.2′′S, 69°51′57.6′′E) (first individuals observed ca. 1970—Chevrier 1996), (3) Port Jeanne d’Arc (49°33′0.3′′S, 69°48′18.2′′E) (first observation in 1991—Chevrier 1996), (4) Ile Guillou (49°28′17.6′′S, 69°48′23.4′′E) (first observation in 1995—Lebouvier, unpublished data) and (5) Pointe Guite (49°25′22.5′′S, 70°16′53.6′′E) (first observation ca. 2003—Lebouvier, unpublished data). Literature and long-term monitoring schemes conducted on Iles Kerguelen since 1974 ensure the accuracy of these arrival dates, except for Port Elizabeth, where the estimated arrival date falls between 1939 and 1970. Based on these data, we can determine the residence time of M. soledadinus in each site (Fig. 2). For the five sites, each batch of measured individuals will be considered as a “population.”
Imagos of M. soledadinus were all hand-collected from December 1st to 15th 2006 in coastal areas under tide drift lines, so there were strong similarities among sampled habitats. Because of the reduced geographical distance between the sites (less than 50 km) and the absence of major topographic barriers impacting temperature or rainfall, all collected individuals experienced similar meteorological conditions whatever the collection site (meteorological data—available for Port-aux-Français since 1950 and for Ile Guillou since 1997—confirmed this assumption). To ensure a sufficient number of insects for each sex to conduct the study and the subsequent statistical analysis, 100 imagos were randomly caught in each site and were immediately stored in vials filled with 70% ethanol before being measured in the lab.
Traits measured
We measured nine morphometric traits (Fig. 3) in 30 males and 30 females for each one of the five sampled sites (except for Port Jeanne d’Arc, where only 26 males were in a preservation state that allowed reliable measurements of all the traits).
Morphological traits measured in adults of Merizodus soledadinus (male shown). TOT Individual total length from the labrum to the end of the right elytra, ELYT Length of the right elytra, INTOC Fore interocular width, PRONO L Length of the pronotum, PRONO W Width of the pronotum, STER Length of the last abdominal sternite, FEMU 1 Metafemur length of the right foreleg, FEMU 3 Metafemur length of the right hind leg, PALP Width of the last article of the right maxillary palp
Pictures of each measured parameter were taken for each specimen with a video camera (720 × 576 pixels) connected to a binocular microscope. Measurements were done by vectorial layouts with JMicroVision 1.2.5 (Geneva, Switzerland). Theoretical precision was 8.8 μm at ×6.4 magnification (used for TOT and ELYT, see Fig. 3 for a detailed description of the traits measured), 3.3 μm at ×16 (used for INTOC, PRONOL, PRONOW, FEMU1, FEMU3 and STER) and 0.9 μm at ×40 (used for PALP). Each appendage was placed perpendicularly to the video camera axis to limit parallax problems. Right legs of the first and the third pairs of legs were detached for measurement of FEMU1 and FEMU3.
After the measurements, each individual (with previously detached legs) was dried for 5 days at 60°C and then weighed with a Mettler H20 microbalance (d = 10 μg). Mass is strongly related to size in ground beetles (Hodar 1996; Jarosik 1989) and this mass measurement (MASS) allowed us to compare individuals between sites, but it does not strictly correspond to dry mass because of the solubilization of several compounds in ethanol (den Nijs et al. 1996). However, den Nijs et al. (1996) showed a significant correlation between alcohol mass and dry mass in adults of Pterostichus cupreus (Coleoptera, Carabidae).
Statistical analysis
A bias was identified in measurements of the total length (TOT) of individuals: because of their articulations, the distance between tagms (head, thorax and abdomen) differed greatly among individuals. The bias was confirmed by the low repeatability of this measure on each specimen. In addition, ethanol storage may modify insect abdomen size by swelling or distension (Gruner 2003). Hence, TOT was not used for the multivariate analyses. High variation was also found for dry mass (MASS), but this measure was reliable and reflects individual variability.
Adequate normality of the distribution of residuals was checked for each variable, each sex and each site by Q–Q plots and Shapiro–Wilk tests; Levene tests were applied to test homoscedasticity of the variables. A two-way Manova was then performed to test the significance of the factors (sex and site) with respect to potential correlations among variables. This multivariate analysis was followed by a Factorial Discriminant Analysis (FDA) for each sex to determine how the individuals from the five sites were structured according to the measured variables. Between- and Within-Group analyses were also used for each sex to determine the proportion of inertia explained by the differences among sites (inter-site variability) on the one hand, and by heterogeneity within sites (intra-site variability) on the other. Separation and neighbourhood of the sites observed on both discriminant planes were fully confirmed using Hierarchical Ascendant Classifications (HAC) on measured variables, with a priori input of five groups (data not shown).
A univariate procedure was performed to explore further the sex × site interaction. Interaction plots showed only an interaction for the STER parameter. A two-way Anova was therefore performed for STER to test (i) differences among sites for STER in each sex (site effect), (ii) sexual dimorphism for STER in each site (sex effect), and (iii) variation among sites in the expression of the sexual dimorphism for STER (sex × site interaction). Pairwise comparisons were performed by the Tukey post hoc procedure.
All statistical tests were done with R™ 2.7.0 with an α threshold = 0.05. Multivariate analyses were run with ADE4 plugin for R™ 2.7.0 (Thioulouse and Dray 2007).
Results
Sexual dimorphism and morphological differences among sites
Mean trait values for each sex and each site are shown in Table 1 with the Manova results. A strong and significant sexual dimorphism appeared, as well as significant morphometric differences among sites. Interaction plots showed the highest sex × site effect for STER (Fig. 4). This suggests that sexual size dimorphism expression was more altered by the site effect for the last abdominal sternite than for the eight other parameters and for the mass. As shown in Fig. 4, STER sexual dimorphism was highest in Port Elizabeth—with females showing the highest values—whereas it was similar in the other sites.
STER sexual dimorphism was further examined using the Tukey post hoc procedure (Fig. 5). Sexual dimorphism for STER was significant only in Port Couvreux, Port Elizabeth and Ile Guillou. Sexual dimorphism was highest in Port Elizabeth and lowest in Port Jeanne d’Arc. Moreover, STER did not differ significantly among males, whereas differences appeared among females (Fig. 5).
Mean STER (SE) in males and females sampled in the five distinct sites. Sites are sorted by colonization dates, from Port Couvreux (1913) to Pointe Guite (ca. 2003). Between sexes: *** P ≤ 0.001, ** P ≤ 0.005; n.s. not significant. Between sites: different letters (males) and numbers (females) indicate significant differences between samples
Variation of morphological traits in males
The Manova confirmed the significant differences among populations. FDA was performed to determine which traits act to separate the populations. In males, the first factorial plane (F1 × F2) accounted for 82.2% of the total inertia (Fig. 6). Groups were significantly separated by the first and second axes (Wilks λ ≈ 0, Bartlet χ², P < 0.001 for F1 and F2). The correlation circle and structure matrix (data not shown) indicated that F1 is a general body size axis (contributions from ELYT, FEMU1, FEMU3, PRONOL and MASS), with negligible contributions from STER, PALP, INTOC and PRONOW. The second axis (F2) mainly corresponded to a contrast between STER and PALP plus INTOC.
Factorial Discriminant Analysis: projection of variables (correlation circle) and males of each population onto the first factorial plane. Lines link individuals to the centre of gravity of their group. Gray curves illustrate density distribution of projected individuals on each axis. The amounts of inertia explained by axes F1 and F2 are expressed as percentages. 1 Port Couvreux, 2 Port Elizabeth, 3 Port Jeanne d’Arc, 4 Ile Guillou, 5 Pointe Guite
Individuals coming from the habitats with the longest (Port Couvreux) and shortest (Point Guite) residence times were opposed on the first discriminant axis (Fig. 6): the smallest individuals were from Port Couvreux (1) and the largest from Pointe Guite (5). Individuals from populations 2, 3 and 4 had intermediate positions and were poorly discriminated on F1 axis. The F2 axis sorted populations as follows: 1; 5; 2; 4 and 3. Individuals from Port Couvreux (1) were well discriminated owing to the particularly low values of their PALP (see Table 1).
Per site discriminations were not completely achieved, and overlapping between populations appeared. In Port Couvreux (1) and Pointe Guite (5), males were the best assigned to their group (respectively 78 and 70%). Individuals from Port Jeanne d’Arc (3) and Ile Guillou (4) were well separated too (respectively 65 and 63%), but most of the males from Port Elizabeth (2) were misclassified, with only 30% well assigned. This finding may be linked to the results of the Between- and Within-Group Analyses, which showed that inter-site differences accounted for only 15% of the total inertia, while intra-site heterogeneity accounted for most of the inertia (85%). These results point to the significant effect of the different sites as well as important intra-site variability caused by high inter-individual heterogeneity. The relative standard deviation (RSD) was used to quantify the variability between males within each population. For all variables, the RSD reached 6.0% in Port Couvreux, 5.6% in Port Elizabeth, 5.4% in Port Jeanne d’Arc, 5.4% in Ile Guillou, and 5.7% in Pointe Guite.
Variation of morphological traits in females
Overall, conclusions were similar for females except that the first factorial plane was rotated. The sign relationship of the F1 and F2 axes was inverted and did not account for the same amount of inertia as in males (Fig. 7). This first factorial plane accounted for 77.6% of the total inertia, with significant discrimination on the two-first axes (Wilks λ ≈ 0, Bartlet χ², P < 0.001 for F1 and F2). The F1 axis mainly discriminated populations by the contrast between STER and PALP plus INTOC (as for the males’ F2 axis). F2 was an increasing general body size axis, mainly constructed by MASS, ELYT, FEMU1 and FEMU3. Port Couvreux (1) and Port Jeanne d’Arc (3) individuals were opposed on the F1 axis, and Pointe Guite (5) individuals were well separated on the F2 axis.
Factorial Discriminant Analysis: projection of variables (correlation circle) and females of each population onto the first factorial plane. Lines link individuals to the centre of gravity of their group. Gray curves illustrate density distribution of projected individuals on each axis. The amounts of inertia explained by axes F1 and F2 are expressed as percentages. 1 Port Couvreux, 2 Port Elizabeth, 3 Port Jeanne d’Arc, 4 Ile Guillou, 5 Pointe Guite
Misclassifications also appeared in females. In Port Couvreux (1), Port Jeanne d’Arc (3) and Pointe Guite (5), females were the best assigned to their groups (respectively 73, 70 and 67%), whereas only 50 and 40% of the individuals from Port Elizabeth (2) and Ile Guillou (4) were well assigned, respectively. As in males, Between- and Within-Group Analyses indicated high intra-site variability: inter-site and intra-site differences accounted for 13.7 and 86.3% of the total inertia, respectively. For the eight reliable measurements, the RSD were 6.4% in Port Couvreux, 5.6% in Port Elizabeth, 5.6% in Port Jeanne d’Arc, 6.9% in Ile Guillou, and 5.7% in Pointe Guite.
Discussion
The importance of examining variation of morphological traits was recently re-emphasised because these traits (i) are used extensively for taxonomy, (ii) are partially under genetic control, (iii) are the target of selection, and (iv) reflect intraspecific clinal divergence (Garnier et al. 2005). Moreover, variation in morphology can exhibit clear patterns of differentiation that molecular markers may not detect (Nice and Shapiro 1999).
Imagos of M. soledadinus were collected at the same times on the Péninsule Courbet (Eastern part of Iles Kerguelen) in similar microhabitats (under tide drift lines and stones along the seashore). Climatic conditions differ greatly between the western and eastern part of Iles Kerguelen. The western region is mountainous and experiences Foehn winds. The eastern region, and more particularly the Péninsule Courbet, is composed of large coastal plains, where climatic conditions at sea level are similar. This assumption is supported by our meteorological data collected on Ile Guillou and at Port-aux-Français at a depth of five centimetres below ground level. The size-trait divergence patterns observed among the five distinct sites on Iles Kerguelen can thus be related to the residence time of M. soledadinus in each location, i.e. to the distinct abundance and availability of the trophic resources as a result of predation pressure (Chevrier 1996; Chevrier et al. 1997; Laparie, Lebouvier and Renault, unpublished data), rather than to differential abiotic conditions in the microhabitats.
Variation in body size, either at the individual level or in the frequency distribution of individuals’ body sizes within a population, may indicate different types of environmental stress (McGeoch 1998). Nutrition is one of the best studied factors that affect morphometry and that can vary among habitats (Shingleton et al. 2007). In our study, in both sexes, morphometry of individuals from Port Couvreux (highest residence time) was always opposed to that one of individuals from Pointe Guite (shortest residence time), both being extremes in terms of general body size. As assumed by the “decreasing body size hypothesis” (Blake et al. 1994; Gray 1989; Szyszko 1983), highly disturbed habitats support smaller ground beetles more than less disturbed areas do (Magura et al. 2006). This probably results from their lowest energetic requirements for growth and their shortest durations of development (Peters 1983). Given the low arthropod diversity on Iles Kerguelen, the two native wingless flies Anatalanta aptera (Diptera, Sphaeroceridae) and Calycopteryx moseleyi (Diptera, Micropezidae) represent two of the most profitable prey for M. soledadinus in terms of energy (Vernon 1986) and handling time (including spotting, capture, eating and digesting—see Krebs and Davies 1993). Over the last 25 years, no A. aptera or C. moseleyi were observed at Port Couvreux and very few were observed near Port Elizabeth although they used to be abundant in both sites (Chevrier 1996; Lebouvier, unpublished data). These species disappeared from Port aux Français as soon as M. soledadinus arrived there (1995) and could still be observed some few kilometers away at Pointe Guite in 2006, where some adults of M. soledadinus were also observed for the first time. Populations of M. soledadinus were found to have a strong impact on invertebrate diversity and abundance (Chevrier 1996; Lalouette, Lebouvier and Renault, unpublished data). A potential predation switch to microinvertebrates such as springtails when the more profitable macroinvertebrate prey like the native flies become scarcer could explain the body size decrease. In addition, a morphometric differenciation also appeared in populations characterized by recent and close residence times, supporting earlier conclusions of Chevrier et al. (1997) that M. soledadinus quickly becomes the most abundant species in invaded habitats.
Besides the overall body size, shape and size of trophic appendages were found to vary between individuals supplied with distinct diets (Thompson 1992; Thompson 2001). Divergence in mouthparts may result in an asymmetry of the prey consumed (Pearson and Stemberger 1980). The thinnest maxillary palps and smallest heads were found in individuals from Port Couvreux, but there were no significant differences among individuals from other sites for those variables. In light of the significant morphological differences among individuals from Port Elisabeth, Port Jeanne d’Arc, Ile Guillou and Pointe Guite, biometric evolution of both head and maxillary palps cannot be coupled to changes in size of the other morphological variables. The residence time in each habitat could be an influential variable in the morphology of M. soledadinus. We hypothesize that alteration of available resources, and the resulting prey-switching, act on the general morphology of the individuals and determine the evolution of the mouthparts parts’ size.
Body size of M. soledadinus can also be altered by intrapopulation competition. Lenski (1982) showed that body size was reduced when density and competition increased in Carabus limbatus (Coleoptera, Carabidae). During the survey of the geographic distribution and abundance of M. soledadinus in 2005–2006, we quantified population densities on Iles Kerguelen. We defined 4 abundance levels from 1 (no individual found after a 10 min search by one person to 4 (very high densities, more than 250 individuals found in 10 min by one person). The five locations sampled in the present study were characterized by the highest abundance level (level 4, very high densities), thus suggesting intrapopulation competition. Despite significant discrimination of the five sampled populations, high variability occurred within each population. In the parasitic beetle Brachinus lateralis (Coleoptera, Carabidae), host size is of prime importance to individual size, but the limited opportunity for host choice may maintain size variation despite evidence of natural selection pressure on size (Juliano 1985). Intrapopulation competition may maintain size heterogeneity in M. soledadinus owing to different food intakes between individuals, regardless of the overall habitat resources. Developmental instability, i.e. intra-individual stochastic variation and phenotypic noise during growth (Nijhout and Davidowitz 2003; Dongen 2006), could also maintain variability within populations regardless of the factors studied here. In addition, the life cycle of M. soledadinus lasts about 1 year and its lifespan exceeds 1 year (Jeannel 1940; Ernsting 1993), suggesting that we should have more than a single cohort within each population. Variation in adult body size may also result from different thermal conditions larval instars endured during their development (Angilletta 2009). Although there are no hints suggesting different population structure in different sites, we can therefore not rule out a possible effect on body size.
Cannibalism may also impact body size when both quality and quantity of food are reduced. This phenomenon was described as prevalent when food resources are limiting because it reduces competition for these resources (Dong and Polis 1992; Currie et al. 1996). More cannibalistic interactions may occur in the first habitats colonized by M. soledadinus. When breeding M. soledadinus populations under controlled conditions, we observed predation only of imagos on larvae (no predation by larvae of one another). As smaller individuals develop faster than bigger ones under similar conditions (as shown by Blanckenhorn (1998) in Scatophaga stercoraria (Diptera, Scatophagidae)), small individuals of M. soledadinus may be less penalized by reaching the adult stage more quickly. Indeed, Chown and Nicholson (2004) reported that traits promoting large body size such as extended development time or increased growth rate (which depends on increased feeding rate) increase the risk of predation. In our study, the residence time could promote a body size diminution and a faster development owing to resource limitation and higher intrapopulation competition in the most altered sites. However, we also must keep in mind that larvae have a different space-approaching behaviour than adults, i.e. they bury themselves and are thus found in the soil, whereas adults are usually found at ground level.
As was previously demonstrated by Chevrier (1996), we found a significant sexual dimorphism in adults of M. soledadinus, with females larger than males. This result is consistent with the literature, since several insect species have already been characterized by marked sexual dimorphism in either mass, size, shape or even physiological capabilities (Day et al. 1994; Fairbairn 1997; Renault et al. 2003; Svensson 1977). Because of their different roles in reproduction, males and females are often under selection that favours their divergent morphological appearance (Badyaev 2002). Energetic allocation contributes greatly to explaining sexual size dimorphism, with females having a higher energy cost associated with gamete production (Nylin and Gotthard 1998; Tammaru et al. 2002). On the other hand, male size may affect their dispersal and be important in sexual selection and competition for females (Nylin and Gotthard 1998).
The morphological traits covarying with reproductive and dispersal functions are of particular interest with respect to the invasive success of a species. They represent the level of developmental and morphological plasticity of the species to the environmental and ecological characteristics of the newly colonized habitat (Chown et al. 2007; Rosecchi et al. 2001). Reproductive traits and more particularly copulatory organs often exhibit reduced variation relative to body size within species of arthropods (Eberhard 1998). We found that STER variation was not paired with body size variation. STER values in males were similar in all sites regardless of body size variation. In females, STER values did not differ between the smallest individuals (Port Couvreux and Port Elizabeth) and the biggest ones (Pointe Guite). Energetic allocation during diet restriction thus involves differential developmental plasticity for STER. The target of sexual selection could be reproduction-related traits rather than body size, as was found previously by Preziosi and Fairbairn (1996). Because insects may have greater success with larger genitalia and not because they are bigger per se, the general body size-independent variation of STER is ecologically relevant. In addition, a stronger influence of reproduction on morphological traits in females than in males can be hypothesized. In Port Elizabeth, the sternites are short in males and long in females compared to the sternites of individuals from other populations. This may result from a greater difference in energetic allocation to reproduction between females and males in this population. Port Elizabeth could be a transitional stage in which reaction norms optimizing fecundity are expressed earlier in females. The relationship between STER and fecundity should be investigated further in M. soledadinus by measuring the number and size of ovarioles and eggs.
An integrative conjecture can be hypothesized to explain the relationship between morphometry and the invasion process in populations of M. soledadinus. In both sexes, differentiation occurred through body size (MASS, ELYT, FEMU1, FEMU3, PRONOL) and reproduction (STER) versus feeding strategy (PALP, INTOC). Life histories promoting increased body size might be prominent when a species colonizes new sites, as was found in individuals from Pointe Guite. Larger individuals may be characterized by higher dispersal ability (Thiele 1977) and may therefore be more efficient than smaller ones in colonizing and establishing populations in new sites. Subsequently, reproduction strategies and adjustments of the feeding behavior as a consequence of the available prey in the microhabitat may be important for the settlement of the populations, as suggested by the characteristics of Port Couvreux individuals.
Blanckenhorn (2000) highlighted the need for experimental and comprehensive studies that address the fitness costs at the ecological level of being larger. Selection of large body size occurs in most organisms and empirical evidence is needed to determine if sporadic selection in time and space suffices to counterbalance this major evolutionary force (Blanckenhorn 2000). In the present study, we demonstrated morphological differentiations at a local scale among several populations differing from both geographical and chronological standpoints. We suggest that the observed size diminution of M. soledadinus may reflect both a priori (development under diet restriction, survival) and a posteriori (intrapopulation competition, cannibalism) effects on development and selection. Our study indicates that being smaller might be advantageous in habitats where optimal trophic resources are altered by M. soledadinus, a view that supports some of the mechanisms proposed by Blanckenhorn (2000).
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Angilletta J, Dunham AE (2003) The temperature-size rule in ectotherms: simple evolutionary explanations may not be general. Am Nat 162:332–342
Arthur AL, Weeks AR, Sgrò CM (2008) Investigating latitudinal clines for life history and stress resistance traits in Drosophila simulans from eastern Australia. J Evol Biol 21:1470–1479
Atchley WR (1971) A comparative study of the causes and significance of morphological variation in adults and pupae of Culicoides: a factor analysis and multiple regression study. Evolution 25:563–583
Badyaev AV (2002) Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol 17:369–378
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Integr Comp Biol 23:85–97
Blake S, Foster GN, Eyre MD, Luff ML (1994) Effects of habitat type and grassland management practices on the body size distribution of carabid beetles. Pedobiologia 38:502–512
Blanckenhorn WU (1998) Adaptive phenotypic plasticity in growth, development, and body size in the yellow dung fly. Evolution 52:1394–1407
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–487
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Blanckenhorn WU, Stillwell CR, Young KA, Fox CW, Ashtons KG (2006) When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution 60:2004–2011
Boggs CL, Freeman KD (2005) Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144:353–361
Bommarco R (1998) Reproduction and energy reserves of a predatory carabid beetle relative to agroecosystem complexity. Ecol Appl 8:846–853
Butler MG (1986) Life history of aquatic insects. In: Resh VH, Rosenberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 24–35
Cabanita R, Atkinson D (2006) Seasonal time constraints do not explain exceptions to the temperature size rule in ectotherms. Oikos 114:431–440
Chevrier M (1996) Introduction de deux espèces d’insectes aux Îles Kerguelen: processus de colonisation et exemples d’interactions. Ph.D thesis, Université de Rennes 1, France, p 187
Chevrier M, Vernon P, Frenot Y (1997) Potential effects of two alien insects on a subantarctic wingless fly in the Kerguelen Islands. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 424–431
Chown SL, Klok CJ (2003) Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography 26:445–455
Chown SL, Nicholson SW (2004) Letal temperatura limits. In: Chown SL, Nicholson SW (eds) Insect physiological ecology: mechanisms and patterns. Oxford University Press, Oxford, pp 115–153
Chown SL, Slabber S, McGeoch MA, Janion C, Leinaas HP (2007) Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc R Soc B 274:2531–2537
Currie CR, Spence JR, Niemelä J (1996) Competition, cannibalism and intraguild predation among ground beetles (Coleoptera:Carabidae): a laboratory study. Coleopts Bull 50:135–148
Day KE, Kirby RS, Reynoldson TB (1994) Sexual dimorphism in Chironomus riparius (Meigen): impact on interpretation of growth in whole-sediment toxicity tests. Environ Toxicol Chem 13:35–39
den Nijs LJMF, Lock CAM, Noorlander J, Booij CJH (1996) Search for quality parameters to estimate the condition of Pterostichus cupreus (Col., Carabidae) in view of population dynamic modelling. J Appl Entomol 120:147–151
Dong Q, Polis GA (1992) The dynamics of cannibalistic populations: a foraging perspective. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford Scientific Publications, Oxford, pp 13–37
Dongen SV (2006) Fluctuating asymmetry and developmental instability in evolutionary biology: past, present and future. J Evol Biol 19:552–563
Eberhard WG (1998) Sexual behavior of Acanthocephala declivis guatemalana (Hemiptera:Coreidae) and the allometric scaling of their modified hind legs. Ann Entomol Soc Am 91:863–871
Ernsting G (1993) Observations on life cycle and feeding ecology of two recently introduced predatory beetle species at South Georgia, sub-Antarctic. Polar Biol 13:423–428
Fairbairn DJ (1997) Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu Rev Ecol Syst 28:659–687
Garnier S, Magniez-Janin F, Rasplus JY, Alibert P (2005) When morphometry meets genetics: inferring the phylogeography of Carabus solieri using Fourier analyses of pronotum and male genitalia. J Evol Biol 18:269–280
Gray JS (1989) Effects of environmental stress on species rich assemblages. Biol J Linn Soc 37:19–32
Gruner DS (2003) Regressions of length and width to predict arthropod biomass in the Hawaiian Islands. Pac Sci 57:325–336
Hallas R, Schiffer M, Hoffmann AA (2002) Clinal variation in Drosophila serrata for stress resistance and body size. Genet Res 79:141–148
Hodar JA (1996) The use of regression equations for estimation of arthropod biomass in ecological studies. Acta Oecol 17:421–433
Jarosik V (1989) Mass vs. length relationship for carabid beetles (Col., Carabidae). Pedobiologia 33:87–90
Jeannel R (1940) Croisière du Bougainville aux îles australes françaises. III. Coléoptères. Mem du Museum National d’Histoire Naturelle, France, Sér A 14:63–202
Juliano SA (1985) The effects of body size on mating and reproduction in Brachinus lateralis (Coleoptera: Carabidae). Ecol Entomol 10:271–280
Juliano SA (1986) Food limitation of reproduction and survival for population of Brachinus (Coleoptera:Carabidae). Ecology 67:1036–1045
Karino K, Seki N, Chiba M (2004) Larval nutritional environment determines adult size in Japanese horned beetles Allomyrina dichotoma. Ecol Res 19:663–668
Kenis M, Auger-Rozenberg MA, Roques A, Timms L, Péré C, Cock MJW, Settele J, Augustin S, Lopez-Vaamonde C (2008) Ecological effects of invasive alien insects. Biol Invasions 11:21–45
Krebs JR, Davies NB (1993) An introduction to behavioural ecology. Blackwell Scientific Publications, Oxford, 432 p
Lenski RE (1982) The impact of forest cutting on the diversity of ground beetles (Coleoptera:Carabidae) in the southern Appalachians. Ecol Entomol 7:385–390
Magura T, Tóthmérész B, Lövei GL (2006) Body size inequality of carabids along an urbanisation gradient. Basic Appl Ecol 7:472–482
McGeoch MA (1998) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev 73:181–201
Moczek AP (1998) Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behav Ecol 9:636–641
Nice CC, Shapiro AM (1999) Molecular and morphological divergence in the butterfly genus Lycaeides (Leipdoptera: Lycaenidae) in North America: evidence of recent speciation. J Evol Biol 12:936–950
Nijhout HF, Davidowitz G (2003) Developmental perspectives on phenotypic variation: canalization, and fluctuating asymmetry. In: Polak M (ed) Developmental instability: causes and consequences. Oxford University Press, Oxford, pp 3–13
Nylin S, Gotthard K (1998) Plasticity in life-history traits. Ann Rev Entomol 43:63–83
Pearson DL, Knisley CB (1985) Evidence for food as limiting resource in the life cycle of tiger beetles (Coleoptera:Cicindelidae). Oikos 45:161–168
Pearson DL, Stemberger SL (1980) Competition, body size and the relative energy balance of adult tiger beetles (Coleoptera:Cicindelidae). Am Midl Nat 104:373–377
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Preziosi RF, Fairbairn DJ (1996) Sexual size dimorphism and selection in the wild in the waterstrider Aquarius remigis: Body size, components of body size and male mating success. J Evol Biol 9:317–336
Renault D, Hance T, Vannier G, Vernon P (2003) Is body size an influential parameter in determining the duration of survival at low temperatures in Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae)? J Zool 259:381–388
Rosecchi E, Thomas F, Crivelli AJ (2001) Can life-history traits predict the fate of introduced species? A case study of two cyprinid fish in southern France. Freshw Biol 46:845–853
Schmidt-Nielsen K (1984) Scaling: why is body size so important? Cambridge University Press, Cambridge, 241 p
Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ (2007) Size and shape: the developmental regulation of static allometry in insects. Bioessays 29:536–548
Snyder W, Evans E (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Syst 37:95–122
Sota T (1985) Limitation of reproduction by feeding condition in a carabid beetle, Carabus yaconinus. Res Popul Ecol 27:171–184
Stern DL, Emlen DJ (1999) The developmental basis for allometry in insects. Development 126:1091–1101
Stern DL, Moon A, Martinez del Rio C (1996) Caste allometries in the soldier-producing aphid Pseudoregma alexanderi (Hormaphididae: Aphidoidea). Insectes Soc 43:137–147
Svensson B (1977) Life history, energy fluctuations, and sexual differentiation in Ephemera danica (Ephemeroptera), a stream-living mayfly. Oikos 29:78–86
Szyszko J (1983) State of Carabidae (Col.) fauna in fresh pine forest and tentative valorisation of this environment. Warsaw Agricultural University Press, Warsaw
Tammaru T, Esperk T, Castellanos I (2002) No evidence for costs of being large in females of Orgyia spp. (Lepidoptera Lymantriidae): larger is always better. Oecologia 133:430–438
Thiele HU (1977) Carabid beetles in their environments. Springer, Berlin
Thioulouse J, Dray S (2007) Interactive multivariate data analysis in R with the ade4 and ade4TkGUI packages. J Stat Soft 22:1–14
Thompson DB (1992) Consumption rates and the evolution of diet-induced plasticity in the head morphology of Melanoplus femurrubrum (Orthoptera: Acrididae). Oecologia 89:204–213
Thompson DB (2001) Genotype-environment interaction and the ontogeny of diet-induced phenotypic plasticity in size and shape of Melanoplus femurrubrum (Orthoptera: Acrididae). J Evol Biol 12:38–48
Vernon P (1986) Evolution des réserves lipidiques en fonction de l’état physiologique des adultes dans une population expérimentale d’un Diptère subantarctique: Anatalanta aptera Eaton. Bull Soc Ecophysiol 11:95–116
Wheeler D (1996) The role of nourishment in oogenesis. Ann Rev Entomol 41:407–431
Wise DH (1979) Effects of an experimental increase in prey abundance upon reproductive rates of two orb-weaving spider species (Araneae: Araneidae). Oecologia 41:289–300
Acknowledgments
This research was supported by the Institut Polaire Francais (IPEV, programme 136), the CNRS (Zone-Atelier de Recherches sur l’Environnement Antarctique et Subantarctique), and the Agence Nationale de la Recherche (programme EVINCE 2007, Vulnerability of native communities to invasive insects and climate change in sub-Antarctic Islands). This research is linked to the SCAR Evolution and Biodiversity in the Antarctic research programme. We are grateful to Y. Frenot, P. Vernon and two anonymous referees for helpful comments and improvement of earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laparie, M., Lebouvier, M., Lalouette, L. et al. Variation of morphometric traits in populations of an invasive carabid predator (Merizodus soledadinus) within a sub-Antarctic island. Biol Invasions 12, 3405–3417 (2010). https://doi.org/10.1007/s10530-010-9739-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9739-2