Abstract
Micronutrient silicon (Si) is receiving increasing attention in agriculture for its benefits to plant growth and stress tolerance. Plants have developed a highly efficient Si-transport mechanism that entails the localization of Si-transporter proteins such as Low silicon1 (Lsi1), Low silicon2 (Lsi2), Low silicon3 (Lsi3), and Low silicon6 (Lsi6), as well as the expression profiling that establishes a highly coordinated network between these proteins, facilitating Si uptake and accumulation. It has also been discovered that silicon (Si) can promote plant growth and alleviate a variety of biological and abiotic stressors. In this review paper, the effects of Si on plant–pathogen interactions are analyzed from physical, biochemical, and molecular perspectives. The addition of silica improves the plant’s physiological and chemical characteristics, including its defence mechanisms, hormonal modulation, and gene expression patterns. Si activates defence-related enzymes, promotes the production of antimicrobial compounds, regulates signal pathways, and induces the expression of defence-related genes. This results in combined resistance that dominates the biochemical/molecular resistance during plant–pathogen interactions. Furthermore, Si alleviates the toxic effects of abiotic stresses such as salt stress, drought, and heavy metals. Silicon’s ability to manage various plant stressors, the mechanisms of silicon-enhanced resistance and silicon’s inhibitory effects on pathogens in vitro are also discussed in this review paper. By integrating the information presented, a clear relationship between silicon treatments and plant growth promotion can be established. This information is valuable for understanding the role of Si in agriculture and improving the utilization of Si fertilizers and sources for agricultural production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
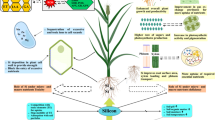
Although silicon (Si) is the most abundant element in the lithosphere, its inclusion in a range of essential components has not yet been provided. Si has a critical role in stimulating plant growth and development. The physiological and metabolic features of plants are greatly influenced by Si. Even though it is a non-essential element, its presence is 30%, the majority of which may be found in minerals. In general, Si is regarded as a non-essential element for plant species, although its inclusion in fertilizer formulations as a favourable element helps plants cope with stress circumstances (López-Pérez et al. 2018). When growing plants in soil-free settings, Si have a positive effect on their growth (Luyckx et al. 2017). Abiotic stressors such as drought, salinity, heat, cold, metal toxicity and lodging can all be alleviated by adding Si to plants. Silicon’s varied contribution to plant growth and yield, increased mechanical strength, improved light absorption, and resilience to several stresses, has earned it the designation of “quasi-essential” from the agronomic industry (Bhardwaj et al. 2022). Additionally, Si enhances resistance to biotic stressors, such as plant diseases and insect pests (Van Bockhaven et al. 2013). Plant tissues such as roots, leaves, stems and hulls are mostly responsible for Si’s beneficial impacts (Fig. 1). The coating of silicon dioxide (SiO2) acts as a physical barrier, preventing fungus from penetrating the plant tissues and insects from probing and biting (Ma 2004). The plant’s stress signalling system interacts with numerous important molecules in soluble Si, suggesting that it may have a role in increasing host resistance to disease (Rodrigues et al. 2004). A wide variety of important genes, associated with stress and regulating plant growth and development, are activated in response to Si absorption (Luyckx et al. 2017; Zargar et al. 2019; Islam et al. 2020; Mir et al. 2022). Multiple specialized and non-specific transporters function together to deliver Si into the aerial portions of crop plants. Numerous genes, including Low silicon 1 (Lsi1), Low silicon 2 (Lsi2), Low silicon 3 (Lsi3), and Low silicon 6 (Lsi6), are involved in Si absorption in roots and aerial parts in several field crops (Wang and Munshi 2015; Ratcliffe et al. 2017; Ouellette et al. 2017).
Beneficial effects of Si under various abiotic stresses. The degree of accumulation of Si in plant roots and shoots affects the degree of impact of micronutrient in the plant. The amount of Si accumulation in shoots is different in different plant spp. Although the micronutrient is available in the soil itself, still all the plant’s roots do not possess the ability to uptake and accumulate Si
Silicon effectively inhibits the toxicity of elements such as Al and Mn, confers resistance to pests and diseases, and even allows the production of nanostructures utilizing organic molecules, enzymes, or organisms as catalysts. It improves photosynthesis, reduces transpiration, and increases plant resistance to biotic and abiotic stresses by regulating the electron transport chain, cellular homeostasis, oxidative phosphorylation, and photosynthetic complex. Proposed mechanisms include creating cell-wall barriers [made through Si(OH)4 polymerization] for tolerance to infection or chemical resistance, modulating antioxidant enzymes and compound synthesis, as well as nutrient uptake mechanisms during water stress (Soundararajan et al. 2016). This review synthesizes the findings of multiple studies to gain a deeper understanding of the interactions between Si and various factors that impact plant growth and health. The paper aims to summarize the mechanism, regulation, and interaction between Si and phytohormones, the role of Si in mediating biotic and abiotic stress, and the processes involved in the influx of Si into the plant through various Si-transporters after its absorption. By summarizing the current state of knowledge on these topics, this review will provide valuable insights for further research and a foundation for developing new strategies for optimizing plant growth and stress tolerance.
Transporters of silicon: their roles and expression patterns in different plant species
There must be some transport mechanism for Si in plants to reap its benefits. In soils with a pH below 9 (Ma and Takahashi 2002), plants absorb silicon as silicic acid, an uncharged monomeric molecule. The uptake, transport, accumulation and distribution of Si necessitate many transporters (Table S1). There are two types of Si transporters in higher plants: channel-type and efflux transporters.
Channel-type/influx Si transporters
Rice necessitates a significant amount of Si for growth, development, and yield. Hence the first Si-influx transporter was discovered in rice (Li et al. 2022). Si is passively transported between the external solution (i.e., apoplast) and the plant cells via the channel-type transporter (Lsi1; Low silicon 1) of Si. All of the channel-type transporters that have been discovered so far, including rice (Lsi6) (Yamaji et al. 2008) in various crops such as barley (Chiba et al. 2009; Yamaji et al. 2012), wheat (Montpetit et al. 2012), maize, cucumber (Mitani et al. 2009a), pumpkin, and soybean, are homologs of rice Lsi1. They are aquaporin (AQP)-like proteins, which are major intrinsic proteins (MIPs), a member of the NIP (Nod26-like intrinsic proteins) subfamily. The AQP family contains unique NIPs, which are exclusive to plants and not found in animals (Saitoh et al. 2021). Aquaporins are a class of membrane channel proteins that facilitate the translocation of aqueous molecules and other small ions across biological membranes (Mitani et al. 2009a). Despite being a passive bidirectional channel, Lsi1 collaborates with an efflux Si transporter to perform the role of an inflow transporter of silicon in plants. The rice gene OsLsi1 brings Si from soil solutions into the roots. Like Lsi1, Lsi6 transports Si across plasma membranes. Lsi6 differs from Lsi1 in expression and subcellular localization. The plant’s roots express Lsi1, but it is missing from the shoots, whereas both roots and shoots express Lsi6. It was found that Lsi6 expression is higher in the developing roots nearest to the root tip, while Lsi1 expression is higher in the mature roots. The side of Lsi6 closest to the vessel shows polar localization. This suggests that Lsi6 transports Si from the xylem to leaf tissues (Yamaji et al. 2008).
Efflux Si transporters
Si is exported from plant cells by the efflux transporter (Lsi2) of Si (Chain et al. 2009; Ma and Yamaji 2015; Deshmukh et al. 2015). Lsi2 was initially discovered in rice (Ma et al. 2007), and its homologs have since been isolated from barley (HvLsi2), maize (ZmLsi2) (Mitani et al. 2009b), and pumpkin (CmLsi2). While Lsi1 relies on passive transport, Lsi2 relies on an active process driven by the plasma membrane proton gradient to transport Si (Mitani-Ueno et al. 2011). Depending on the type of plant, Lsi1 and Lsi2 have distinct patterns of expression in terms of their tissue and/or cellular localization (Ma et al. 2008). The rice and barley roots express Lsi2, while the roots and the shoots express GmNIP2-1, GmNIP2-2, CmLsi1, CSiT1, and CSiT2, as well as additional homologous genes, including OsS6, HvLsi6, and ZmLsi6. Another transporter, OsLsi3, is positioned apolarly between the dilated and enlarged vascular networks in the parenchyma. Si distributions in panicles were decreased in Lsi2 and Lsi3 knockouts, however, they were elevated in flag leaves, similar to what was observed in Lsi6 knockouts (Li et al. 2022).
Efficient coupling of Si influx and efflux transporters
Si uptake is facilitated by the expression of Lsi1 and Lsi2 in the roots. The different polarity of cell layers in rice indicates that cooperation between Lsi1 and Lsi2 is necessary to uptake nutrients (Ma et al. 2007). Exodermis and endodermis of rice roots have two Casparian strips that prevent the apoplastic movement of water and other solutes across each cell layer (Mitani et al. 2009a). Root maturation destroys the majority of cortical cells, most of which are lined by sclerenchyma, and forms aerenchyma, with the cell wall remnants that are left forming narrow, spoke-like apoplastic connections between the exodermis and endodermis (Yamaji and Ma 2009; Yamaji et al. 2012). As a result, Lsi1 imports Si from the exodermal cell’s distal side into the symplast, and Lsi2 exports it to the apoplastic connections from the proximal side. Endodermis-located Lsi1 and exodermis-located Lsi2 are responsible for importing and exporting Si from the endodermis to the stele, respectively (Sakurai et al. 2015). Si uptake can be efficiently transported in a polar orientation due to the polarization of Lsi1 and Lsi2. The similarity in their expression patterns showed that root Si absorption significantly reduced when either Lsi1 or Lsi2 was knocked out (Yamaji et al. 2012).
Mechanism of uptake, transportation, and absorption of silicon
Since Si undergoes many chemical changes throughout the storing, depositing, and transporting stages, its concentration varies widely among plant tissues (Fig. S1A). Plants have their unique method for absorption and transportation of nutrients (Kaur and Greger 2019). Physiological observations such as plant Si content and water uptake rates have been used to predict three distinct Si uptake mechanisms depending on water absorption (Mitani 2005; Kaur and Greger 2019). Plants are referred to as active systems when they have higher silicon absorption ability than water absorption ability. Plants with silicon uptake rates comparable to water uptake rates are called passive systems. In contrast, plants with lower silicon uptake rates than water uptake rates are referred to as rejective system (Marron et al. 2016).
Active system
This mechanism is exemplified by silicon-accumulating plants such as barley and maize. Lsi1 and Lsi2 are Si transporters that participate in both influx and efflux activities. Moreover, either one or both of them display polar localization. Aerenchyma is present in the majority of rice root cortical cells, which have the morphology of two Casparian strips in the exodermis and endodermis. Lsi1, which is polarized distally, imports Si into the exodermis before Lsi2, which is polarized proximally, exports Si to the aerenchyma. Figure S1 illustrates that Lsi1 and Lsi2 transport Si from the apoplastic region to the endodermis and then to the xylem. Lsi1 is a concentration-dependent passive transporter, whereas Lsi2 is a concentration-dependent active efflux transporter (Ma et al. 2006). Due to the polar localization of Lsi1 and Lsi2, which generate an effective directed transport system, considerable quantities of Si accumulate in the shoots (Ma and Yamaji 2015). Lsi1 and Lsi2 are found in the same cell, and Casparian strips have been identified as important components of an active Si absorption system (Sakurai et al. 2015).
Passive system
This absorption system is used in cucumber and pumpkin to obtain Si from the environment. It has been shown that CmLsi1 and CmLsi2 in pumpkin and CsLi1 and CsLsi2 in cucumber have been partially identified (Mitani et al. 2011; Sun et al. 2017, 2018). Researchers found that CmLsi1 and CsLsi1 are expressed in the majority of root cells, while CsLsi2 is found in endodermal cells (Sun et al. 2017). Most other Si-accumulating plants, excluding CsLsi1, show no polar localization at the cortical cells, unlike Lsi1 and Lsi2 (Mitani-Ueno et al. 2011). The polarity of CsLsi1 at the endodermis may be seen (Sun et al. 2017). Furthermore, Lsi1 and Lsi2 are not found in the same cell in these plant species (Sun et al. 2018). Because Lsi1 is a bidirectional transporter, the presence of Lsi1 and Lsi2 in the same environment makes it difficult for Si to be absorbed (Mitani et al. 2009b).
Rejective system
Non-Si accumulator, such as tomato, employs this uptake method. The larger distance between NPA domains in tomato Lsi1 renders it inactive (Deshmukh et al. 2015). According to a recent study, tomato roots, on the other hand, have a functional Lsi1 called SlLsi1 (Sun et al. 2020). There is no polar localization of SlLsi1 in the root cells. It appears that the lack of a Lsi2-type transporter in tomatoes is the cause for the tomato’s low levels of accumulative accumulation. When a functional Lsi2 from cucumber was produced in tomato, the transgenic tomato plants showed an increase in Si accumulation (Sun et al. 2020). Depending on the processes involved in Si absorption, plants’ capacities for accumulating Si vary substantially. Accumulators (10–15% dry weight), intermediate (1–3% dry weight), and excluders or non-accumulators (1% dry weight); are the three types of plants (Liang et al. 2007). According to recent studies, Si-accumulators, intermediates, and excluders have active and passive systems for absorption and transport. After absorption by the roots, silicic acid is transferred from the cortex to the stele, then moved through the xylem, and finally translocated via a transpiration stream into the shoots.
Reproductive stages of husks of rice and barley are rich in Si. Si increases grain fertility in the husk by reducing water loss and protecting against disease infestation. No stomata can be found on the husk, and the grains have a smaller surface area than the enlarged leaves. This means that transpiration contributes to the uptake of Si by the grain. The nodes of graminaceous plants are important for redistributing absorbed minerals (Fig. S2). Several transporters congregate in the first node under the panicles, providing selective mineral element delivery to the grains with the lowest transpiration (Mostofa et al. 2021).
Si is collected in shoots by transpiration and subsequently polymerized into amorphous silica (SiO2–nH2O) through Si polymerization (Ma et al. 2006). Therefore, this amorphous silica is concentrated in plant cell walls and can also be deposited in root cells. When silicon content in the plant grows, monosilicic acid polymerizes into silica gel through a non-enzymatic process. The silicon remains in the plant tissue (Mitani 2005). The pathway of Si from leaf xylem via Lsi6 is shown in Fig. 2.
Accumulation of Si in leaves. Rice leaves must have a physical barrier (Casparian strip) in order to efficiently assimilate Si from the environment. Lsi6 removes Si from the xylem sap and deposits it in specified cells. Guttation fluid is the only means of removing Si from the roots, and most of the remaining Si is deposited as amorphous hydrated silica for future deposition (Liang et al. 2015a)
Involvement of Si in enhancing plant growth by down-regulation of stresses via various cellular signaling cascade
Plant species such as wheat, rice, maize, and bamboo have already been shown to benefit from Si, although it is still not listed as an essential element for plants. Si provides mechanical support for plant species that are prone to lodging and makes them more resistant to disease. This mechanical support may be owing to the adhesion of Si to cell walls, which increases cell wall rigidity (Collin et al. 2014). Excessive water loss by transpiration can be prevented by depositing silicates in the epidermal tissues of the plants, as well. There are many ways in which Si might help plants recover from environmental stressors such as heavy metals, disease and radiation. Under stressful conditions, Si has a positive impact on plants and soils because of its several roles (Côté-Beaulieu et al. 2009).
Biotic stresses
Silicon is mostly found in the epidermal cells of leaves, stems, and roots. In rice leaf blades, Si is deposited as a 2.5 µm thick film slightly below the cuticular film (0.1 µm thick), forming a double coating of Si-cuticle (Liang et al. 2015b). The blockage is thought to be caused mostly by Si deposition in the cell wall and foliage. Si moves from roots to leaves via the apoplast area. Si is polymerized in intercellular gaps, while Si is deposited in xylem vessels and leaf forming walls (Etesami and Jeong 2018). The polymerized Si of the apoplast and cell wall region can effectively prevent pathogen invasion (Fleck et al. 2011). Additionally, Si has been shown to increase resistance to various diseases. Bacterial blight, brown spot, Magnaporthe grisea, leaf blast, sheath blight, stalk rot in rice, and the powdery mildew of Triticum aestivum, Hordeum vulgare, and Cucumis sativus are some of the diseases that are suppressed by application of silicon (Liang et al. 2007; Marchenkov et al. 2018). Furthermore, Ratnayake et al. (2016) believed that biochemical processes derived from Si is a major tool for providing plant resistance to pathogens than physical mechanisms. For example, Si diminishes disease by developing several chemical barriers such as β-1,3-glucanases, chitinases (CHI) (Cruz et al. 2013), peroxidase (POX) (Mburu et al. 2016), superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), polyphenol oxidases (PPO), phenylalanine ammonia lyase (Zhang et al. 2013). Cruz et al. (2013) discovered that Si increases chitinase activity in response to Asian soybean rust. Rice plants treated with Si showed increased POX activity, while cucumber plants supplied with Si exhibited significant chitinase activity (Dallagnol et al. 2011). H2O2 hydrolysis and cell wall lignification are accelerated in POX-enhanced hosts (Torres et al. 2006). Peroxide also attaches to phenolic polymers that promote tissue lignification. A vital task of PPO is the synthesis of quinines that are much more hazardous to plant pathogens than phenols due to the oxidation of phenolic compounds. In the synthesis of lignin, PPO is critical (Song et al. 2016). By reducing the mechanical disintegration of leaves, silica protects grass chlorenchyma cells from locusts (Schistocerca gregaria). The abaxial surface with a thick wax coating provides coffee plants protection from insect-eating, which is influenced by the presence of Si. Rice plants treated with silica had silicified trichomes resembling a ladder (Alhousari and Greger 2018). Microstructures generated by Si can also act as a mechanical barrier against planthoppers and stem borers.
Induction of plant resistance to plant pathogens by Si (plant–pathogen interaction)
There are two ways in which Si plays an important role in plant defence: (i) physical and (ii) biochemical, and molecular mechanisms (Wang et al. 2017). The formation of silica layer and papillae as well as the depositing of callose are the physical mechanisms of plant defense. Different defence-related enzymes are activated, antimicrobial chemicals are produced, and numerous plant signalling pathways are activated as part of the biochemical mechanism of defence.
(i) Physical defense mechanism
There are two main types of physical defense reactions induced by Si in plant cells such as silica layer formation and papillae formation.
Silica layer formation (silicification): plant mechanical strength has been linked to Si. When silicon is present in the epidermal cells of plants, it encourages silicification, which leads to the formation of papillae and the deposition of complex organic compounds in the epidermal cell walls. Since fungal pathogens cannot penetrate the physical barriers that protect plants, their cells are less vulnerable (Van Bockhaven et al. 2013). The cuticle-Si double layer prevents pathogen entry and disease, reducing disease’s prevalence by building silicon beneath the plant’s cuticle (Ma and Yamaji 2006; Yamaji et al. 2008). There is a Si–cuticle double layer in rice leaf blades, where silica is discovered in a 2–5 µm layer immediately beneath the cuticle layer.
Slp1 (Siliplant1) is a basic protein high in proline, lysine, and glutamic acid and has seven repeat units. Studies on the overexpression and localization of Slp1 indicate that it plays a significant role in silicification in the silica cells of sorghum leaves (Fig. 3) (Kumar et al. 2020). During the process of cell extension, silica cells begin transcribing Slp1. Vesicles containing translated Slp1 molecules are stored in the cytoplasm. As the cell matures and prepares for silicification, its vesicle contents are secreted into the apoplasm (Kumar et al. 2021). In that spot, Slp1 molecules interact with the supersaturated silicic acid, leading to the rapid deposition of silica. The production of the mineral within silica cells generates an inner siliceous secondary wall (Kumar and Elbaum 2018). This reduces the amount of the silica cell’s cytoplasm while the cell is still alive and maintains cell-to-cell connection via plasmodesmata (Kumar et al. 2017). Within a few hours, the thickened silica wall nearly fills the cell volume as the silica cell completes its development and the cell dies. Transient overexpression of Slp1 in sorghum causes ectopic silica accumulation in all leaf epidermal cells (Kumar et al. 2020).
Process of silicification in plants. A Different forms of silicon in soil. B Silicon can only be taken up by the plant in the form of monosilicic acid [Si(OH)4]. C Siliplant1 (Slp1) influences sorghum silica cell silica biogenesis. The silicification of plants improved their physical barrier resistance to fungi. Slp1 is transcribed, translated, encapsulated in membrane-bound vesicles (Slp1v), and preserved until silicification in the cytoplasm. When the silica cells are prepared for silicification, the apoplastic region receives Slp1 from the cytoplasm. In the apoplastic solution, the protein template (secreted Slp1; Slp1s) deposits solid silica from supersaturated silicic acid. Additional diffusion of silicic acid from the apoplastic fluid decreases the cytoplasmic volume of silica cells that is constrained by the growing cell wall of silica deposits. D Si application under drought stress restricts leaf transpiration by (1) physically preventing cuticular transpiration through cuticle layer thickening caused by silica deposits and (2) controlling stomatal movement via turgor loss of guard cell and cell wall physical and mechanical modifications (Kumar et al. 2017, 2020, 2021; Kumar and Elbaum 2018)
Papillae formation: In response to fungal attempts to enter the cell wall, plants commonly produce cell wall appositions called papillae, whose chemical composition differs from that of primary and secondary cell walls (Hückelhoven 2014). It has also been demonstrated that Si promotes papillae formation upon pathogen infection. Silicon accumulation was found in the haustorial neck and collar region, as well as in the papillae, preventing pathogen access (Akhtar et al. 2018). The papilla is a complex structure that develops between the plasma membrane and the cell wall of a plant (Voigt 2014).
In response to Blumeria graminis f. sp. hordei infection, the application of Si results in the development of papillae in the epidermal cells of barley. Si availability extended the range of papillae in rose leaf cells in response to infection by Podosphaera pannosa (Shetty et al. 2012). The predominance of papillae after Si treatment could boost rice resistance to blast, and wheat and barley resistance to powdery mildew and rust (Cai et al. 2008). The application of silica increases the deposition of callose and phenolics, resulting in the creation of effective papillae during the barley–Blumeria graminis f. sp. hordei interaction and restricting fungal growth by trapping the penetration peg in the papillae (Chowdhury et al. 2014).
(ii) Biochemical and molecular defenses as induced by silicon
Despite the physical barriers, Si induced a range of chemical defence mechanisms involving the rapid creation of defence chemicals via primary and secondary metabolic pathways (Ahanger et al. 2020). Increased production and accumulation of antimicrobial compounds such as phenolics, anthocyanins (I), lignin, callose (CAL), phytoalexins, and defence-related enzyme activities such as PAL, PPO, POX, lipoxygenase (LOX), chalcone synthase (CHS), chalcone isomerase, β-1,3-glucanase (GLU), and pathogenesis-related (PR) proteins are all examples (Akhtar et al. 2018; Ahammed and Yang 2021). Si acts upon several signaling pathways such as salicylic acid (SA), jasmonic acid (JA), and ethylene signaling pathways, in order to cascade defence signalling in plant’s immunity systems and in the regulation of plant defence responses (Van Bockhaven et al. 2013). The mechanism of inducing resistance by Si is classified into two categories such as inducing secondary metabolites, and defense-related enzymes and compounds.
Secondary metabolites in silicon-induced defense
Phenylpropanoid-derived secondary metabolites, such as phenols and flavonoids, have long been recognized for their antifungal properties. An in vitro study demonstrated that both Si and Si-induced secondary metabolites can significantly restrict the growth of fungal pathogens (Ahammed and Yang 2021). Secondary metabolism and phenylpropanoid accumulation are aided by exogenous Si administration in response to microbial pathogens (Ahanger et al. 2020). For example, in bitter gourd, enhanced accumulation of phenols and flavonoids caused by Si is a major factor in controlling powdery mildew (Ahammed and Yang 2021). Antifungal toxic chemicals, such as aglycones and phytoalexins, can be found in the leaves of plants that receive silica from the soil. The sclerenchyma and vascular tissues of banana roots may be more resistant to Fusarium oxysporum f. sp. cubense because of the increased accumulation of phenols, flavonoids, lignin, and dopamine that is generated by Si (Fortunato et al. 2014).
The stem strength of Paeonia lactiflora was improved by an average of 24.86% when Si was applied (Zhao et al. 2021). It was found that Si-induced phenolic acids, such as chlorogenic acid, and the relative amounts of genes that encode PAL and lipoxygenase, led to the strengthening of tolerance to grey leaf spot disease (Magnaporthe oryzae) in perennial ryegrass systems (Rahman et al. 2015). Furthermore, the presence of damaged fungal hyphae in the endodermal cell layers and vascular veins of cotton roots demonstrates a strong Si-stimulated chemical defence in addition to physical barriers (Ahammed and Yang 2021). During the interaction between rice and Cochliobolus miyabeanus, applying Si not only improves the rate of photosynthesis but also increases photorespiration. Hence, it increases rice’s susceptibility to brown spot disease (Van Bockhaven et al. 2015).
Defense-related enzymes and compounds in Si-induced defense
Enzymes associated with host defence, which include β-1,3-glucanase, chitinases, and peroxidases, are essential for plant resistance to diseases (Ramamoorthy et al. 2002; Brunings et al. 2009; Ghareeb et al. 2011; Suresh et al. 2022) (Table 1). Si supplementation increases the activity of PAL, PPO, GLU, CHI, and POX, possibly through defence priming, which greatly contributes to plant immunity against pathogens and several host–pathogen interactions (Bakhat et al. 2018). Phenolic compounds and lignin are more abundant in cotton roots treated with potassium silicate (Whan et al. 2016). Si-induced elevation of phenolic acids, particularly chlorogenic acid and flavonoids, and relative levels of genes encoding PAL and lipoxygenase contributed to better resistance to grey leaf spot disease in perennial ryegrass (Magnaporthe oryzae) (Rahman et al. 2015).
Upregulation of various systemic signals in Si-induced defense
To protect against biotrophic diseases, SA primarily generates defence mechanisms, while JA and ET-mediated defence strategies are more commonly used to protect against necrotizing infections (Ramamoorthy et al. 2001; Pieterse et al. 2012). In consequence of lesions, pathogen attacks, and herbivory, silicon treatment increases the buildup of phytohormones (Kim et al. 2014). For example, rice administered with Si has exhibited tolerance to insect herbivores through the buildup of JA, but it also affects the wound-induced production of the JA protein (Ye et al. 2013).
Several studies indicated that Si regulated plant stress responses by modulating phytohormone homeostasis and boosting different signalling pathways (Brunings et al. 2009; Ghareeb et al. 2011). Si-treated plants accumulate plant phytohormones in response to alter the pathogen’s attack (Kim et al. 2014). During infection of powdery mildew pathogen in Arabidopsis, Si increases the enzymes engaged in the SA pathway by the upregulation of the gene expression. Resistant phenotypes demonstrate a substantial increase in the rate of SA production and the related defense genes compared to the controls, which indicates that Si plays an indispensable role in the defense mechanism along with the SA pathway (Vivancos et al. 2015). Resistance mechanism to powdery mildew pathogen (Erysiphe cichoracearum) infection was observed in Arabidopsis plants treated with Si by encouraging the synthesis of SA, JA, and ET (Vivancos et al. 2015). Si induced resistance in tomato infected with Ralstonia solanacearum and rice infected with Magnaporthe oryzae by activating JA and ET signaling pathways (Ramamoorthy et al. 2002; Brunings et al. 2009; Ghareeb et al. 2011).
-
i.
SA pathway: SA biosynthesis involves two sets of genes: EDS1/PAD4 & EDS5/SID2, which are necessary for SA biosynthesis (Shah 2003). Plants with higher Si (TaLsi) concentrations were more susceptible to Golovinomyces cichoracearum infection in comparison to plants with lower amounts of Si (TaLsi) concentrations (Vivancos et al. 2015). Si injection suppressed the area under the disease progression curve (AUDPC) in PAD4 and SID2 mutant lines, indicating Si’s ability to boost Arabidopsis resistance to Golovinomyces cichoracearum is maintained in mutants engineered to absorb Si more efficiently (Vivancos et al. 2015). The NPR1 (Nonexpresser of PR Genes1) regulatory protein promotes PR gene expression in response to SA, and numerous SA-inducible WRKY proteins, whose activity is enhanced by Si injection, positively regulate NPR1 (Li et al. 2004). In tomato plants, an infection with Ralstonia solanacearum stimulates the WRKY1 transcription factor, which in turn activates defense genes, and Si increases their activity (Suresh et al. 2022). The level of endogenous SA and consequent PRs expression has increased as a result of Si enhancing the expression of multiple defence genes, the activity of various transcription factors, and the upregulation of numerous signalling pathways (Kurabachew et al. 2013) (Fig. 4).
-
ii.
JA pathway: silicon facilitates increased JA-mediated defense mechanisms, such as increased production of enzymes and proteins involved in defense, as well as increased expression of transcription factors producing proteins engaged in JA signaling; by boosting JA-mediated defense mechanisms, JA also promotes enhanced leaf silicification and the development of silica cells containing phytoliths (Ye et al. 2013). As part of the fine-tuning of the JA signaling system, ubiquitin protein ligase destroys the negative regulator of the JA signaling pathway, JAZ1 (Jasmonate ZIM-domain protein 1) domain (Thines et al. 2007) (Fig. 4). Current study suggests that the anti-feeding protein JA is essential for rice defense responses (Kim et al. 2014).
-
iii.
ET pathway: JERF3, TSRF1, and ACCO are marker genes linked with the ET signalling system. The transcription factor JERF3 leads to the activation of the ET and JA signalling pathway, ACCO is responsible for ethylene production, and TSRF1 is known to be an ET-responsive transcription factor (Pirrello et al. 2012). Si treatment upregulated the expression of JERF3, TSRF1, and ACCO genes in tomato plants infected with R. solanacearum, providing evidence that Si-induced resistance was mediated by ET and JA signalling pathways (Ghareeb et al. 2011). When a pathogen infects a cell, the ET and JA pathways are responsible in order to regulate the production of specific defence-related genes, such as PDF1.2 (Plant Defensin 1.2.) (Pieterse et al. 2009) (Fig. 4).
Crosstalk between signalling pathways in the plant defence response regulated by Si. Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) all have effects on plant defence, and these effects, along with their interactions, are shown to modify the defensive response that is mediated by silica. T, negative effect; purple stars, positive effect; red, increased or up-regulated by Si supply. The networking of signalling pathways is modified from Pieterse et al. (2009). (Color figure online)
Silicon-mediated expression of defense-responsive genes
Several plant biologists reported that Si had a protective effect against environmental threats. Furthermore, Si-mediated defense against powdery mildew and rice blast diseases has already been widely investigated. Extensive research (Rodrigues et al. 2004) revealed that Si positively regulates genes related to the defense mechanism like CHS, PAL, PR1, POX, CHI, and β-1, 3-glucanases in response to the Magnaporthe grisea infection (Fig. 5). This is consistent with the findings of previous studies that have shown Si is an important factor in the defense mechanism. M. grisea can be prevented by supplementing rice with Si, which alters the expression profile of rice’s defensive genes (Brunings et al. 2009). To alleviate the toxicity of heavy metals, Si treatment in rice also stimulated the expression of genes involved in heavy metal transportation and detoxification (Brunings et al. 2009). As a whole, Si controls the genes that control important plant processes, especially in the presence of a stressful environment.
Schematic illustration of Si-mediated regulation of vital genes associated with defence and phytohormones upon biotic stress. Rcht2 chitinase; Prla PR-1; Lox lipoxygenase; PAL phenylalanine ammonia-lyase; CAD cinnamyl alcohol dehydrogenase; CHS chalcone synthase; PGIP polygalactouranase inhibitor protein; PA phosphatase associated to defence; PR-1 pathogenesis-related protein; ERF ethylene response factor; JERF jasmonate and ethylene-responsive factor 3; TSRF Tomato stress-responsive factor; ACCO 1-aminocyclopropane-1-carboxylate oxidase; FD-1 ferredoxin-I; POD peroxidase; WRKY II WRKY group II transcription factor; SA salicylic acid; JA jasmonic acid
Abiotic stresses
Silicon regulates the response of plants to many abiotic stresses such as salinity, extreme temperature fluctuations, metallic toxicity, drought and flood damage, as well as overexposure to nutrients and ultraviolet (UV) light. Recent research indicates that overexpressing the Lsi1 gene in Dular rice improves the plant’s proline concentration and tolerance to cold weather conditions. This enhancement is attributed to the maintenance of cellular osmotic balance and increased calcium deposition in the root tips (Xie et al. 2022). Silicon formed underneath the cuticular layer reduces water loss through cuticular transpiration and helps plants mitigate water stress during drought conditions. Additionally, Si decreases stomatal conductance in response to guard cell turgor loss brought on by Si deposition and altered cell wall characteristics (Zhu and Gong 2014). Si helps plants extract water from the soil (Savvas and Ntatsi 2015). Silicon helps to reduce salt stress by preventing the uptake of Na+ and Cl. Si feeding enhances potassium uptake and permits the maintenance of K/Na, which directly stabilizes proton pump activity in salt-treated root tips (Xu et al. 2015). Si could affect the bioavailability of hazardous substances in soil contaminated with metals. Hydrolysis of soluble silicate results in the production of gelatinous metasilicic acid (H2SiO3) which is heavy metal-retentive. The apoplast also produces hydroxyl-aluminium silicate, which aids in Al detoxification. Endodermis accumulation of heavy metals is enhanced by adding silicates to roots (Keller et al. 2015). In response to Si treatment, manganese (Mn) buildup at the shoot level was primarily found in the epidermis. By interacting with phytohormones, polyamines, hydrogen sulphide, and nitric oxide, Si also ameliorates several abiotic stresses (Tripathi et al. 2021; Raza 2021; Sabagh et al. 2021). A study showed that the combined application of SA and Si improves physiological and biochemical mechanisms and photosynthetic efficiency in mustard seedlings, reducing lanthanum (La) toxicity. This is achieved by suppressing H2O2 and electrolytic leakage, increasing antioxidant enzyme activity and nutrient content and improving carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase activity. The results also show an increase in glycine, betaine and cysteine accumulation (Siddiqui et al. 2022). The crosstalk network enhances antioxidant defense, reduces oxidative damage, and enhances resistance to multiple abiotic stresses by applying Si treatment (Fig. 6). Si enhanced phytohormone synthesis and metabolism during abiotic stress (Zhu and Gong 2014; Kim et al. 2016b). Abscisic Acid (ABA), JA, Gibberellic Acid (GA), ET, SA, Brassinosteroid (BR), and Indole acetic acid (IAA) are the predominant phytohormones induced by Si in response to abiotic stress (Arif et al. 2021). Si may therefore be crucial in generating an adapted plant response, although the particular chemical cues involved in adaptative processes must yet be fully characterized.
Crosstalk network of Si with various phytohormones in response to oxidative stress in plants exposed to various abiotic stresses. Si and other signaling molecules such as BR, SA, GA, H2S, PRO, and NO activate antioxidant defence systems synergistically in response to abiotic stimuli such as salt, heat, and heavy metals. Si can also affect endogenous phytohormones such as ABA, SA, JA, Cytokinin (CK), and GA in plants subjected to diverse abiotic stressors and activates antioxidant defenses. Thus, active antioxidant systems restrict ROS formation, decrease oxidative stress and enhance plant tolerance to abiotic stressors (Zhu and Gong 2014; Kim et al. 2016a). Arrow with a thunder indicates an increase in the specific molecule; dotted arrow with an end line indicates decrease in the specific molecule; arrow with a knob end indicates no specific changes in the molecules; double-sided arrowhead indicates interaction between Si and the other signaling molecules
Crosstalk between silicon and other plant signaling molecules
ROS
Reactive oxygen species (ROS) signalling molecules play a role in various biological processes that influence the health of living organisms. ROS formation in plant cells can be induced by abiotic stresses such as salinity, cold, heat, dehydration etc. Even a minute change in the ROS balance in plants can trigger a cell response (Khan et al. 2021). At high concentrations, ROS destroy biomolecules, resulting in oxidative stress, whereas at low to moderate concentrations, they act as signalling molecules. It should be emphasized that despite the fact that ROS induce cell death, their production is a crucial stage in conferring stress resistance. Stress-induced ROS activation reactions must have a quick initiation, followed by a rapid cessation when the stress is no longer present (Huang et al. 2019). An efficient anti-oxidative mechanism is engaged in order to regulate the ROS equilibrium within a cell. Such non-enzymatic antioxidants encompass ascorbic acid, phenolics, carotenoids, glutathione, and tocopherols. Enzymatic antioxidants include ROS-scavenging enzymes such as SOD, CAT, APX, glutathione S-transferases (GSTs), thioredoxins, and peroxiredoxins (Huang et al. 2019).
Salinity stress: salt stress is caused by the buildup of ROS (Farouk et al. 2020). Exogenous Si boosts SOD activity while decreasing CAT and APX activity in borage plants during salinity (Torabi et al. 2015). CAT, peroxidase, and polyphenol peroxidase levels are decreased in rice plants under salt stress when Si is applied to the leaves (Kim et al. 2014). In the presence of Si, several other investigations have found that salt stress regulates the activity of several enzymes, including SOD, GPX, APX, GR, and CAT. Hasanuzzaman et al. (2018) reported that Si modulated the antioxidant system to reduce the negative effects of salt stress in soybean.
Drought stress: the excessive formation of ROS in plants under drought stress is detrimental to growth, but Si treatment dramatically reduces this effect by activating antioxidant mechanisms (Gong et al. 2005). Due to the increased activity of the antioxidant defence system, ROS-induced oxidative damage on Triticum aestivum plant growth was reduced by Si treatment (Gong et al. 2008). By altering antioxidant enzyme activity, proline and chlorophyll metabolism, redox homeostasis and nutrient intake from the soil, Si slowed premature leaf senescence inflicted by drought and salt stress (Alamri et al. 2020). The antioxidant defences of tomato plants are activated during drought stress by Si, which enhances SOD and CAT activity and water uptake in tomato plants (Shi et al. 2014).
Thermal stress: during periods of thermal stress, Si has been discovered to be a ROS homeostasis regulator (cold and heat stress). Exogenous Si promotes antioxidants such as APX, SOD, GSH-Px, GSH (Glutathione), MDHAR (monodehydroascorbate reductase), GR, and AsA that reduce ROS as well as lipid peroxidation in cucumbers under chilling stress conditions (15/8 °C, day/night) (Liu et al. 2009). SOD, APX, and GPX activities were enhanced in Salvia splendens treated with Si at 35 °C, but CAT activities were reduced (Soundararajan et al. 2014). The detoxification of ROS by enhanced anti-oxidative system action was demonstrated in another investigation to reduce cold stress in maize when Si was used as a treatment. Si treatment in heat-stressed tomato plants boosted the expression of SlCAT, SlAPX, and SlPOD genes (Alberto Moldes et al. 2013; Sahebi et al. 2017).
UV stress: protecting plants from UV radiation stress can also be achieved through Si’s ability to regulate the plant’s physiological and biochemical processes. Si and Si nanoparticles (SiNPs) have been shown to dramatically alleviate UV-B radiation damage in wheat by modulating oxidative stress (Tripathi et al. 2017). Si application reduced POD and CAT activities in soybean seedlings exposed to UV-B exposure (Shen et al. 2010).
Heavy metal stress: as an additional element, rice plants under heavy metal stress can regulate their metal transport, preventing damage as indicated by decreased MDA activity (Kim et al. 2016a). As a result of lower POD activity, manganese toxicity in cucumbers can be alleviated by adding silicates (Dragišić Maksimović et al. 2012). After applying SiNP with chromium (Cr) to pea seedlings, stress tolerance phenotypes were noticed.
Polyamines
Polyamines are essential regulators in plants against abiotic stressors and in maintaining normal metabolism, growth, and survival in adverse situations. Polyamine-rich plants are better able to withstand environmental stressors (Gupta et al. 2013). To make abiotic stress resistance, polyamine biosynthesis and Si accumulation work in concert in Sorghum bicolor to improve salinity stress tolerance while delaying leaf senescence (Gupta et al. 2013). Antioxidant defenses are regulated by Si, stimulating the synthesis and storage of endogenous polyamines such as spermine, spermidine, and putrescine to mediate salt tolerance (Wang and Munshi 2015). Si can reduce abiotic stress by boosting polyamine and ethylene metabolism (Manivannan and Ahn 2017). According to a recent study (Yin et al. 2019), the application of Si-mediated salt tolerance in cucumber was linked to the balance between polyamines and ethylene synthesis, increasing polyamine levels in favour of low ethylene production and, consequently, reducing Na+ buildup. Si also improves Na+/K+ homeostasis by regulating polyamine levels in salt-stressed cucumber seedlings (increasing putrescine and spermidine) (Wang and Munshi 2015). Si-induced polyamine synthesis alleviates abiotic stress and increases plant vitality and yield.
Nitric oxide
Environmental stress triggers a wide range of metabolic processes to release the ubiquitous plant-signaling chemical nitric oxide (NO) (Xia et al. 2015; Prakash et al. 2019; Rather et al. 2020). Due to the coupling of Si with sodium nitroprusside (NO source), Cd tolerance and biomass increased, as well as the antioxidant defense system of wheat seedlings (Singh et al. 2020). According to the researchers, exposure to Si may also enhance plant defenses by improving endogenous NO synthesis. Si works with NO to enhance plant growth and increase resilience to stress (Ahmad et al. 2021). The combined addition of Si and NO lowered heavy metal absorption in Brassica juncea while raising oxidative stress tolerance by enhancing plant length, shoot/root dry mass, chlorophyll and carotenoid content, and antioxidant activity and ROS accumulation (Ahmad et al. 2021).
Silicon interaction with hydrogen sulfide (H2S) and calcium (Ca2+)
Hydrogen sulphide (H2S) and calcium (Ca2+) influence signalling cascades in silicon. Si protects pepper plants from boron toxicity through the buildup of endogenous H2S (Kaya et al. 2020a). Furthermore, endogenous H2S modulates Si-mediated Cd tolerance with NO in pepper plants (Kaya et al. 2020b). Maintaining plasma membrane permeability and increasing Ca and K levels in shoots are achieved by administering Si to maize seedlings, resulting in improved water stress tolerance (Kaya et al. 2006). Ca2+ and silica have also been shown to alleviate the oxidative stress produced by Cd poisoning in rice seedlings (Srivastava et al. 2015). The stress tolerance of other heavy metals, such as aluminium, boron, chrome, copper, and zinc, has improved dramatically when Si is applied (Tripathi et al. 2012).
Conclusion and future challenges for silicon in plant biology
Exogenous (foliar or root) Si supplementation encourages and facilitates plant development, particularly under stress. Si deposition in the plant influences cellular processes such as development, stomatal control, nutrient absorption, metal detoxification, and the plant’s resilience to abiotic and biotic stressors. The existence of multiple transporters involved in the intake, transportation, and translocation of Si from root to shoot emphasizes the purpose of Si to plant growth. A complex interplay among phytohormones, ROS, other signaling molecules such as NO, calcium and transcriptional factors, and antioxidant systems, is established when stress is present. As a result, the plants can withstand possible biotic challenges better by Si-mediated regulation of signalling pathways (e.g., ET; SA; JA). Although specific effects of Si on plant metabolism and gene expression have been demonstrated, the mechanisms by which Si affects plant growth and development are still uncertain. While the studies in this manuscript illustrate our present understanding of the absorption, transport, deposition, and signaling of Si in plants, there are still many issues, some of which are basic, that went unanswered. For example, we do not know or have a limited understanding regarding how Si is loaded into the xylem or the identity of the transporter that pumps Si to plant cells, and we do not know, on a fundamental level, how Si provides plants with such a wide range of advantages. To date, no data indicates that Si plays an active part in any biochemical or metabolic pathway that might define the advantages of Si supplementation for plants. Similarly, we have insufficient knowledge of the optimal amounts of Si required for optimal plant growth at each developmental stage. Hundreds of researchers have found that applying Si to poor Si-accumulator species has favorable impacts, which is difficult to explain given our current understanding. Other unsolved problems include the role of Si in interactions with signaling molecules under normal and stress conditions, its impact on the intake of nutrients, its influence on the photosynthetic machinery, and its involvement in the integration of phytohormones. Numerous disciplines, including agriculture, industrial uses, and ecology, will benefit from a deeper knowledge of the biology of Si.
Data availability
As this is a review article, the availability of data and materials are not required for this submission.
Abbreviations
- Si:
-
Silicon
- SiO2 :
-
Silicon dioxide
- (Si(OH)4):
-
Monosilicic acid
- NIP:
-
Nodulin-26 like intrinsic proteins
- NPA:
-
Asn-Pro-Ala
- CHI:
-
Chitinases
- POX/POD:
-
Peroxidase
- EVB:
-
Enlarged vascular bundle
- DVB:
-
Diffuse vascular bundle
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- PPO:
-
Polyphenol oxidases
- PAL:
-
Phenylalanine ammonia-lyase
- H2O2 :
-
Hydrogen peroxide
- K2SiO3 :
-
Potassium silicate
- EDX:
-
Energy-dispersive x-ray
- SiO2 :
-
Silicon dioxide
- ACN:
-
Anthocyanins
- CAL:
-
Callose
- LOX:
-
Lipoxygenase
- CHS:
-
Chalcone synthase
- GLU:
-
β-1,3-Glucanase
- PR:
-
Pathogenesis-related protein
- SA:
-
Salicylic acid
- JA:
-
Jasmonic acid
- ET:
-
Ethylene
- GA:
-
Gallic acid
- TEM:
-
Transmission electron microscopy
- AUDPC:
-
Area under the disease progression curve
- NPR1:
-
Nonexpresser of PR Genes1
- JAZ1:
-
Jasmonate ZIM-domain protein 1
- UV:
-
Ultraviolet
- H2SiO3 :
-
Metasilicic acid
- PSII:
-
Photosystem II
- Nramp:
-
Natural resistance-associated macrophage protein
- OsHMA2:
-
Oryza sativa heavy metal ATPase 2
- PDF 1.2:
-
Plant defensin 1.2
- ROS:
-
Reactive oxygen species
- GSTs:
-
Glutathione S-transferases
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- SiNPs:
-
Silver nanoparticles
- Cr:
-
Chromium
- NO:
-
Nitric oxide
- H2S:
-
Hydrogen sulphide
- CAD:
-
Cinnamyl alcohol dehydrogenase
- PGIP:
-
Polygalactouranase inhibitor protein
- PA:
-
Phosphatase associated to defense
- ERF:
-
Ethylene response factor
- JERF3:
-
Jasmonate and ethylene responsive factor 3
- TSRF:
-
Tomato stress-responsive factor
- ACCO:
-
1-Aminocyclopropane-1-carboxylate oxidase
- FD-1:
-
Ferredoxin-I
- WRKY II:
-
WRKY group II transcription factor
References
Ahammed GJ, Yang Y (2021) Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol Biochem 165:200–206. https://doi.org/10.1016/j.plaphy.2021.05.031
Ahanger MA, Bhat JA, Siddiqui MH et al (2020) Integration of silicon and secondary metabolites in plants: a significant association in stress tolerance. J Exp Bot 71:6758–6774. https://doi.org/10.1093/jxb/eraa291
Ahmad A, Khan WU, Ali Shah A et al (2021) Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 262:128384. https://doi.org/10.1016/j.chemosphere.2020.128384
Akhtar N, Chandra R, Mazhar Z (2018) Silicon based defence mechanism in plants. Trends Biosci 32:3663–3674
Alamri S, Hu Y, Mukherjee S et al (2020) Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol Biochem 157:47–59. https://doi.org/10.1016/j.plaphy.2020.09.038
Alberto Moldes C, de Lima F, Filho O, Manuel Camiña J et al (2013) Assessment of the effect of silicon on antioxidant enzymes in cotton plants by multivariate analysis. J Agric Food Chem 61:11243–11249. https://doi.org/10.1021/jf4039088
Alhousari F, Greger M (2018) Silicon and mechanisms of plant resistance to insect pests. Plants 7:33. https://doi.org/10.3390/plants7020033
Arif Y, Singh P, Bajguz A et al (2021) Silicon mediated abiotic stress tolerance in plants using physio-biochemical, omic approach and cross-talk with phytohormones. Plant Physiol Biochem 166:278–289. https://doi.org/10.1016/j.plaphy.2021.06.002
Bakhat HF, Bibi N, Zia Z et al (2018) Silicon mitigates biotic stresses in crop plants: a review. Crop Prot 104:21–34. https://doi.org/10.1016/j.cropro.2017.10.008
Bhardwaj S, Sharma D, Singh S et al (2022) Physiological and molecular insights into the role of silicon in improving plant performance under abiotic stresses. Plant Soil. https://doi.org/10.1007/s11104-022-05395-4
Bi Y, Tian SP, Guo YR et al (2006) Sodium silicate reduces postharvest decay on Hami melons: induced resistance and fungistatic effects. Plant Dis 90:279–283. https://doi.org/10.1094/PD-90-0279
Brunings AM, Datnoff LE, Ma JF et al (2009) Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann Appl Biol 155:161–170. https://doi.org/10.1111/j.1744-7348.2009.00347.x
Cai K, Gao D, Luo S et al (2008) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 134:324–333. https://doi.org/10.1111/j.1399-3054.2008.01140.x
Chain F, Côté-Beaulieu C, Belzile F et al (2009) A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol Plant Microbe Interact 22:1323–1330. https://doi.org/10.1094/MPMI-22-11-1323
Chérif M, Asselin A, Bélanger R (1994) Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology. https://doi.org/10.1094/PHYTO-84-236
Chiba Y, Mitani N, Yamaji N, Ma JF (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57:810–818. https://doi.org/10.1111/j.1365-313X.2008.03728.x
Chowdhury J, Henderson M, Schweizer P et al (2014) Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol 204:650–660. https://doi.org/10.1111/nph.12974
Collin B, Doelsch E, Keller C et al (2014) Evidence of sulfur-bound reduced copper in bamboo exposed to high silicon and copper concentrations. Environ Pollut 187:22–30. https://doi.org/10.1016/j.envpol.2013.12.024
Côté-Beaulieu C, Chain F, Menzies JG et al (2009) Absorption of aqueous inorganic and organic silicon compounds by wheat and their effect on growth and powdery mildew control. Environ Exp Bot 65:155–161. https://doi.org/10.1016/j.envexpbot.2008.09.003
da Cruz MFA, Rodrigues FÁ, Polanco LR et al (2013) Inducers of resistance and silicon on the activity of defense enzymes in the soybean–Phakopsora pachyrhizi interaction. Bragantia 72:162–172. https://doi.org/10.1590/S0006-87052013005000025
Dallagnol LJ, Rodrigues FA, DaMatta FM et al (2011) Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice–Bipolaris oryzae interaction. Phytopathology® 101:92–104. https://doi.org/10.1094/PHYTO-04-10-0105
Dallagnol LJ, Rodrigues FA, Pascholati SF et al (2015) Comparison of root and foliar applications of potassium silicate in potentiating post-infection defences of melon against powdery mildew. Plant Pathol 64:1085–1093. https://doi.org/10.1111/ppa.12346
Dann EK, Muir S (2002) Peas grown in media with elevated plant-available silicon levels have higher activities of chitinase and β-1,3-glucanase, are less susceptible to a fungal leaf spot pathogen and accumulate more foliar silicon. Australas Plant Pathol 31:9–13. https://doi.org/10.1071/AP01047
Deshmukh RK, Vivancos J, Ramakrishnan G et al (2015) A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J 83:489–500. https://doi.org/10.1111/tpj.12904
Domiciano GP, Cacique IS, Chagas Freitas C et al (2015) Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology® 105:738–747. https://doi.org/10.1094/PHYTO-10-14-0280-R
Dragišić Maksimović J, Mojović M, Maksimović V et al (2012) Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J Exp Bot 63:2411–2420. https://doi.org/10.1093/jxb/err359
Etesami H, Jeong BR (2018) Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Saf 147:881–896. https://doi.org/10.1016/j.ecoenv.2017.09.063
Farouk S, Elhindi KM, Alotaibi MA (2020) Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol Environ Saf 206:111396. https://doi.org/10.1016/j.ecoenv.2020.111396
Filha MSX, Rodrigues FA, Domiciano GP et al (2011) Wheat resistance to leaf blast mediated by silicon. Australas Plant Pathol 40:28–38. https://doi.org/10.1007/s13313-010-0010-1
Fleck AT, Nye T, Repenning C et al (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot 62:2001–2011. https://doi.org/10.1093/jxb/erq392
Fortunato AA, da Silva WL, Rodrigues FÁ (2014) Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology® 104:597–603. https://doi.org/10.1094/PHYTO-07-13-0203-R
Fortunato AA, Debona D, Bernardeli AMA, Rodrigues FA (2015) Defence-related enzymes in soybean resistance to target spot. J Phytopathol 163:731–742. https://doi.org/10.1111/jph.12370
Ghareeb H, Bozsó Z, Ott PG et al (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89. https://doi.org/10.1016/j.pmpp.2010.11.004
Gong H, Zhu X, Chen K et al (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321. https://doi.org/10.1016/j.plantsci.2005.02.023
Gong HJ, Chen KM, Zhao ZG et al (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol Plant 52:592–596. https://doi.org/10.1007/s10535-008-0118-0
Guo Y, Liu L, Zhao J, Bi Y (2007) Use of silicon oxide and sodium silicate for controlling Trichothecium roseum postharvest rot in Chinese cantaloupe (Cucumis melo L.). Int J Food Sci Technol 42:1012–1018. https://doi.org/10.1111/j.1365-2621.2006.01464.x
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036. https://doi.org/10.1007/s11738-013-1239-4
Hasanuzzaman M, Nahar K, Rohman MM et al (2018) Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanz 70:185–194. https://doi.org/10.1007/s10343-018-0430-3
Huang H, Ullah F, Zhou D-X et al (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800. https://doi.org/10.3389/fpls.2019.00800
Hückelhoven R (2014) The effective papilla hypothesis. New Phytol 204:438–440. https://doi.org/10.1111/nph.13026
Islam W, Tayyab M, Khalil F et al (2020) Silicon-mediated plant defense against pathogens and insect pests. Pestic Biochem Physiol 168:104641. https://doi.org/10.1016/j.pestbp.2020.104641
Kaur H, Greger M (2019) A review on Si uptake and transport system. Plants 8:81. https://doi.org/10.3390/plants8040081
Kaya C, Tuna L, Higgs D (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J Plant Nutr 29:1469–1480. https://doi.org/10.1080/01904160600837238
Kaya C, Akram NA, Ashraf M et al (2020a) Exogenously supplied silicon (Si) improves cadmium tolerance in pepper (Capsicum annuum L.) by up-regulating the synthesis of nitric oxide and hydrogen sulfide. J Biotechnol 316:35–45. https://doi.org/10.1016/j.jbiotec.2020.04.008
Kaya C, Ashraf M, Al-Huqail AA et al (2020b) Silicon is dependent on hydrogen sulphide to improve boron toxicity tolerance in pepper plants by regulating the AsA-GSH cycle and glyoxalase system. Chemosphere 257:127241. https://doi.org/10.1016/j.chemosphere.2020.127241
Keller C, Rizwan M, Davidian J-C et al (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 241:847–860. https://doi.org/10.1007/s00425-014-2220-1
Khan MIR, Ashfaque F, Chhillar H et al (2021) The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: an entrancing crosstalk between stress alleviators. Plant Physiol Biochem 162:36–47. https://doi.org/10.1016/j.plaphy.2021.02.024
Kim Y-H, Khan AL, Kim D-H et al (2014) Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol 14:13. https://doi.org/10.1186/1471-2229-14-13
Kim Y-H, Khan AL, Lee I-J (2016a) Silicon: a duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit Rev Biotechnol 36:1099–1109. https://doi.org/10.3109/07388551.2015.1084265
Kim Y-H, Khan AL, Waqas M et al (2016b) Silicon-mediated mitigation of wounding stress acts by up-regulating the rice antioxidant system. Cereal Res Commun 44:111–121. https://doi.org/10.1556/0806.43.2015.031
Kumar S, Elbaum R (2018) Interplay between silica deposition and viability during the life span of sorghum silica cells. New Phytol 217:1137–1145. https://doi.org/10.1111/nph.14867
Kumar S, Milstein Y, Brami Y et al (2017) Mechanism of silica deposition in sorghum silica cells. New Phytol 213:791–798. https://doi.org/10.1111/nph.14173
Kumar S, Adiram-Filiba N, Blum S et al (2020) Siliplant1 protein precipitates silica in sorghum silica cells. J Exp Bot 71:6830–6843. https://doi.org/10.1093/jxb/eraa258
Kumar S, Natalio F, Elbaum R (2021) Protein-driven biomineralization: comparing silica formation in grass silica cells to other biomineralization processes. J Struct Biol 213:107665. https://doi.org/10.1016/j.jsb.2020.107665
Kurabachew H, Stahl F, Wydra K (2013) Global gene expression of rhizobacteria-silicon mediated induced systemic resistance in tomato (Solanum lycopersicum) against Ralstonia solanacearum. Physiol Mol Plant Pathol 84:44–52. https://doi.org/10.1016/j.pmpp.2013.06.004
Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16:319–331. https://doi.org/10.1105/tpc.016980
Li R, Sun Y, Wang H, Wang H (2022) Advances in understanding silicon transporters and the benefits to silicon-associated disease resistance in plants. Appl Sci 12:3282. https://doi.org/10.3390/app12073282
Liang Y, Si J, Römheld V (2005) Silicon uptake and transport is an active process in Cucumis sativus. New Phytol 167:797–804. https://doi.org/10.1111/j.1469-8137.2005.01463.x
Liang Y, Sun W, Zhu Y-G, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428. https://doi.org/10.1016/j.envpol.2006.06.008
Liang Y, Nikolic M, Bélanger R et al (2015a) Silicon and insect pest resistance. In: Silicon in agriculture. Springer Netherlands, Dordrecht, pp 197–207
Liang Y, Nikolic M, Bélanger R et al (2015b) Silicon uptake and transport in plants: physiological and molecular aspects. In: Silicon in agriculture. Springer Netherlands, Dordrecht, pp 69–82
Liu J, Lin S, Xu P et al (2009) Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric Sci China 8:1075–1086. https://doi.org/10.1016/S1671-2927(08)60315-6
López-Pérez MC, Pérez-Labrada F, Ramírez-Pérez LJ et al (2018) Dynamic modeling of silicon bioavailability, uptake, transport, and accumulation: applicability in improving the nutritional quality of tomato. Front Plant Sci 9:647. https://doi.org/10.3389/fpls.2018.00647
Luyckx M, Hausman J-F, Lutts S, Guerriero G (2017) Silicon and plants: current knowledge and technological perspectives. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00411
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18. https://doi.org/10.1080/00380768.2004.10408447
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier, Amsterdam
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Ma JF, Yamaji N (2015) A cooperative system of silicon transport in plants. Trends Plant Sci 20:435–442. https://doi.org/10.1016/j.tplants.2015.04.007
Ma JF, Tamai K, Yamaji N et al (2006) A silicon transporter in rice. Nature 440:688–691. https://doi.org/10.1038/nature04590
Ma JF, Yamaji N, Mitani N et al (2007) An efflux transporter of silicon in rice. Nature 448:209–212. https://doi.org/10.1038/nature05964
Ma JF, Yamaji N, Mitani N et al (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Manivannan A, Ahn Y-K (2017) Silicon regulates potential genes involved in major physiological processes in plants to combat stress. Front Plant Sci 8:1346. https://doi.org/10.3389/fpls.2017.01346
Marchenkov AM, Petrova DP, Morozov AA et al (2018) A family of silicon transporter structural genes in a pennate diatom Synedra ulna subsp. danica (Kütz.) Skabitsch. PLoS ONE 13:e0203161. https://doi.org/10.1371/journal.pone.0203161
Marron AO, Ratcliffe S, Wheeler GL et al (2016) The evolution of silicon transport in eukaryotes. Mol Biol Evol 33:3226–3248. https://doi.org/10.1093/molbev/msw209
Mburu K, Oduor R, Mgutu A, Tripathi L (2016) Silicon application enhances resistance to xanthomonas wilt disease in banana. Plant Pathol 65:807–818. https://doi.org/10.1111/ppa.12468
Mir RA, Bhat BA, Yousuf H et al (2022) Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front Plant Sci 13:819658. https://doi.org/10.3389/fpls.2022.819658
Mitani N (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261. https://doi.org/10.1093/jxb/eri121
Mitani N, Chiba Y, Yamaji N, Ma JF (2009a) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21:2133–2142. https://doi.org/10.1105/tpc.109.067884
Mitani N, Yamaji N, Ma JF (2009b) Identification of maize silicon influx transporters. Plant Cell Physiol 50:5–12. https://doi.org/10.1093/pcp/pcn110
Mitani N, Yamaji N, Ago Y et al (2011) Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation: pumpkin silicon transporter. Plant J 66:231–240. https://doi.org/10.1111/j.1365-313X.2011.04483.x
Mitani-Ueno N, Yamaji N, Ma JF (2011) Silicon efflux transporters isolated from two pumpkin cultivars contrasting in Si uptake. Plant Signal Behav 6:991–994. https://doi.org/10.4161/psb.6.7.15462
Montpetit J, Vivancos J, Mitani-Ueno N et al (2012) Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol Biol 79:35–46. https://doi.org/10.1007/s11103-012-9892-3
Mostofa MG, Rahman MdM, Ansary MdMU et al (2021) Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit Rev Biotechnol 41:918–934. https://doi.org/10.1080/07388551.2021.1892582
Ouellette S, Goyette M-H, Labbé C et al (2017) Silicon transporters and effects of silicon amendments in strawberry under high tunnel and field conditions. Front Plant Sci 8:949. https://doi.org/10.3389/fpls.2017.00949
Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316. https://doi.org/10.1038/nchembio.164
Pieterse CMJ, Van der Does D, Zamioudis C et al (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055
Pirrello J, Prasad BN, Zhang W et al (2012) Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biol 12:190. https://doi.org/10.1186/1471-2229-12-190
Polanco LR, Rodrigues FA, Nascimento KJT et al (2014) Photosynthetic gas exchange and antioxidative system in common bean plants infected by Colletotrichum lindemuthianum and supplied with silicon. Trop Plant Pathol 39:35–42. https://doi.org/10.1590/S1982-56762014000100005
Prakash V, Singh VP, Tripathi DK et al (2019) Crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ Exp Bot 161:41–49. https://doi.org/10.1016/j.envexpbot.2018.10.033
Rahman A, Wallis CM, Uddin W (2015) Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology® 105:748–757. https://doi.org/10.1094/PHYTO-12-14-0378-R
Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot 20:1–11. https://doi.org/10.1016/S0261-2194(00)00056-9
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68. https://doi.org/10.1023/A:1014904815352
Ratcliffe S, Jugdaohsingh R, Vivancos J et al (2017) Identification of a mammalian silicon transporter. Am J Physiol Cell Physiol 312:C550–C561. https://doi.org/10.1152/ajpcell.00219.2015
Rather BA, Mir IR, Sehar Z et al (2020) The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol Biochem 155:523–534. https://doi.org/10.1016/j.plaphy.2020.08.005
Ratnayake RMRNK, Daundasekera WAM, Ariyarathne HM, Ganehenege MYU (2016) Some biochemical defense responses enhanced by soluble silicon in bitter gourd-powdery mildew pathosystem. Australas Plant Pathol 45:425–433. https://doi.org/10.1007/s13313-016-0429-0
Raza A (2021) Eco-physiological and biochemical responses of rapeseed (Brassica napus L.) to abiotic stresses: consequences and mitigation strategies. J Plant Growth Regul 40:1368–1388. https://doi.org/10.1007/s00344-020-10231-z
Rodrigues FÁ, McNally DJ, Datnoff LE et al (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology® 94:177–183. https://doi.org/10.1094/PHYTO.2004.94.2.177
Sabagh AE, Mbarki S, Hossain A et al (2021) Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front Agron 3:648694. https://doi.org/10.3389/fagro.2021.648694
Sahebi M, Hanafi MM, Rafii MY et al (2017) Screening and expression of a silicon transporter gene (Lsi1) in wild-type indica rice cultivars. BioMed Res Int 2017:1–13. https://doi.org/10.1155/2017/9064129
Saitoh Y, Mitani-Ueno N, Saito K et al (2021) Structural basis for high selectivity of a rice silicon channel Lsi1. Nat Commun 12:6236. https://doi.org/10.1038/s41467-021-26535-x
Sakurai G, Satake A, Yamaji N et al (2015) In silico simulation modeling reveals the importance of the Casparian strip for efficient silicon uptake in rice roots. Plant Cell Physiol 56:631–639. https://doi.org/10.1093/pcp/pcv017
Savvas D, Ntatsi G (2015) Biostimulant activity of silicon in horticulture. Sci Hortic 196:66–81. https://doi.org/10.1016/j.scienta.2015.09.010
Schurt DA, Cruz MFA, Nascimento KJT et al (2014) Silicon potentiates the activities of defense enzymes in the leaf sheaths of rice plants infected by Rhizoctonia solani. Trop Plant Pathol 39:457–463. https://doi.org/10.1590/S1982-56762014000600007
Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6:365–371. https://doi.org/10.1016/S1369-5266(03)00058-X
Shen X, Zhou Y, Duan L et al (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol 167:1248–1252. https://doi.org/10.1016/j.jplph.2010.04.011
Shetty R, Jensen B, Shetty NP et al (2012) Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa: silicon induced resistance in roses. Plant Pathol 61:120–131. https://doi.org/10.1111/j.1365-3059.2011.02493.x
Shi Y, Zhang Y, Yao H et al (2014) Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem 78:27–36. https://doi.org/10.1016/j.plaphy.2014.02.009
Siddiqui MH, Mukherjee S, Al-Munqedhi BMA et al (2022) Salicylic acid and silicon impart resilience to lanthanum toxicity in Brassica juncea L. seedlings. Plant Growth Regul. https://doi.org/10.1007/s10725-021-00787-5
Singh S, Prasad SM, Sharma S et al (2020) Silicon and nitric oxide-mediated mechanisms of cadmium toxicity alleviation in wheat seedlings. Physiol Plant. https://doi.org/10.1111/ppl.13065
Song A, Xue G, Cui P et al (2016) The role of silicon in enhancing resistance to bacterial blight of hydroponic- and soil-cultured rice. Sci Rep 6:24640. https://doi.org/10.1038/srep24640
Soundararajan P, Sivanesan I, Jana S, Jeong BR (2014) Influence of silicon supplementation on the growth and tolerance to high temperature in Salvia splendens. Hortic Environ Biotechnol 55:271–279. https://doi.org/10.1007/s13580-014-0023-8
Soundararajan P, Manivannan A, Jeong BR (2016) Chapter 3 regulatory mechanisms by silicon to overcome the salinity-induced imbalance of essential nutrient elements. In: Tripathi DK, Singh VP, Ahmad P et al (eds) Silicon in plants. CRC Press, Taylor & Francis Group, Boca Raton, pp 47–66
Srivastava RK, Pandey P, Rajpoot R et al (2015) Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma 252:959–975. https://doi.org/10.1007/s00709-014-0731-z
Sun H, Guo J, Duan Y et al (2017) Isolation and functional characterization of CsLsi1, a silicon transporter gene in Cucumis sativus. Physiol Plant 159:201–214. https://doi.org/10.1111/ppl.12515
Sun H, Duan Y, Qi X et al (2018) Isolation and functional characterization of CsLsi2, a cucumber silicon efflux transporter gene. Ann Bot 122:641–648. https://doi.org/10.1093/aob/mcy103
Sun H, Duan Y, Mitani-Ueno N et al (2020) Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ 43:732–744. https://doi.org/10.1111/pce.13679
Suresh P, Shanmugaiah V, Rajagopal R et al (2022) Pseudomonas fluorescens VSMKU3054 mediated induced systemic resistance in tomato against Ralstonia solanacearum. Physiol Mol Plant Pathol 119:101836. https://doi.org/10.1016/j.pmpp.2022.101836
Thines B, Katsir L, Melotto M et al (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448:661–665. https://doi.org/10.1038/nature05960
Torabi F, Majd A, Enteshari S (2015) The effect of silicon on alleviation of salt stress in borage (Borago officinalis L.). Soil Sci Plant Nutr 61:788–798. https://doi.org/10.1080/00380768.2015.1005540
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378. https://doi.org/10.1104/pp.106.079467
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012) Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant 34:279–289. https://doi.org/10.1007/s11738-011-0826-5
Tripathi DK, Singh S, Singh VP et al (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81. https://doi.org/10.1016/j.plaphy.2016.06.026
Tripathi DK, Vishwakarma K, Singh VP et al (2021) Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J Hazard Mater 408:124820. https://doi.org/10.1016/j.jhazmat.2020.124820
Van Bockhaven J, De Vleesschauwer D, Höfte M (2013) Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot 64:1281–1293. https://doi.org/10.1093/jxb/ers329
Van Bockhaven J, Spíchal L, Novák O et al (2015) Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol 206:761–773. https://doi.org/10.1111/nph.13270
Vivancos J, Labbé C, Menzies JG, Bélanger RR (2015) Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway: prophylactic role of silicon against plant diseases. Mol Plant Pathol 16:572–582. https://doi.org/10.1111/mpp.12213
Voigt CA (2014) Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00168
Wang S, Munshi JA (2015) Repair of prestressed concrete cylinder with localized delamination. Nucl Eng Des 295:759–766. https://doi.org/10.1016/j.nucengdes.2015.07.032
Wang M, Gao L, Dong S et al (2017) Role of silicon on plant–pathogen interactions. Front Plant Sci 8:701. https://doi.org/10.3389/fpls.2017.00701
Whan JA, Dann EK, Aitken EAB (2016) Effects of silicon treatment and inoculation with Fusarium oxysporum f. sp. vasinfectum on cellular defences in root tissues of two cotton cultivars. Ann Bot 118:219–226. https://doi.org/10.1093/aob/mcw095
Xia X-J, Zhou Y-H, Shi K et al (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66:2839–2856. https://doi.org/10.1093/jxb/erv089
Xie Y, Waqas M, Khan MU et al (2022) Overexpression of the rice gene Lsi1 (low silicon gene 1) enhances plant–microbe interactions that result in improved chilling tolerance. Plant Growth Regul 98:525–538. https://doi.org/10.1007/s10725-022-00890-1
Xu CX, Ma YP, Liu YL (2015) Effects of silicon (Si) on growth, quality and ionic homeostasis of aloe under salt stress. South Afr J Bot 98:26–36. https://doi.org/10.1016/j.sajb.2015.01.008
Yamaji N, Ma JF (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21:2878–2883. https://doi.org/10.1105/tpc.109.069831
Yamaji N, Mitatni N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389. https://doi.org/10.1105/tpc.108.059311
Yamaji N, Chiba Y, Mitani-Ueno N, Feng Ma J (2012) Functional characterization of a silicon transporter gene implicated in silicon distribution in barley. Plant Physiol 160:1491–1497. https://doi.org/10.1104/pp.112.204578
Ye M, Song Y, Long J et al (2013) Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1305848110
Yin J, Jia J, Lian Z et al (2019) Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol Environ Saf 169:8–17. https://doi.org/10.1016/j.ecoenv.2018.10.105
Zargar SM, Mahajan R, Bhat JA et al (2019) Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech 9:73. https://doi.org/10.1007/s13205-019-1613-z
Zhang G, Cui Y, Ding X, Dai Q (2013) Stimulation of phenolic metabolism by silicon contributes to rice resistance to sheath blight. J Plant Nutr Soil Sci 176:118–124. https://doi.org/10.1002/jpln.201200008
Zhao D, Xu C, Luan Y et al (2021) Silicon enhances stem strength by promoting lignin accumulation in herbaceous peony (Paeonia lactiflora Pall.). Int J Biol Macromol 190:769–779. https://doi.org/10.1016/j.ijbiomac.2021.09.016
Zhu Y, Gong H (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472. https://doi.org/10.1007/s13593-013-0194-1
Acknowledgements
The authors are grateful to the entire administration of Madurai Kamaraj University, Madurai, Tamil Nadu, India, for their unwavering support in completing the aforementioned work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The study was a collaborative effort, with all authors contributing to its conception and design. VS planned and structured the review. Material preparation, data collection, analysis, preparation of the first draft and all the illustrations were created by AG and SKH. RP, VR, and MPS commented and critically reviewed all the versions of the manuscript. Supervision was done by VS. All the authors critically revised the work and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
We agreed with the journal policy and provided our consent for the publication.
Additional information
Communicated by Tariq Aftab.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shanmugaiah, V., Gauba, A., Hari, S.K. et al. Effect of silicon micronutrient on plant’s cellular signaling cascades in stimulating plant growth by mitigating the environmental stressors. Plant Growth Regul 100, 391–408 (2023). https://doi.org/10.1007/s10725-023-00982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-00982-6