Abstract
The present study was undertaken to examine the possible roles of calcium (Ca2+) and silica (Si) in protection against oxidative damage due to Cd2+ toxicity in rice (Oryza sativa L.) seedlings grown in hydroponics. Rice seedlings raised for 12 days in hydroponics containing Cd(NO3)2 (75 μM) showed reduced growth; increase in the level of reactive oxygen species (ROS) (O2 ·− and H2O2), thiobarbituric acid reactive substances (TBARSs) and protein carbonylation; and increase in the activity of antioxidant enzymes—superoxide dismutase (SOD), catalase (CAT) and guaiacol peroxidase (GPX) compared to untreated controls. Exogenously added Ca2+ (2 mM) and Si (200 μM) significantly alleviated negative effect of Cd2+ by restoration of growth of the seedlings, suppression of Cd2+ uptake and restoration of root plasma membrane integrity. The levels of O2 ·−, H2O2, lipid peroxidation and protein carbonyls were much lower when Ca2+ and Si were added in the growth medium along with Cd2+ as compared to Cd-alone-treated seedlings. Ca2+ and Si lowered Cd-induced increase in SOD, GPX and APX activities while they elevated Cd-induced decline in CAT activity. Using histochemical staining of O2 ·− and H2O2 in leaf tissues, it was further confirmed that added Ca2+ and Si suppressed Cd-induced accumulation of O2 ·− and H2O2 in the leaves. The results suggest that exogenous application of Ca2+ and Si appears to be advantageous for rice plants in alleviating Cd2+ toxicity effects by reducing Cd2+ uptake, decreasing ROS production and suppressing oxidative damage. The observations indicate that Ca2+ and Si treatments can help in reducing Cd2+ toxicity in rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd2+) is a widespread long-range transported, potent heavy metal pollutant of the environment. It is toxic to all life forms, and at a very low concentration, it causes many morphological, physiological, biochemical and structural changes in growing plants ultimately leading to decline in productivity (Shah et al. 2001; Sharma and Dubey 2006). The sources primarily responsible for release and accumulation of Cd2+ in the environment include metallurgic industries, waste incinerators, urban traffic, cement factories, application of commercial fertilizers, etc. (Shah et al. 2001). Cd2+ is taken up by plants mainly by roots from the soil; however, some amount of Cd2+ also enters in the shoots via leaves (Gallego et al. 2012). In higher plants, uptake of Cd2+ mainly depends on its availability and concentration in the surrounding root zone (Clemens 2006). It is suggested that in a concentration more than 5–10 μg g−1 leaf dry mass, Cd2+ is toxic to most of the plants (White and Brown 2010).
For its transport inside the plant body, Cd2+ uses the transporters of many essential elements, mainly the transporters involved in micronutrient uptake. These transporter proteins lack specificities for particular metal. Various metal transporters engaged in transport of calcium (Ca2+), Fe2+, Mg2+ and Zn2+ allow Cd2+ transport in the cells (Clemens 2006). High uptake of Cd2+ causes reduction in plant growth (Sandalio et al. 2009), decreased uptake of nutrients from the soil, decrease in photosynthesis, damage to root tips, inhibition of root metabolism, inhibition in activities of many enzymes and induction of oxidative stress in the tissues (Sandalio et al. 2001). Involvement of Cd2+ in increased production of reactive oxygen species (ROS) such as superoxide radical (O2 ·−), hydrogen peroxide (H2O2) and hydroxyl radical (·OH) and induction of oxidative stress has been documented in many crop species (Shah et al. 2001; Andresen and Küpper 2013). Cd2+ is a redox non-active metal and does not involve directly in cellular redox processes (Clemens 2006), but it has been shown that Cd2+ promotes ROS production that leads to oxidative damage to cellular biomolecules and causes impairment to redox homeostasis of the cell (Sandalio et al. 2001; Andresen and Küpper 2013). Cd2+ can indirectly activate NADPH oxidase present on membranes leading to enhanced production of O2 ·− and H2O2 (Romero-Puertas et al. 2004). Oxidative stress is regarded as important event in expression of Cd2+ toxicity in rice plants (Shah et al. 2001). To quench increasingly produced ROS under Cd2+ toxicity and other abiotic stresses and to prevent the cells against oxidative damage caused by ROS, plants possess antioxidative defence system comprising the non-enzymic components such as ascorbate (AsA), glutathione (GSH) and the antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX) and the enzymes of Halliwell–Asada pathway such as ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (GR) (Mittler et al. 2004; Moller et al. 2007; Gill and Tuteja 2010; Sharma et al. 2012; Andresen and Küpper 2013).
Attempts have been made by various groups of workers to alleviate Cd2+ toxicity effects in plants by application of mineral nutrients and compounds such as salicylic acid and nitric acid (Hayat et al. 2010; Xu et al. 2013). Ca2+ is an important micronutrient for plants and is actively involved in the regulation of plant cell metabolism and in signal transduction (Hall 2002). Ca2+ has high physical resemblance with Cd2+ including charge and radius, and at the same pH, Cd2+ competes with Ca2+ for its accumulation in the plants through root cells (White 2000). Many workers have reported reversing of Cd2+-induced damage by exogenous application of Ca2+ in plant species such as Arabidopsis thaliana, Sedum alfredii and leguminous plants (Talukdar 2012). Tian et al. (2011) showed that exogenous Ca2+ protected S. alfredii plants from Cd2+-induced damage by decreasing the accumulation of Cd2+ in the shoots. In Lens culinaris plants, exogenous Ca2+ alleviated the impact of Cd2+-induced oxidative stress by modulating the activity of antioxidative enzymes (Talukdar 2012). It has been observed that exogenous application of moderate Ca2+ promotes membrane stability and re-stabilizes Ca2+-mediated signalling inside the plants (Cramer 1985). Silica (Si) is the second most abundant element on earth surface and in the soil (Zeng et al. 2011). Though Si is a non-essential metal, it is considered beneficial for the growth of higher plants and it also protects plants against various abiotic stresses (Liang et al. 2005). Si has been shown to promote resistance in plants against the toxicity of the metals Cd2+ and Mn2+ (Liang et al. 2005; Shi et al. 2005a, b, 2010; Song et al. 2009). Rice seedlings grown in presence of Cd2+ + Si in nutrient medium showed significant increase in biomass in comparison with seedlings grown in presence of Cd2+ alone (Shi et al. 2005b).
As oxidative stress is an important component in the expression of Cd2+ toxicity in rice plants (Shah et al. 2001), in order to explore the possibilities to minimize the toxic effects of Cd2+ in growing rice plants by using minerals and other chemical agents such as Ca2+ and Si2+, the present study was undertaken to examine the effects of toxic level of Cd2+ on growth, production of ROS, induction of oxidative stress and status of antioxidative defence components in growing indica rice seedlings and to explore the possible role of exogenously applied Ca2+ and Si in alleviation of Cd2+ toxicity-induced oxidative stress effects in the seedlings.
Materials and methods
Plant material and metal treatments
Seeds of rice (Oryza sativa L.) cv. Malviya-36, commonly grown in India and sensitive to Cd2+, were used. Sensitivity of this cultivar towards Cd2+ was evaluated on the basis of root/shoot growth inhibition and other morphological parameters (data not reported here). Seeds were surface sterilized with 0.1 % sodium hypochlorite solution for 10 min, rinsed with glass-distilled water and imbibed for 24 h in water. Seedlings were then raised in plastic pots in hydroponics containing either Yoshida nutrient solution (Yoshida et al. 1976) which served as control or nutrient solutions supplemented with 75 μM Cd(NO3)2, 75 μM Cd(NO3)2 + 2 mM CaCl2 and 75 μM Cd(NO3)2 + 200 μM K2SiO3 which served as treatment solutions. Treatment concentration of Cd2+ was standardized following similar studies conducted by us earlier (Shah et al. 2001). Pots were kept for the growth of seedlings for 12 days in a green house at 28 ± 1 °C under 80 % relative humidity and 12-h light/dark cycle with 190–200 μmol m−2 s−1 irradiance as described earlier (Shah et al. 2001). Seedlings were uprooted at 12 days of growth, and all the measurements, physiological analyses and biochemical determinations were done in triplicate.
Rice seedling vigour, relative water content and metal uptake
At 12-day growth of seedlings, the lengths of roots and shoots as well as fresh biomass were determined. Root/shoot samples were dried in an oven at 70 °C for 3 days, and dry mass was determined on the basis of ten random samplings in triplicate. Relative water content (RWC) was determined in roots and shoots of control and metal-treated seedlings according to Weatherley (1950). RWC was calculated using the formula: RWC = (FW − DW) / (TW − DW) × 100, where FW = fresh weight, DW = dry weight and TW = turgid weight. Concentration of absorbed Cd2+ in roots/shoots was determined using inductively coupled plasma–optical emission spectrometer (ICP-OES, Optima 7000 DV, PerkinElmer) following the protocol of Allen et al. (1986). Plant samples were washed thoroughly with ion-free water (Milli-Q) and oven dried at 70 °C for 3 days. Dried samples weighing 150 mg were used for quantification of intracellular Cd2+. Samples were ground to fine powder and digested in diacid mixture (nitric acid/perchloric acid). Digested samples were diluted to 25 ml with ion-free water and filtered with 0.45-μM membrane filter. Filtrates were then subjected to analysis of Cd2+ using ICP-OES, and the data obtained were compared with standards taken form PerkinElmer (USA).
Measurement of the level of ROS, lipid peroxidation and membrane damage
The rate of O2 ·− production was measured by observing the oxidation of epinephrine to adrenochrome following the method of Mishra and Fridovich (1972). Results were expressed as change in absorbance at 480 nm min−1 g−1 tissue fresh weight. The level of H2O2 was measured in the tissues as described by Jana and Choudhuri (1981). Fresh root/shoot samples weighing 150 mg were homogenized in 3 ml of 50 mM sodium phosphate buffer (pH 6.5). After centrifugation at 10,000g for 10 min, the supernatant was used to determine H2O2 content by reaction with titanium sulphate. The intensity of yellow colour developed was recorded at 410 nm, and the amount of H2O2 was calculated using extinction coefficient 0.28 μM−1 cm−1 and expressed as nmol g−1 tissue fresh weight. The level of lipid peroxidation products was measured in terms of thiobarbituric acid reactive substances (TBARSs) according to the method of Heath and Packer (1968). The concentrations of lipid peroxides were expressed as nmol TBARS g−1 fresh weight of the tissues using an extinction coefficient of 155 mM−1 cm−1. The extent of plasma membrane injury due to Cd2+ treatment was determined in roots of the plants by a spectrophotometric assay of Evans blue stain retained by cells as described by Schützendübel et al. (2001). For this, roots from Cd2+-, Cd2+ + Ca2+- and Cd2+ + Si-treated seedlings were stained with 0.025 % (w/v) Evans blue solution and washed with 100 μM CaCl2. The stained portions from the root tips were removed with a razor blade. Ten root pieces from the identical positions were placed together, and the trapped Evans blue was released by homogenizing the root portions in 1 ml of 1 % (w/v) aqueous sodium dodecyl sulphate (SDS). The homogenate was centrifuged at 13,500×g for 10 min. The optical density of supernatant was measured spectrophotometrically at 600 nm.
Histochemical localization of ROS, lipid peroxidation and membrane damage
Localization of O2 ·− in situ was detected in the leaves of the control and treated seedlings using the dye nitroblue tetrazolium (NBT) as substrate following the method of Frahry and Schopfer (2001). Leaf discs (3–5 mm) were immersed in 6 mM NBT (prepared in 10 mM Na-citrate buffer, pH 6.0) at 25 °C for 8 h under light. The leaves were then immersed in boiling ethanol (95 %) for 10 min to decolourize the leaves except the dark blue insoluble formazan deposits produced by the reaction of NBT with O2 ·−. After cooling, leaf discs were photographed. Histochemical detection of H2O2 in situ was done according to Thordal-Christensen et al. (1997). Leaf discs, 3–5 mm, were incubated in a solution containing 1 mg ml−1 3,3′-diaminobenzidine (DAB) prepared in HCl-acidified (pH 3.8) water. After 8 h of incubation at 25 °C, leaf discs were kept in boiling 95 % ethanol for 10 min and were then cleared in saturated chloral hydrate. Leaf discs were then examined under a light microscope. Reddish brown-coloured spots appeared to be characteristic of the reaction of DAB with H2O2. Histochemical detection of lipid peroxides was done in roots as described by Pompella et al. (1987). Excised root tips (5–10 mm) were stained with Schiff’s reagent for 20 min, which detects aldehydes that originate from lipid peroxidation. Stained root tips were rinsed with sulphite solution (0.5 % [w/v] K2S2O5 in 0.05 M HCl) and then placed in this solution for 10 min to retain the staining colour. The loss of plasma membrane integrity was evaluated in root tips of the seedlings microscopically as described by Schützendübel et al. (2001) by examining the Evans blue stain retained by cells. Roots from control and Cd2+-, Cd2+ + Ca2+- and Cd2+ + Si2+-treated intact seedlings were stained with 0.25 % (w/v) aqueous Evans blue solution, for 30 min, and after rinsing with water, root tips were visualized under light microscope.
Determination of protein thiol, protein carbonylation and proteolytic activity
To assess the extent of protein oxidation, protein thiol content was determined in roots and shoots. Fresh samples weighing 150 mg were homogenized in 0.15 % (w/v) sodium ascorbate solution, and the homogenate was centrifuged at 22,000×g for 15 min at 4 °C. Total thiol and non-protein thiol contents were determined in the supernatant using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) following the method of de Kok and Kuiper (1986). The content of protein thiol was calculated by subtracting the content of non-protein thiol from total thiol and expressed as nmol g−1 fresh weight of tissues. The content of protein-bound carbonyls was determined following the method of Levine et al. (1994). Root and shoot samples weighing 1 g were homogenized in 2.0 ml of 10 mM sodium phosphate buffer (pH 7.4) containing 1 mM EDTA, 2 mM dithiothreitol, 0.2 % (v/v) Triton X-100 and 1 mM phenylmethane sulphonyl fluoride (PMSF). After centrifugation at 25,000×g for 30 min at 4 °C, supernatants containing 500 μg protein were mixed with 1 % (w/v) streptomycin sulphate for 20 min to remove the nucleic acids. After centrifugation at 2000×g, supernatants in 200-μl volumes were mixed with 300 μl of 10 mM 2,4-dinitrophenyl hydrazine (DNPH) (freshly prepared) in 2 M HCl. Individual blank samples were incubated in 2 M HCl. After 1-h incubation at 25 °C, proteins were precipitated with pre-cooled 10 % (w/v) trichloroacetic acid (TCA) and the pellets were washed three times with 500 μl of ethanol/ethyl acetate (1:1). The pellets were then dissolved in 6 M guanidine hydrochloride in 20 mM potassium phosphate buffer (pH 2.3), and the absorbance was measured at 370 nm using UV–vis spectrophotometer (PerkinElmer, LAMBDA EZ 201, USA). Carbonyl content was calculated using a molar absorption coefficient for aliphatic hydrazones as 22,000 M−1 cm−1 and expressed in terms of nmol carbonyl mg−1 protein. Proteolytic activity was measured in enzyme extracts prepared from root and shoot samples as described by Polge et al. (2009). Tissues weighing 300 mg were homogenized using chilled mortar and pestle in 2 ml of 50 mM Tris–HCl buffer (pH 7.5) and centrifuged at 15,000×g for 15 min at 4 °C, and supernatants were dialyzed in cellophane membrane. Proteolytic activity was assayed in dialyzed enzyme (0.25 ml) by reaction with 0.25 ml of 2 % azocasein prepared in 0.1 M Tris–HCl (pH 8.4). After 6 h of incubation at 28 °C, the reaction was stopped by adding 1.2 ml of 10 % TCA. After placing on ice for 10 min, the contents were centrifuged at 3000×g for 20 min. Then, 1.0 ml of the resulting supernatant was mixed with 1.0 ml of 1.0 M NaOH. After 30 min, absorbance was recorded at 440 nm using spectrophotometer. The extinction coefficient of azocasein 1 % in 1 M NaOH was taken as 37 L cm−1 g−1. One unit of proteolytic activity is expressed as mg of azocasein degraded min−1 mg−1 protein.
Determination of ascorbate and glutathione pool
The contents of reduced AsA, oxidized ascorbate (dehydroascorbate (DHA)) and total ascorbate (AsA + DHA) were determined following the method of Law et al. (1983). Fresh root/shoot samples weighing 200 mg were homogenized using a chilled mortar and pestle in 5 ml of 5 % (w/v) m-phosphoric acid and centrifuged at 22,000×g for 10 min at 4 °C. For estimation of total ascorbate, 100 μl of the supernatant was incubated with 110 mM KH2PO4, 3.6 mM EDTA and 1.5 mM dithiothreitol (DTT) in a total volume of 700 μl to reduce all DHA to AsA. One hundred microlitres of 0.5 % N-ethylmaleimide (NEM) was added to remove excess DTT. AsA was analyzed in a similar manner except that 200 μl of deionized water was substituted for DTT and NEM. After the addition of 400 μl 10 % (w/v) TCA, 400 μl 44 % O-phosphoric acid, 400 μl 65 mM α,α′-bipyridyl in 70 % ethanol and 200 μl 100 mM FeCl3 were added. After incubation at 40 °C for 1 h in a shaking water bath, colour was developed. Absorbance was measured at 525 nm. Standards for AsA and DHA were prepared between 0 and 5 mM in 5 % (w/v) m-phosphoric acid. For each sample, DHA was estimated from the difference between total ascorbate and AsA. Total glutathione (GSH + GSSG), oxidized glutathione (GSSG) and reduced glutathione (GSH) contents were determined following the method of Griffith (1980). Samples weighing 200 mg were homogenized in cold in 5 % (w/v) sulphosalicylic acid and centrifuged at 22,000×g for 10 min at 4 °C. Two solutions, namely, solution A (pH 7.2) consisting of 100 mM Na2HPO4·7H2O, 40 mM NaH2PO4·H2O, 15 mM EDTA, 1.8 mM DTNB and 0.04 % bovine serum albumin (BSA, Sigma) and solution B (pH 7.2) consisting of 1 mM EDTA, 50 mM imidazole, 0.2 % BSA and 2 U ml−1 GR, were prepared. Total glutathione was measured in a reaction mixture consisting of 400 μl of solution A, 320 μl of solution B, 400 μl of a 1:5 dilution of supernatant in 0.5 M KH2PO4 (pH 7.0) and 80 μl of 3.0 mM NADPH. The reaction rate was measured following change in absorbance at 412 nm for 5 min. To estimate GSSG, a similar method was followed except that 1 ml of 1:2 diluted supernatant in 0.5 M KH2PO4 (pH 6.0) was prior incubated with 20 μl of 2-vinylpyridine at 25 °C for 1 h to derivatize GSH. GSH and GSSG standards were between 0 and 18 μM in 5 % (w/v) sulphosalicylic acid diluted appropriately with 0.5 M KH2PO4 (pH 7.0). For each sample, GSH was estimated from the difference between total glutathione and GSSG.
Assays of antioxidative enzymes
The activity of SOD was determined using the method of Beauchamp and Fridovich (1971) based on the inhibition of p-nitro blue tetrazolium chloride (NBT) reduction by O2 ·− under light. Excised leaves weighing 200 mg were homogenized using a chilled mortar and pestle in 5 ml of 100 mM K-phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 0.1 % (v/v) Triton X-100 and 2 % (w/v) polyvinyl pyrrolidone (PVP). After centrifugation at 22,000×g for 10 min at 4 °C, supernatants were dialyzed in cellophane membrane tubing for 6 h against the extraction buffer in cold with three to four changes of the buffer. In the supernatant, total SOD activity was determined. One unit of SOD activity is expressed as the amount of enzyme required to cause 50 % inhibition of the rate of NBT reduction measured at 560 nm. CAT activity was assayed according to Beers and Sizer (1952) by measuring the decomposition of H2O2 at 240 nm (extinction coefficient of 0.036 mM−1 cm−1) by observing the decrease in absorbance using a UV–vis spectrophotometer (PerkinElmer, LAMBDA EZ 201, CA, USA). The activity of GPX was assayed in enzyme extracts according to Egley et al. (1983). Fresh tissue (±200 mg) was homogenized in 3 ml of chilled 50 mM Na-phosphate buffer (pH 7.0). After centrifugation and dialysis, enzyme activity was assayed by measuring increase in absorbance due to formation of tetraguaiacohinone at 420 nm (extinction coefficient of 26.6 mM−1 cm−1) at 30-s intervals up to 2 min using spectrophotometer (Bausch and Lomb, Spectronic 20, USA). Enzyme specific activity is expressed as μmol H2O2 reduced mg−1 protein min−1. The activity of APX was assayed according to Nakano and Asada (1981). H2O2-dependent oxidation of AsA was followed by measuring the decrease in absorbance at 290 nm (extinction coefficient of 2.8 mM−1 cm−1). Enzyme specific activity is expressed as μmol AsA oxidized mg−1 protein min−1. MDHAR activity was assayed following the method of Hossain et al. (1984). Samples weighing 200 mg were homogenized using a chilled mortar and pestle in 3 ml of extraction medium containing 100 mM K-phosphate buffer (pH 7.5), 1 mM EDTA, 2 % (w/v) PVP and 1 mM AsA (added prior to use). Homogenate was centrifuged at 22,000×g for 10 min at 4 °C and dialyzed. The assay mixture in 1-ml reaction medium contained 90 mM potassium phosphate buffer (pH 7.5), 0.01 mM EDTA, 0.0125 % Triton X-100, 0.25 U ascorbate oxidase (units as described by Sigma Chemicals Co., USA), 0.2 mM NADH and enzyme extract. The reaction was followed by measuring the decrease in absorbance at 340 nm due to NADH oxidation. Enzyme specific activity is expressed as nmol NADH oxidized mg−1 protein min−1. The activity of DHAR was assayed by measuring the reduction of DHA in the presence of GSH at 265 nm according to Hossain et al. (1984) using a UV–vis spectrophotometer after accounting for the non-enzymic reduction of DHA by GSH. Enzyme specific activity is expressed as nmol DHA reduced mg−1 protein min−1. GR activity was assayed using the method of Schaedle and Bassham (1977) following the oxidation of NADPH at 340 nm. Enzyme specific activity is expressed as nmol NADPH oxidized mg−1 protein min−1.
Protein estimation
In all preparations, protein content was estimated according to the method of Bradford (1976) using bovine serum albumin (BSA, Sigma) as standard.
Statistical analysis
All experiments were performed in triplicate. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Differences among control and treatments were analyzed by one-way ANOVA multiple comparison analysis using GraphPad Prism. Asterisks (*), (**) and (***) indicate values that differ significantly from controls, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas (#), (##) and (###) indicate values that differ significantly from Cd2+ treatment to Cd2+ + Ca2+ and Cd2+ + Si2+ treatments, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively.
Results
Effect of exogenous Ca2+ and Si on growth of Cd2+-treated rice seedlings
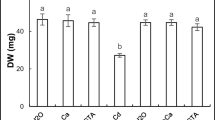
When growth of rice seedlings was recorded for 12 days, it was observed that 75 μM Cd(NO3)2 in the growth medium significantly inhibited length and fresh weights of roots and shoots (Table 1). Rice seedlings grown for 12 days in presence of 75 μM Cd(NO3)2 showed 40 % (p ≤ 0.01) reduction in length of roots and 56 % (p ≤ 0.001) reduction in shoots compared to the control-grown seedlings. Presence of Ca2+ and Si resulted in significant alleviation of Cd2+-induced retardation in the growth of the seedlings. Presence of Ca2+ resulted in 29 % (p ≤ 0.01) increase in root length and 40 % (p ≤ 0.01) increase in shoot length compared to Cd2+-treated seedlings, whereas presence of Si led to 17 % increase in length of roots and 30 % (p ≤ 0.05) increase in the length shoots compared to Cd2+-treated seedlings. Similar to the decline in length, Cd2+ treatment for 12 days caused 41 % (p ≤ 0.01) decline in fresh weight of roots and 42 % (p ≤ 0.01) decline in fresh weight of shoots in the seedlings (Table 1). Exogenous Ca2+ treatment resulted in 60 % (p ≤ 0.01) increase in fresh weight of roots and 36 % (p ≤ 0.05) increase in fresh weight of shoots compared to Cd2+-treated seedlings. Similarly, 200-μM Si treatment along with Cd2+ resulted in 45 % (p ≤ 0.01) increase in fresh weight of roots and 25 % (p ≤ 0.05) increase in fresh weight of shoots compared to Cd2+-alone-treated seedlings. With Cd treatment, an insignificant decline in RWC of roots and shoots was observed. Treatments with Ca2+ and Si along with Cd2+ resulted in higher level of RWC in the seedlings compared to Cd-treated seedlings (Table 1).
Effect of Ca2+ and Si treatments on Cd2+ content in the tissues
Treatment of rice seedlings with Cd(NO3)2 resulted in uptake of Cd2+ in roots as well as shoots (Table 2). ICP-OES analysis of Cd2+ revealed that the concentration of absorbed Cd2+ was greater in roots than in shoots. Rice seedlings exposed to 75 μM Cd(NO3)2 for 12 days showed 1.421 μmol Cd2+ g−1 dry wt in roots and 0.898 μmol Cd2+ g−1 dry wt in shoots. We also analyzed the seedlings which were co-treated with Ca2+ or Si along with Cd2+ for the same timescale. Results indicated that Ca2+ and Si treatments reduced Cd2+ accumulation in the roots and shoots. Ca2+ treatment resulted in lesser Cd2+ accumulation in the seedlings compared to Si. Ca2+ treatment resulted into decreased accumulation of Cd in roots and shoots of the seedlings. Seedlings treated with 75 μM Cd(NO3)2 + 2 mM CaCl2 for 12 days showed 0.831 μmol Cd2+ g−1 dry wt in roots and 0.507 μmol Cd2+ g−1 dry wt in shoots. This showed that exogenous Ca2+ treatment of seedlings along with Cd2+ resulted in 41–43 % decreased uptake of Cd2+, compared to Cd2+-alone-treated seedlings. Similar to Ca2+, treatment of seedlings with Si along with Cd2+ also resulted in decreased uptake of Cd2+ by the seedlings. With 200-μM Si treatment, uptake of Cd2+ was reduced by 20 % in roots and by 25 % in shoots compared to Cd2+-alone-treated seedlings.
Effect of Ca2+ and Si on Cd-induced ROS generation, lipid peroxidation and membrane damage
Cd2+ treatment for 12 days caused 73 % (p ≤ 0.001) increase in O2 ·− level in roots and 68 % (p ≤ 0.001) increase in shoots of the seedlings (Fig. 1a). Presence of Ca2+ and Si in the growth medium suppressed Cd-induced O2 ·− generation in both roots and shoots. Similarly, Cd2+ treatment caused increased production of H2O2 in rice seedlings compared to controls (Fig. 1b). With Cd2+ treatment of the seedlings for 12 days, H2O2 level increased by 94 % (p ≤ 0.001) in roots and 83 % (p ≤ 0.001) in shoots. A decline in Cd-induced H2O2 production was observed in roots and shoots when Ca2+ or Si was present in the medium. In Cd2+-treated seedlings, increased ROS content in the cells resulted in oxidative damage of lipids leading to increased level of lipid peroxides, expressed in terms of TBARS content. Lipid peroxidation is a direct measure of oxidative stress in the tissues, and after 12 days of Cd2+ treatment, we observed 90 % (p ≤ 0.001) increase in TBARS content in roots and 109 % (p ≤ 0.001) increased content in shoots compared to controls (Fig. 1c). In the present investigation, we observed that exogenous application of Ca2+ and Si ameliorated Cd-induced oxidative damage in rice seedlings by reducing the level of O2 ·−, H2O2 and lipid peroxides. Ca2+ appeared to be a better ameliorator than Si in protecting the cells against oxidative damage. Uptake of the dye Evans blue has been widely used as an indicator of loss of plasma membrane integrity as well as an indicator of cell death. In our experiments, nearly 5.32-fold (p ≤ 0.001) higher uptake of dye was observed in the root tips of Cd2+-treated seedlings compared to controls (Table 2). This indicated a significant loss of root plasma membrane integrity due to Cd2+ treatment. Presence of Ca2+ or Si in the treatment medium resulted in considerable protection of membrane integrity marked by less uptake of dye in the roots, compared to the uptake of dye in Cd-treated roots. Treatment with Ca2+ and Si caused 47 % (p ≤ 0.01) and 42 % (p ≤ 0.01), respectively, less absorption of Evans blue through root membrane compared to Cd2+-treated seedlings, showing that the presence of Ca2+ and Si protected integrity of cell membrane against Cd2+-induced damage.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on contents of a superoxide anion, b hydrogen peroxide (H 2 O 2 ) and c thiobarbituric acid reactive substances (TBARS, a measure of lipid peroxidation) in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#) and (##) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 and p ≤ 0.01, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Localization of H2O2 and O2 ·− in leaves of seedlings
To examine histochemical localization of O2 ·− in situ in the cells, the dye NBT was used. This dye reacts with O2 ·− and forms blue-coloured formazan spots in the cells. More intense dark-blue-coloured patches were observed in Cd2+-treated rice leaves compared to untreated controls, suggesting that Cd2+ treatment of seedlings enhanced O2 ·− generation in the leaves (Fig. 2a). Intensity and number of formazan spots decreased in the leaves of the seedlings grown in presence of Cd2+ + Ca2+ or Cd2+ + Si in comparison with Cd2+-treated seedlings. This suggests that treatment with Ca2+ and Si suppressed Cd-induced O2 ·− generation in rice leaves. The generation of H2O2 was histochemically detected in the leaves of seedlings using DAB, which reacts with H2O2, yielding dark brown precipitates. We observed very few spots with very less intensity on leaf surface of control grown seedlings, whereas in leaves of Cd2+-treated seedlings, more intensely stained spots were seen, showing greater accumulation of H2O2 due to Cd2+ treatment (Fig. 2b). Intensity and number of formazan spots decreased in the leaves of the seedlings grown under Cd2+ + Ca2+ or Cd2+ + Si2+ in comparison with Cd2+-treated seedlings, though they were higher than control grown seedlings. These visual results reflect the protective role of Ca2+ as well as Si over Cd2+-induced generation of ROS and oxidative damages.
a Microscopic view of localization of superoxide anion (O2 ·−) in situ in rice leaves using NBT staining. Leaves of the seedlings grown for 12 days either in nutrient solution (control, a) or in nutrient solution containing Cd2+ (b), Cd2+ + Ca2+ (c) and Cd2+ + Si (d) were used. Dark-stained patches represent O2 ·− produced in mesophyll cells. b Histochemical detection of hydrogen peroxide in situ by 3,3′-diaminobenzidine (DAB) uptake method in rice leaves. Seedlings grown for 16 days either in nutrient solution (control, a) or in nutrient solution containing Cd2+ (b), Cd2+ + Ca2+ (c) and Cd2+ + Si (d) were used. Dark spots represent presence of H2O2
Histochemical detection of lipid peroxidation and membrane damage in root tips
Lipid peroxidation was examined histochemically in the roots using Schiff’s reagent. Root surfaces got stained with this reagent depicting formation of lipid peroxides (Fig. 3a). Increased formation of lipid peroxides marked by intensely coloured root surfaces was observed in roots of Cd2+-treated seedlings compared to controls. Presence of Ca2+ and Si reduced colour formation on root surfaces compared to Cd2+-treated seedlings which reflects decreased lipid peroxidation in the seedlings due to Ca2+ and Si treatments compared to Cd2+-treated seedlings. Similar results were obtained when root tips of the seedlings were stained with Evans blue and examined microscopically for histochemical localization of the dye (Fig. 3b). Seedlings exposed to Cd(NO3)2 (75 μM) showed intense staining of Evans blue in root tips, suggesting that Cd2+ treatment resulted in alteration of cell membrane integrity in roots. Comparatively lesser staining with Evans blue in Ca2+- or Si-treated root tips compared to root tips of Cd-treated seedlings indicates that both Ca2+ and Si protect root tips against Cd2+-induced plasma membrane damage.
a Histochemical detection of lipid peroxidation in root tips of rice seedlings. Pink-red-stained root portions represent extent of lipid peroxidation in the cells. Control represents untreated seedlings. b Histochemical detection of loss of plasma membrane integrity in root tips of rice seedlings treated with Cd2+, Cd2+ + Ca2+ or Cd2+ + Si. Intense blue-stained root portions represent increased Evans blue uptake by the cells, showing loss of plasma membrane integrity
Effect of Ca and Si on Cd-induced alterations in protein and non-protein thiols
In response to Cd2+ treatment, a significant decline in protein thiol level was observed in the seedlings (Fig. 4a). Rice seedlings grown for 12 days in presence of 75 μM Cd(NO3)2 showed 58 % (p ≤ 0.01) decline in protein thiol level in roots and 25 % (p ≤ 0.05) decline in the level in shoots compared to controls. The level of protein thiol was always higher in shoots than in roots in both control as well as Cd2+ treatments. Treatment with Ca2+ or Si considerably alleviated Cd-induced decline in protein thiol level. Both Ca2+ and Si appeared to be equally effective in restoring Cd-induced decline in protein thiol level in the seedlings. Non-protein thiol level was elevated in both roots and shoots of Cd2+-treated seedlings compared to controls (Fig. 4b). With Cd2+ treatment nearly 70 % (p ≤ 0.01), increase in non-protein thiol level was observed in roots and 43 % (p ≤ 0.01) increase in shoots compared to controls. Ca2+ and Si promoted a further increase in the level of non-protein thiol in both roots and shoots of the Cd-stressed seedlings. These results reflect the protective role of Ca2+ and Si by promoting increased synthesis of thiol-containing compounds in Cd-stressed seedlings and in maintaining a high reduction pool in the cells.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on a protein thiol and b non-protein thiol in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#) and (##) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 and p ≤ 0.01, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Effect of Ca2+ and Si on Cd2+-induced protein carbonylation and proteolytic activity
Exposure of rice seedlings to Cd2+ led to increased level of protein carbonyls (Fig. 5a). Seedlings treated with Cd2+ for 12 days showed 88 % (p ≤ 0.001) increase in protein carbonyls in roots and 78 % (p ≤ 0.001) increase in shoots compared to controls. Exogenous treatment of rice seedlings with Ca2+ and Si along with Cd2+ led to considerable decline in the level of protein carbonyls compared to the level in Cd2+-treated seedlings. Parallel to increase in the content of protein-bound carbonyls, the proteolytic activity also increased in the seedlings with Cd2+ treatment (Fig. 5b). Rice seedlings subjected to Cd2+ treatment for 12 days showed 46 % (p ≤ 0.01) increased proteolytic activity in roots and 48 % (p ≤ 0.01) increased activity in shoots compared to controls. With the presence of Ca2+ and Si in the medium, a considerable decline in total protease activity was observed in the seedlings compared to the activity in Cd-stressed seedlings.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on a carbonyl content and b proteolytic activity in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#) and (##) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 and p ≤ 0.01, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Effect of Ca and Si on Cd-induced changes in non-enzymic antioxidants
The content of reduced form of ascorbate (AsA) and its oxidized form (DHA) as well as the ratio of AsA/DHA declined in Cd2+-treated seedlings compared to controls (Fig. 6). We observed 42 % (p ≤ 0.01) decline in AsA level in roots and 46 % (p ≤ 0.01) decline in shoots in rice seedling exposed to 75 μM Cd2+ for 12 days (Fig. 6a), whereas under similar conditions, DHA level declined by 14 % in roots and by 26 % (p ≤ 0.05) in shoots compared to controls (Fig. 6b). Changes in the reduced and oxidized forms of ascorbate under Cd2+ toxicity also resulted into drop in AsA/DHA ratio in the cell. In rice seedlings grown for 12 days under Cd2+ treatment, 32 % (p ≤ 0.05) decline in AsA/DHA ratio was observed in roots and 25 % (p ≤ 0.05) decline in shoots (Fig. 6c). Ca2+ and Si treatments resulted in increased levels of AsA in the seedlings compared to Cd-treated seedlings. Increase in AsA level increased AsA/DHA ratio in Cd2+ + Ca2+- and Cd2+ + Si-treated seedlings compared to Cd2+-alone-treated seedlings. The level of another antioxidant GSH declined significantly in rice seedlings due to Cd2+ treatment, whereas the level of its oxidized form GSSG increased (Fig. 7). In seedlings exposed to 75 μM Cd2+ for 12 days, 26 % (p ≤ 0.05) decline in GSH level was observed in roots and 40 % (p ≤ 0.01) decline in shoots compared to the level in controls (Fig. 7a). The ratio GSH/GSSH declined in the seedlings with Cd2+ treatment (Fig. 7c). Ca2+ and Si application resulted in a positive change in total glutathione pool in roots and shoots of Cd2+ stressed seedlings. GSH level increased in Cd2+-treated seedlings compared to controls, whereas GSSG level was regulated towards control. This increased GSH and regulated GSSG level resulted in increase in GSH/GSSG ratio in both roots and shoots of Cd2+ + Ca2+- and Cd2+ + Si-treated rice seedlings as compared to Cd-treated seedlings.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on the levels of a reduced ascorbate (AsA), b dehydroascorbate (DHA) and c ratio of AsA/DHA in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number sign (#) indicates values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on the contents of a reduced glutathione (GSH), b oxidized glutathione (GSSG) and c ratio of GSH/GSSG in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#), (##) and (###) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Effect of Ca and Si on Cd-induced alterations in the activities of antioxidative enzymes
Cd2+ treatment resulted in increase in the activities of almost all antioxidative enzymes studied except CAT. Cd2+ + Ca2+ and Cd2+ + Si treatment for 12 days resulted in 85 % (p ≤ 0.001) increase in SOD activity in roots and 54 % (p ≤ 0.01) increased activity in shoots (Fig. 8a). With the presence of 2 mM CaCl2 or 200 μM Si in the medium along with 75 μM Cd2+, apparently distinct patterns of antioxidative enzyme activities were observed in the seedlings (Fig. 8). Ca2+ treatment resulted in suppression of Cd-induced increase in SOD activity, whereas exogenous application of Si resulted in further increase in SOD activity in both roots and shoots. The activity of CAT showed distinct behaviour compared to other antioxidative enzymes under various treatments. The activity of CAT decreased in both roots and shoots due to Cd2+ treatment. Rice seedlings grown for 12 days under Cd2+ treatment showed 15 % decline in CAT activity in roots and 20 % decline in shoots compared to controls (Fig. 8b). Significant increase in CAT activity was observed in the seedlings grown in presence of Ca2+ +Cd2+ or Si + Cd2+ compared to Cd-alone-treated or control-grown seedlings. With the presence of Ca2+ in the medium, we observed 30 % (p ≤ 0.05) and 45 % (p ≤ 0.01) increase in CAT activity in roots and 35 % (p ≤ 0.05) and 50 % (p ≤ 0.01) increase in shoots compared to the activity in control and Cd2+-treated plants. Whereas the presence of Si in the medium resulted in 30 % (p ≤ 0.05) and 51 % (p ≤ 0.01) increase in root CAT activity and 23 % and 45 % (p ≤ 0.01) increase in shoot activity in the seedlings as compared to control-grown and Cd2+-treated seedlings. Unlike CAT, the activity of GPX increased in both roots as well as shoots of the seedlings with Cd2+ treatment. Cd-induced increase in GPX activity was much higher in roots than in shoots. Seedlings exposed to 75 μM Cd2+ for 12 days showed 70 % (p ≤ 0.001) increase in GPX activity in roots and 39 % (p ≤ 0.01) increase in activity in shoots compared to controls (Fig. 8c). In presence of Ca2+ or Si in the treatment medium, there was suppression in Cd-induced increase in GPX activity. Ca2+ treatment resulted into a marked and conspicuous suppression in Cd-induced increase in root GPX activity compared to Si.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on activities of a superoxide dismutase (SOD), b catalase (CAT) and c guaiacol peroxidase (GPX) in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#) and (##) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 and p ≤ 0.01, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
To elucidate the mechanism of the maintenance of redox balance under Cd2+ toxicity, we examined the activities of the enzymes of ascorbate–glutathione cycle. A marked increase in the activity of APX was observed in both roots and shoots of Cd-treated seedlings compared to controls (Fig. 9). Roots always maintained higher APX activity than shoots under both control and Cd2+ treatments. Seedlings grown under 75 μM Cd2+ for 12 days showed 56 % (p ≤ 0.01) increased APX activity in roots and 47 % (p ≤ 0.01) increased activity in shoots compared to controls. Ca2+ treatment resulted in decline in APX activity in the seedlings compared to Cd2+-alone-treated seedlings; however, the activity was higher than control-grown seedlings (Fig. 9a). Treatment with Si promoted APX activity to a significantly higher level compared to control 69 % (p ≤ 0.001) as well as Cd2+-alone-treated seedlings. Cd2+ treatment resulted in significant increase in MDHAR activity in the seedlings (Fig. 9b). Seedlings grown under 75 μM Cd2+ for 12 days showed 60 % (p ≤ 0.01) increase in MDHAR activity in roots and 76 % (p ≤ 0.001) increased activity in shoots compared to the controls. MDHAR activity was greater in shoots than in roots in controls as well as under different treatments. Similar to MDHAR, the activity of DHAR also increased in seedlings from Cd2+ treatment. Seedlings grown under 75 μM Cd2+ for 12 days showed 77 % (p ≤ 0.001) increased DHAR activity in roots and 41 % (p ≤ 0.01) increased activity in shoots (Fig. 9c). GR, an important enzyme needed for reduction of GSSG using NADPH, showed increased activity in the rice seedlings with Cd2+ treatment (Fig. 9d). Rice seedlings raised under 75 μM Cd2+ for 12 days showed 71 % (p ≤ 0.01) increased GR activity in roots and 115 % (p ≤ 0.001) increased activity in shoots compared to the activity in controls (Fig. 9d). Ca2+ treatment resulted in little increase in MDHAR activity in roots and almost similar level of activity in shoots compared to Cd2+-treated seedlings, whereas DHAR activity was lower in Ca2+ + Cd2+-treated seedlings compared to Cd2+-alone-treated seedlings. GR activity was also lower in the roots of Ca2+ + Cd2+-treated seedlings compared to Cd2+-alone-treated seedlings. With the presence of Si, however in the medium, the activities of all the three enzymes MDHAR, DHAR and GR increased further as compared to the activities in Cd2+-treated seedlings.
Effect of Cd2+, Cd2+ + Ca2+ and Cd2+ + Si in the growth medium on activities of a ascorbate peroxidase (APX), b monodehydroascorbate reductase (MDHAR), c dehydroascorbate reductase (DHAR) and d glutathione reductase (GR) in roots and shoots of rice seedlings at 12 days of growth. Values are mean ± SD based on three independent determinations, and bars indicate standard deviations. Asterisks (*), (**) and (***) indicate values that differ significantly from control, at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, whereas number signs (#) and (##) indicate values significantly differing from Cd2+ to Cd2+ + Ca2+ and Cd2+ + Si, at p ≤ 0.05 and p ≤ 0.01, respectively, according to one-way ANOVA multiple comparison analysis using GraphPad Prism
Discussion
Increasing concentrations of various metals like Cd2+, Pb2+, Hg+, Cr5+ and Ni2 in the agricultural land cause either directly or indirectly increased production of ROS and thereby intense oxidative damage to growing plants. Being a redox-inactive metal, Cd2+ does not participate in Fenton-type ROS-producing reactions; it indirectly activates NADPH oxidases in membranes giving rise to ROS generation and also promotes Fenton-type reactions by increasing free Fe++ ion by replacing it from its active sites (Romero-Puertas et al. 2004). Increasing pieces of evidence suggest that oxidative damage is an important factor in the expression of toxicity caused due to many metals in plants (Sandalio et al. 2009; Sharma et al. 2012). It has been shown that exogenous application of Ca2+ and Si alleviates the Cd2+-induced growth inhibition in many plant species (Liang et al. 2005; Tian et al. 2011). In our experiments performed in hydroponics, 75 μM Cd2+ significantly inhibited the growth of rice seedlings. It has been observed that Cd2+ irreversibly inhibits proton pump responsible for elongation of cells (Aidid and Okamoto 1993). Whereas the presence of Ca2+ and Si in the medium protected the seedlings against Cd2+-induced growth inhibition. Restoration of growth of the seedlings due to presence of Ca2+ or Si paralleled with decreased accumulation of Cd2+ by the seedlings. In S. alfredii plants, exogenous treatment of Ca2+ significantly restored root growth inhibited due to Cd2+ (Tian et al. 2011). It is suggested that addition of Ca2+ to the growth medium reduces Cd2+ activity at plasma membrane surface of root cells (Tian et al. 2011). Supplying exogenous Si has been shown to alleviate Cr5+-induced reduction in root and shoot growth and metabolism in rice (Zeng et al. 2011). Our knowledge related to the mechanism of Si-induced growth promotion in plants is insufficient; however, it is believed that Si increases cell wall extensibility (Hossain et al. 2002). Our results indicate that the presence of Ca2+ and Si in nutrient medium plays important role in restoring plant growth due to Cd2+ toxicity in rice plants.
Cd2+ treatment resulted in significant decline in plant biomass in comparison with control, whereas significant restoration in biomass was observed with presence of Ca2+ and Si. It has been shown that Ca2+ or Si reduces Cd2+-induced decline in biomass in different plant species (Sandalio et al. 2009; Farzadfar et al. 2013). In our experiments, we observed that treatment of rice seedlings with 75 μM Cd(NO3)2 promoted accumulation of Cd2+ within the plants, whereas with the presence of Ca2+ or Si in the nutrient medium, the accumulation of Cd2+ was significantly reduced by the roots. Due to high resemblance in chemical character, Cd2+ uses Ca2+ transporters for its uptake inside the cells; therefore, increased Ca2+ in the nutrient medium might compete with Cd2+ for its uptake and internalization in the cells. It has been observed that exogenous application of Ca2+ alleviates Cd2+-induced damage in plants by decreasing the accumulation of Cd2+ in plant parts promoting plant growth (Tian et al. 2011) and membrane stability and re-stabilizing Ca2+-mediated signalling inside the plants (Cramer 1985). Presence of Si inside the cell has been shown to reduce accumulation as well as translocation of available heavy metals in the cell. In cowpea plants, it has been reported that Si modifies cation-binding capacity of cell wall and lowers soluble symplastic Mn2+ concentration (Horst et al. 1999). Si has been shown to detoxify the toxic Mn present in symplast (Iwasaki et al. 2002). In peanut plants, Si pretreatment reduced Cd2+ accumulation in the tissues (Shi et al. 2010). Si in its secondary action enhances the uptake of Ca2+ in plant roots, which competes with Cd2+ for uptake and lowers its transport from roots to shoots (Song et al. 2009). Though there is a possibility of competition of Cd2+ with Ca2+ and Si for decreased Cd2+ accumulation in growing rice plants as observed in our studies, the possibility of reduced uptake of free ionic species of Cd2+ due to ionic interactions with Ca2+ and Si also cannot be ruled out.
Increased production of the ROS (O2 ·− and H2O2) is the key contributor in Cd2+-induced oxidative damage in plants (Shah et al. 2001; Romero-Puertas et al. 2004). Though Cd2+ is not involved in redox reactions in cells, it causes production of ROS in plants. Cd2+ indirectly activates NADPH oxidase present at membrane surface and subsequently promotes formation of ROS leading to oxidative damage in cells (Romero-Puertas et al. 2004). Though increased production of ROS in the cell poses threat to cellular biomolecules, ROS also act as signal molecules and activate stress-responsive and defence-related genes via signalling pathways (Sharma et al. 2012). In our studies, we observed that exogenous application of Ca2+ or Si along with Cd2+ reduced ROS production in the tissues in comparison of Cd-stressed seedlings. Ca2+ supplementation has been shown to reduce Cd-induced oxidative damage by lowering formation of ROS (H2O2 and O2 ·−) in S. alfredii and Matricaria chamomilla L. plants (Tian et al. 2011; Farzadfar et al. 2013). Many workers have shown that Si reduces generation of ROS in cells under toxicities due to the metals Mn2+ (Shi et al. 2005b) and Cd2+ (Song et al. 2009).
The overproduced ROS, if not properly scavenged, causes oxidative damage to membrane lipids, proteins, chloroplastic pigments, enzymes, nucleic acids, etc. Lipid peroxidation is regarded as an effective indicator of cellular oxidative damage (Srivastava and Dubey 2011); therefore, we examined the level of TBARS in roots and shoots of rice seedlings under different treatments. Our results showed significant increase in TBARS level in the seedlings grown in the presence of Cd2+. Interestingly in seedlings grown under Cd2+ + Ca2+ and Cd2+ + Si, we observed remarkable decline in TBARS content compared to Cd2+-treated seedlings. This shows that Ca2+ and Si considerably protect Cd-induced oxidative injury in rice seedlings, and this protection might be related to the avoidance of H2O2 generation and the reduction of Cd2+ uptake (Song et al. 2009; Farzadfar et al. 2013). Besides increased lipid peroxidation, we observed significant increase in membrane damage in root tips of Cd-exposed seedlings. Permeability and integrity of the membrane change due to peroxidation of membrane lipids which damages cell membranes irreversibly. Evans blue uptake assay both histochemically and spectrophotometrically confirmed increased permeability of cell membranes due to Cd2+ treatment. Further, Ca2+ and Si significantly reduced the internalization of Evans blue inside the root cells by promoting membrane integrity and decreasing lipid peroxidation.
A decline in the level of protein thiol and an increase in the level of non-protein thiol were observed in the seedlings grown in presence of Cd2+. A decline in protein thiol level indicates oxidation of –SH groups of proteins under Cd2+ toxicity. Non-protein thiols in rice mainly compose of cysteine, γ-glutamyl cysteine, GSH, hydroxymethyl GSH, phytochelatins [PCs; (γ-Glu-Cys)n-Gly] and hydroxymethyl phytochelatins (hm-PCs) (Klapheck et al. 1994). In our studies, a decline in the level of GSH and an increase in the level of non-protein thiol indicate that Cd2+ toxicity might favour induction of the synthesis of PCs and hm-PCs and other low-molecular-weight thiol-rich compounds which are known to play a role in the detoxification mechanism against heavy metals in plants (Zenk 1996). Rice seedlings grown under Ca2+ and Si treatments showed restoration in protein thiol level. Whereas non-protein thiol content also increased in seedling grown under Cd2+ + Ca2+ and Cd2+ + Si treatments compared to control and Cd-treated seedlings, showing limiting effect of Ca2+ and Si over Cd2+ accumulation, translocation and induced oxidative stress. Klapheck et al. (1994) showed that Cd2+-treated rice plants had lower level of GSH content and increased levels of γ-Glu-Cys peptides and PCs compared to non-treated plants.
Protein carbonylation is another consequence of oxidative damage to proteins, and it is widely used as a marker of protein oxidation (Moller et al. 2007). The oxidation of a number of protein amino acids particularly Arg, His, Lys, Pro, Thr and Trp may lead to inhibition or alteration in protein functioning and increase its susceptibility towards proteolytic attack (Moller et al. 2007). Our studies showed a significant increase in carbonyl content as well as increase in proteolytic activity in roots and shoots of Cd-treated seedlings compared to controls. Srivastava and Dubey (2011) reported that excess Mn2+ led to carbonylation in rice seedlings in dose- and time-dependent manner. In our studies with application of Ca2+ and Si along with Cd2+, a significant decline in carbonyl content as well as proteolytic activity was noticed as compared to the carbonylation and proteolysis in Cd-treated seedlings. A direct correlation was noticed between increase in the level of carbonylation of proteins and proteolytic activity under different treatments. It has been observed that carbonylated proteins when accumulate in the cells become toxic and also become more susceptible to proteolysis due to unfolding of target protein domains (Moller et al. 2007). In Cd2+-exposed pea and Arabidopsis plants, enhanced proteolytic activity leads to more efficient degradation of oxidized proteins (Romero-Puertas et al. 2002). In our studies, increased production of ROS might cause increased oxidation of proteins which in turn would trigger enhanced protease activity under different treatments. As Ca2+ and Si in the treatment medium lowered generation of ROS, compared to Cd2+-treated seedlings, Ca2+ and Si appear to safeguard cellular proteins against oxidative injury and thus indirectly restrict protease activity compared to the activity in Cd2+-treated seedlings.
Increased level of ROS in the cells alters cellular redox status due to oxidative damage resulting in disruption of redox signalling and redox control (Noctor 2006). The cellular redox state in the cells maintains homeostasis and enables the plants to deal with redox events such as oxidative stress (Srivastava and Dubey 2011). Our results showed a decline in the ratio of both AsA/DHA and GSH/GSSG in Cd2+-treated rice seedlings. AsA is a potent ROS scavenger and it provides protection to the cell organelles and biomolecules from oxidative damage by directly scavenging O2 ·− and ·OH (Gill and Tuteja 2010). Transgenic plants with higher AsA contents showed improved tolerance to oxidative stress (Wang et al. 2010). In our studies, a high level of AsA and a higher AsA/DHA ratio in Cd2+ + Ca2+- and Cd2+ + Si-treated seedlings compared to Cd-alone-treated seedlings suggest that Cd2+ tolerance in rice is associated with maintenance of a higher level of reduced form of ascorbate in the tissues. Similarly, the reduced glutathione (GSH) is also a potent scavenger of the ROS (O2 ·−, H2O2 and ·OH) and plays an important role in antioxidative defence system by regenerating AsA via the AsA–GSH cycle (Gill and Tuteja 2010). GSH has an important role in avoiding ROS-induced oxidative damage and in maintaining normal reduced state of the cell (Sharma et al. 2012). A delicate balance between the GSH and GSSG is essential to maintain cellular redox state (Sharma et al. 2012). Due to its crucial role in antioxidative defence, GSH level is used as a stress marker and an elevated GSH level is correlated with the ability of plants to withstand abiotic stress-induced oxidative damage (Gill and Tuteja 2010). Many workers have reported decline in reduction pool of cell under metal toxicity (Shi et al. 2005b; Song et al. 2009; Srivastava and Dubey 2011). Our results indicate that similar to AsA, a high level of GSH and a higher GSH/GSSG ratio under Ca2+ and Si treatments are associated with Cd toxicity tolerance in rice seedlings and that exogenous Ca2+ and Si help in maintaining adequate reduced levels of ascorbate and glutathione in the cells under Cd2+ toxicity to withstand Cd-induced oxidative damage.
Under various abiotic stresses including metal toxicity, the antioxidative defence system of plants scavenges overproduced ROS depending on their concentration in the cell (Gill and Tuteja 2010). A delicate balance always exists between ROS produced and cellular antioxidative defence machinery, in order to minimize cellular damage. Among the antioxidative enzymes, SOD catalyzes the conversion of O2 ·− to less toxic H2O2 and O2 and it is considered as the first line of defence against ROS in plants. We observed a marked increase in SOD activity in Cd2+-exposed rice seedlings. Increased SOD activity in plants is directly related with generation of O2 ·− in the cells and is considered as induction of protection mechanism against abiotic stresses (Srivastava and Dubey 2011). In rice plants grown under Cd2+ + Ca2+ and Cd2+ + Si treatments, we observed two distinct patterns in SOD activity; presence of Ca2+ resulted in considerable decline in SOD activity whereas Si caused increase in SOD activity in comparison with Cd2+-alone-treated seedlings. Similar to our studies, Farzadfar et al. (2013) reported that Ca2+ treatment resulted in decline in SOD activity in Cd-treated M. chamomilla plants. It has been reported that exogenous Si led to an increase in SOD activity in Cucumis sativus plants grown under Mn stress (Shi et al. 2005b). The intracellular level of H2O2 is regulated by a wide range of enzymes; two most importantly are CATs and peroxidases, both of which take care of overproduced H2O2 due to high SOD activity under oxidative stress. We observed decline in CAT activity in roots and shoots of Cd2+-treated seedlings, suggesting that the H2O2 scavenging mechanism by CAT is possibly less effective when seedlings are subjected to prolonged Cd2+ treatment of nearly 12 days. Srivastava and Dubey (2011) reported an increase in CAT activity in rice seedlings exposed to 5–10 days with Mn excess, and the activity declined when Mn stress persisted for longer duration. Decline in CAT activity in metal-exposed plants could be attributed to either inactivation of enzyme due to its direct interaction with metal ions or ROS (Dat et al. 2000) or due to its decreased synthesis or impaired protein assembly (Ushimaru et al. 1999). Ca2+ and Si treatment to rice seedlings significantly increased CAT expression when present along with Cd in growth medium. According to Shi et al. (2005a), Si treatment resulted in increase in CAT activity in C. sativus grown under Mn2+ toxicity. Pyngrope et al. (2013) showed that higher CAT level in drought-tolerant seedlings protects the seedlings from oxidative damages by warding them from overproduced ROS. Peroxidases play important role in scavenging H2O2 in plants. We observed significant increase in APX and GPX activities in Cd2+-treated seedlings compared to controls, and presence of Ca2+ and Si in the medium further regulated this increase. Farzadfar et al. (2013) also observed that Cd2+ treatment resulted in exponential increase in peroxidase activity in M. chamomilla plants, which was significantly regulated by Ca2+ treatment. Shi et al. (2005a), however, reported that exogenous application of Si promoted APX activity but GPX activity declined. The enzymes MDHAR and DHAR together with GR participate in regeneration of antioxidants AsA and GSH and help in maintaining redox cellular balance. Optimum activity of GR is necessary in the cells to maintain an optimum pool of GSH. The activity of these enzymes increased in rice seedlings with Cd2+ treatment. Srivastava and Dubey (2011) reported increased activities of the enzymes MDHAR, DHAR and GR in rice seedlings under Mn2+ exposure and suggested that Mn2+ induced GSH and AsA regenerating system in the seedlings in order to maintain their requisite levels in the tissues to cope up with the adverse conditions of oxidative damage due to Mn2+. Other studies have also revealed enhanced activity of the enzymes of AsA–GSH cycle in different plant species under toxicity of the metals Mn2+ and Cd2+ (Shah et al. 2001; Shi et al. 2005b; Srivastava and Dubey 2011). Presence of Cd2+ and Si, in our studies, helped in maintaining sufficiently high activity levels of the enzymes of AsA–GSH cycle, suggesting further that Ca2+ and Si help the cells in generating sufficiently reduced levels of ascorbate and glutathione under Cd2+ toxicity.
In conclusion, results of the present study suggest that Cd2+ toxicity in rice seedlings is associated with induction of oxidative stress in the seedlings due to overproduced O2 ·− and H2O2. Among the antioxidative enzymes, SOD, GPX APX, MDHAR, DHAR and GR appear to play a key role in scavenging O2 ·− and H2O2 under Cd2+ toxicity. Ca2+ and Si appear to protect rice plants from Cd2+ toxicity by adequately maintaining pool of antioxidants AsA and GSH and higher activity levels of key antioxidant enzymes. Nearly 50 % less Cd2+ was detected in roots as well as shoots when Ca2+ was present in the growth medium. Whereas Si, which was not so efficient as Ca2+ to prevent Cd2+ entry in plant roots, restricted Cd2+ uptake to a considerable extent. But, Si significantly promoted plant’s antioxidative defence system so that plants were able to cope up with overproduced ROS to diminish the effects of oxidative stress. The results suggest that exogenous application of Ca2+ and Si in the growth medium ameliorates Cd-induced oxidative stress in rice seedlings. The observations indicate that Ca2+ and Si treatments can help in reducing Cd2+ toxicity in rice plants.
References
Aidid SB, Okamoto H (1993) Responses of elongation growth rate, turgor pressure and cell wall extensibility of stem cells of Impatiens balsamina to lead, cadmium and zinc. Biometals 6:245–249
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Mooren PD, Chapman SB (eds) Methods in plant ecology. Blackwell Scientific Publication, Oxford, pp 285–344
Andresen E, Küpper H (2013) Cadmium toxicity in plants. In: Sigel A, Sigel H, Sigel RKO (eds) Cadmium: from toxicity to essentiality. Metal Ions in Life Sciences 11:395–413
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Cramer GR (1985) Displacement of Ca2+ and Na+ form the plasma lemma root cells. Plant Physiol 79:207–211
Dat J, Vandenbeele S, Vranova E, Van Montagu M, Inze D, Van Breusegm F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
de Kok LJ, Kuiper PJC (1986) Effect of short-term dark incubation with chloride and selenate on the glutathione content of spinach leaf discs. Physiol Plant 68:477–482
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sidaspinosa L. Planta 157:224–232
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2013) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut Res 20:1413–1422
Frahry G, Schopfer P (2001) NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analysed with a tetrazolium-based assay. Planta 212:175–183
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Griffith OW (1980) Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 6:207–212
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Horst WJ, Fecht M, Naumann A, Wissemeier AH, Maier P (1999) Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. J Plant Nutr Soil Sci 162:263–274
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MT, Ryuji M, Soga K, Wakabayashi K, Kamisaka S, Fuji S, Yamamoto R, Hoson T (2002) Growth promotion and increase in cell wall extensibility by silicon in rice and some Poaceae seedlings. J Plant Res 115:23–27
Iwasaki K, Maier P, Fecht M, Horst WJ (2002) Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 238:281–288
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Klapheck S, Fliegner W, Zimmer I (1994) Hydroxymethyl-phytochelatins [(gamma- glutamyl-cysteine)n,-serine] are metal-induced peptides of the Poaceae. Plant Physiol 104:1325–1332
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid and spinach (Spinacea oleracea) chloroplasts: the effect of hydrogen peroxide and paraquat. Biochem J 210:899–903
Levine RL, Williams J, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liang Y, Wong J, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Mishra HP, Fridovich I (1972) The role of superoxide anion in autooxidation of the epinephrine and sample assay for SOD. J Biol Chem 247:3170–3175
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Ann Rev Plant Biol 58:459–481
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G (2006) Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29:409–425
Polge C, Jaquinod M, Holzer F, Bourguignon J, Walling L (2009) Evidences for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway activation in response to cadmium. J Biol Chem Mol Biol 284:35412–35424
Pompella A, Maellaro E, Casini AF et al (1987) Measurement of lipid peroxidation in vivo: a comparison of different procedures. Lipids 22:206–211
Pyngrope S, Bhoomika K, Dubey RS (2013) Oxidative stress, protein carbonylation, proteolysis, and antioxidative defense system as a model for depicting water deficit tolerance in Indica rice seedlings. Plant Growth Regul 69:149–165
Romero-Puertas MC, Gomez JM, del Rio A, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ 25:677–686
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gomez M, del Rio LA, Sandalio LM (2004) Cd-induced subcellular accumulation of O2 − and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sandalio L, Dalurzo H, Gomez M, Romero-Puertas M, del Rio L (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sandalio LM, Rodriguez-Serrano M, del Rio LA, Romero-Puetas MC (2009) Reactive oxygen species and signalling in cadmium toxicity. In: del Rio LA, Puppo A (eds) Reactive oxygen species and plant signalling. Springer, Berlin, pp 175–189
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schützendübel A, Schwanz P, Teichmann T et al (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content and differentiation in scot pine (Pinus sylvestris) roots. Plant Physiol 127:887–892
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Sharma P, Dubey RS (2006) Cadmium uptake and its toxicity in higher plants. In: Khan NA, Samiullah (eds) Cadmium toxicity and tolerance in plants. Narosa Publishing House, New Delhi, pp 63–86
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanisms in plants under stressful conditions. J Bot. doi:http://dx.doi.org/10.1155/2012/217037
Shi QH, Bao ZY, Zhu ZJ, He Y, Qian QQ, Yu JQ (2005a) Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66:1551–1559
Shi XH, Zhang CC, Wang H, Zhang FS (2005b) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Shi G, Cai Q, Liu C, Wu L (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61:45–52
Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y (2009) Silicon enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172(1):74–83
Srivastava S, Dubey RS (2011) Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul 64:1–16
Talukdar D (2012) Exogenous calcium alleviates the impact of cadmium induced oxidative stress in Lens culinaris Medic. seedlings through modulation of antioxidant enzyme activities. J Crop Sci Biotech 15:325–334
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley powdery mildew interaction. Plant J 11:1187–1194
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84:63–69
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on antioxidant enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:181–187
Wang Z, Xiao Y, Chen W, Tang K, Zhang L (2010) Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol 52:400–409
Weatherley PE (1950) Studies in the water relation of cotton plant 1. The field measurement of water deficits in leaves. New Phytol 49:81–97
White PJ (2000) Calcium channels in higher plants. Biochim Biophys Acta Biomembr 1465:171–189
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
Xu L, Dong Y, Fan Z, Kong J, Liu S, Bai X (2013) Effects of the application of exogenous NO at different growth stage on the physiological characteristics of peanut grown in Cd-contaminated soil. J Plant Interact. doi:10.1080/17429145. 2013.830780
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. International Rice Research Institute, Philippines, 83
Zeng F, Zhao F, Qiu B, Ouyang Y, Wu F, Zhang G (2011) Alleviation of chromium toxicity by silicon addition in rice plants. Agric Sci China 10(8):1188–1196
Zenk MH (1996) Heavy metal detoxification in higher plants: a review. Gene 179:21–30
Acknowledgments
The authors are grateful to the Banaras Hindu University for providing research facility to carry out this work.
Conflict of interest
The authors hereby declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Srivastava, R.K., Pandey, P., Rajpoot, R. et al. Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma 252, 959–975 (2015). https://doi.org/10.1007/s00709-014-0731-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0731-z