Abstract
Chilling is an environmental phenomenon that hampers the growth of plants. Related studies are primarily based on the aerial plant parts. In contrast, the belowground parts of plant that interact with the rhizosphere microbiome under low temperature stress have not been studied adequately. The overexpression of Low silicon gene 1 (Lsi1) in Dular rice (Oryza sativa L.) significantly enhances its concentration of proline compared with the wild type Dular. This overexpression maintains the osmotic balance of cells through an influx of Ca2+, K+, and H+ and an efflux of Na+. The deposition of calcium and activity of plasma membrane H+-ATPase determined in the root tip was consistent with the results of ion flux obtained. In addition, high-throughput sequencing identified significant variation among 84 genera in different rhizocompartments, including the rhizosphere (R), rhizoplane (RS) and endosphere (N). The bacteria identified are associated with photosynthesis, energy metabolism, nitrogen fixation and defense, which increased significantly in the overexpressed plants. In contrast, the number of pathogenic microorganisms associated with the plant were significantly reduced. Our findings suggest that the stability of ion balance in the overexpressed plant roots affects the structure of microbial community in soil. In conclusion, the overexpression of Lsi1 gene enhanced the cold tolerance of rice, and its underlying mechanisms were comprehensively analyzed, which can provide further evidence for its role in the stress tolerance of rice plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In China, cold spells in the late spring, particularly in the month of April, causes damage to rice (Oryza sativa ) seedlings that lead to reductions in yield. (Fang et al. 2017b)Novel methods, such as the development of cold tolerant rice varieties, are required to limit these losses. Many studies suggest that elements, such as silicon (Si), play a significant role in strengthening the defense mechanisms of the plant (Mitani et al. 2005, 2009). Silicon absorption in rice is controlled by the Low silicon gene 1 (Lsi1) that encodes a Nod26-like mojor intrinsic protein (NIP) subfamily (Ma et al. 2006). We unexpectedly found a feature of this overexpressed line that occurs when the temperature decrease to below 15℃. Moreover, our early studies confirmed that the overexpression of Lsi1 gene in Dular rice increased the cold tolerance by mediating both the transcriptional and proteomic regulatory networks Fang et al. 2017a, Azeem et al. 2016). Nevertheless, the study of Lsi1 gene in rice has focused on the aboveground plant parts, and to our knowledge, no research on the belowground parts has been reported. Therefore, it is crucial to reveal the response of root-associated microbes to cold in the rhizosphere.
Plants that grow in the soil are in contact with diverse microbial communities. There are many microbial communities in the rhizosphere area (Bulgarelli et al. 2013). In addition, the microbial community of a plant determines its health (Berendsen et al. 2012).
These studies unveil a new avenue toward plant microbial interactions, structure and the factors that affect them. However, no studies have been conducted to understand how the microbial community in rice responds to cold. Our early results indicated that the overexpression of Lsi1 enhances cold tolerance in rice. In addition, previous studies also noted that the root ions in rice correspondingly change under low temperature stress, which mediates an increase in the cold tolerance of rice plants (Ma et al. 2015). Moreover, many studies have confirmed the close relationship between root ion transport and microbial metabolism (Zhang et al. 2017; Shabala et al. 2009).
We studied the connection between ion transport and the associated root-microbes under cold stress. First, we determined the content of proline (Pro) and silicon(Si) in the wild type (WT) and Lsi1-overexpression Dular (OS) roots. In addition, to further elucidate the balance of ions required to maintain the cell membrane permeability, we performed Non-invasive Micro-test Technology (NMT) to monitor the influx and efflux of the ions. We primarily focused on Ca2+, K+, Na+ and H+ in the OS roots in comparison with the WT. Moreover, we utilized the deep pyrosequencing of 16 S rDNA microbial genes to detect the operational taxonomic units (OTUs) that were related to three distinct compartments, including the rhizosphere (R), rhizoplane (RS), and endosphere (N). The large datasets from the WT and Lsi1 rice were used to identify putative microbial interactions involved in the processes to increase cold tolerance. Therefore, we provide insights into the ionic balance and process of the assembly of root microbiome in response to cold.
Materials and methods
Research material
The study was conducted in the experimental field of the Agricultural Ecological Key Laboratory, Fujian, China. Based on the Coding sequence (CDS) of rice Lsi1, our research group designed the PCR primers to amplify rice Lsi1 and inserted it into a gene-overexpression vector namely Ubiquitin 1301 to construct the recombinant plasmid (Lsi1-Ubiquitin1301), and then transformed it into Agrobacterium EHA105; Using Agrobacterium-mediated genetic transformation system, we obtained the Dular’s mutants with Lsi1 overexpression (Dular-OS).The rice plants were cultivated in pots. The WT (Oryza sativa L., accession: Dular) and OS (cold tolerant) rice lines were used for all the experiments in this study (Azeem et al., 2016; Fang et al. 2017). Plump rice seeds were disinfected with a solution of 25% sodium hypochlorite (NaClO) for 15 min, washed with deionized water, and then soaked for 48 h for incubation at 28 °C in the dark and 85% relative humidity for 24 h. After germination, the seeds were uniformly planted in growing trays (upper surface diameter 13 cm; lower surface diameter 8 cm; height 7.5 cm) containing matrix soil at 85% relative humidity and 28 °C for 14 h with 20,000 lx light intensity, and then 22 °C in dark for 10 h. After the growth of 1.5 leaves, the planting conditions were adjusted to 15 °C for 14 h with 20,000 lx of light intensity, and then 10 °C in the dark for 10 h at 85% relative humidity. Moreover, normal plantings (the control group) were established at regular temperature (28 °C for 14 h with 20,000 lx of light intensity, and then 22 °C in dark for 10 h at 85% relative humidity). After 48 h of treatment, the samples were collected for subsequent analysis.
Determination of proline (pro) content
0.5 g rice roots were taken and placed in glass tubes, and then 5 mL 3% sulfosalicylic acid solution (3% (W/V) sulfosalicylic acid, 100% H2O) was added to each tube respectively. Afterward, the tube was sealed with the bulb plug before initiation of t extraction in boiling water bath for 10 min. After it was cooled, the filtrate was filtered into clean glass tubes. Two ml extract was taken in another clean glass tube containing 2 ml glacial acetic acid and 2 ml 2.5% acidic ninhydrin reagent (2.5% (W/V) ninhydrin, 60% glacial acetic acid, 40% 6 M phosphoric acid), and then heated it in water bath for 30 min. After it was cooled, it was shaken for 30s after addition of 4mL toluene, and the components in the tube were let to sit for a sometime. The supernatant was shifted to 10mL centrifugal tube, and centrifuged at 3,000 rpm for 5 min. The supernatant was taken in a colorimetric cup. Toluene was used as a blank control, the absorbance value was measured at 520 nm, and the concentration was calculated.
Measurement of net Ca2+, K+, Na+ and H+ flux with the NMT system
The net fluxes of Ca2+, K+, Na+ and H+ were measured by Non-invasive Micro-test Technology (NMT-YG-100, Younger USA LLC, Amherst, MA 01002, Beijing, China). The influxes were measured with V2.0 (Younger USA LLC, Amherst, MA 01002, USA) Software (Zhao et al. 2015). This technique provides a high level of resolution and non-invasively method to measure different ions and small molecules in situ with working principle detailed in Li et al. (Li et al. 2010). The root of rice plant was calibrated with measuring solution for 10 min, then transferred to a small petri dish (6 cm diameter) containing 10 ml of measuring solution. While, the roots were immobilized in petri dish containing measuring solution and microelectrode was vibrating between two positions i.e. 30 μm from the root surface and kept perpendicular axis to the root. The background was recorded by vibrating the electrode in measuring solution without containing roots, then the electrode tip was filled with a commercially available ionophore (Ca2+, K+, Na+ and H+). The measuring solution for Ca2+, H+ and K+ contained a mixture 0.1mM CaCl2, 0.1mM KCl and 0.3mM 2-(N-Morpholino) ethanesulfonic acid (MES), pH 6.0. The measuring solutions for Na+ consisted of 0.1mM CaCl2, 0.1mM KCl, 0.1mM NaCl and 0.3mM MES, pH 6.0.
Assay of plasma membrane H+-ATPase activity
Plasma membrane of rice root was prepared according the method of Xu et al. (Xu et al. 2012) and was stored at -80 °C until analysis. Bradford method was used to determine the protein concentration (Bradford 1976). The H+-ATPase activity was determined in a reaction volume of 0.5 mL that contained 30 mM BTP/MES, pH 6.5, 5 mM MgSO4, 50 mM KCl and 4 mM Tris-ATP and plasma membrane. The reaction continued for 30 min at 30 °C and was stopped with 1 mL of stopping solution containing 2% (v/v) concentrated H2SO4, 5% (w/v) sodium dodecyl sulphate 0.7% (w/v) sodium molybdate, followed by 50 mL of 10% (w/v) ascorbic acid. Absorbance at 700 nm was measured with a spectrophotometer.
Calcium concentration
The selected root tips were washed with ddH2O before the concentration of calcium was measured as described by Qin (Qin et al. 2017). Briefly, the root tip samples were incubated in a solution that contained 0.2 mM CaC12, 50 mM sorbitol, 20 µM Fluo-3 AM (Beyotime Biotechnology, Haimen, China), and 1% (v/v) dimethyl sulfoxide. The samples were wrapped in foil and incubated at 4 °C for two hours and washed twice with a solution of 0.2 mM CaC12 and incubated at room temperature for two hours. The sample slides were then observed under 10 X fluorescence microscopy.
Sampling and collection of the rhizocompartments
The roots were collected from rice plants, and the soil was shaken off the roots to leave 1 mm of soil remaining around the roots. The 1 mm of soil was washed off in phosphate-buffer saline (PBS) and kept as the rhizosphere soil. The clean roots were washed twice to remove remaining soil and placed in clean PBS in a 50 ml falcon tube. The rhizoplane microbes were extracted by sonicating the roots after the rhizosphere compartment had been removed. The sonicated roots were then placed in a new, clean falcon tube and sonicated twice more. The PBS in tube was discarded and refilled with clean PBS. These roots were then kept as the endospheric sample (Edwards et al. 2015).
DNA extraction, PCR amplification, PCR purification and pyrosequencing
The total DNA of different rhizocompartments, including the rhizosphere, rhizoplane and endosphere soil, was extracted using a Soil Gen DNA Kit (CWBIO, Beijing, China) following the manufacturer’s instructions. A Nanodrop 2000 C spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used to determine the concentration of DNA. The DNA was then diluted to 1 ng/ul using sterile water. The 515 F (GTGCCAGCMGCCGCGGTAA) and 806 R (GGACTACHVGGGTWTCTAAT) primers were used to amplify the bacterial 16 S rDNA V4 fragments (Osborne et al. 2018, Xu et al. 2017). PCR reactions were conducted, and the PCR products were monitored using 2% agarose gel electrophoresis. The samples that contained bands between 400 and 450 bp were selected for further analysis. The sequencing libraries were obtained using a TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. Pyrosequencing was performed on an Illumina HiSeq 2500 platform, and 250 bp paired-end reads were generated.
Operational taxonomic unit (OTU) analysis and statistical analysis
The barcode and primer sequences were removed, and the raw tags of origin tag data were spliced using FLASH (V1.2.7). After the chimeras were removed, the sequences obtained were denoted as effective tags and used to perform OTU cluster and species annotation. Sequences that had ≥ 97% similarity was assigned to the same OTU using Uparse software v7.0.1001 (Edgar 2013).
The abundance of OTUs in each sample was homogenized, and alpha and beta diversity analyses were performed. The Observed-species and the community richness Chao1, ACE, Shannon, Goods-coverage and PD_whole_tree indices were calculated using QIIME software (Version 1.8). The R software (Version 2.15.3) was used to draw distribution curves, and the differences among groups of alpha diversity indices were analyzed. The function of microorganisms was predicted by the FAPROTAX database and Python 2.7 software. A principal coordinate analysis (PCoA) was conducted using the R language program. A one-way analysis of variance (ANOVA) followed by a Tukey’s test (P ≤ 0.05) was performed for multiple comparison using DPS software version 7.51 (Enfield, UK).
Statistical analysis
All the data presented in the paper are the means of three biological replicates. The differences among the treatments were calculated and statistically analyzed using the variance and the least-significant-difference multiple-range test (LSD, P < 0.05). The Statistical package for OriginPro 8.0 and the Data Processing System (DPS) version 7.05 were used for the statistical analysis.
Results
The OS and WT under chilling stress
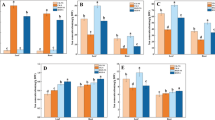
To gain insight into the ability of rice plants to eliminate reactive oxygen species (ROS), we measured the contents of Pro and Si in both the WT and OS plants at low and normal temperatures. The content of Si in OS was significantly increase compare to that in WT both in low and normal temperature (Figure S1). There was no significant difference in the content of Pro between the OS (18.85 µg·g− 1·FW) and WT (19.39 µg·g− 1·FW) at normal temperatures (Fig. 1). The contents of Pro in the OS and WT significantly increased at low temperature compared with normal temperature, and the content of Pro in the OS (41.32 µg·g− 1·FW) was significantly higher than that of the WT (29.25 µg·g− 1·FW), which increased by 41.26% at low temperature. In addition, the content of Pro and Si increased in the OS plants under low temperature stress, which protected the cell membrane system from damage and enhanced the cold resistance of the plants.
The proline content in OS and WT roots. WT: Wild type Dular. OS: Lsi1-overexpression Dular. NT: Normal temperature. LT: Low Temperature. The small letter indicates significance level were between the 4 treatments (two genotypes and two stress conditions). Columns with different letters are statistically significantly different (LSD test, P < 0.05). Error bars are standard error (± SE) of three biological replications
The fluxes of Ca2+, K+, Na+ and H+ in the WT and OS
To estimate the flow of some important ions involved in the response to cold, maintenance of the osmotic balance and defense signaling in roots, we used NMT technology. There was no significant difference in the concentrations of Ca2+, K+, Na+ and H+ between the WT and OS plants at normal temperature, and a small amount was excreted. There were significant differences observed in the fluxes of ions between the WT and OS (Figs. 2 and 3). The flow rate of Ca2+ revealed that the roots of the OS plants assimilated higher amounts of Ca2+ compared with the WT. In addition, the roots of WT plants occasionally absorbed and released Ca2+. Furthermore, the OS plants absorbed Ca2+ as a whole, and the rate reached 54.31 pmol·cm− 2·s− 1. However, the WT plants showed an efflux of Ca2+ as a whole, and the rate reached 0.92 pmol·cm− 2·s− 1. In addition, the OS roots significantly absorbed K+, and the rate reached 12.78 pmol·cm− 2·s− 1. However, the roots of the WT plants emitted a larger quantity of K+, and the rate reached 157.02 pmol·cm− 2·s− 1. The flow rate of Na+ showed that the roots of the OS plants emitted more Na+, and the rate reached 43.02 pmol·cm− 2·s− 1, which was significantly different from the efflux in WT, which was 14.04 pmol·cm− 2·s− 1. Similarly, there was a distinct difference in the efflux of H+ ions between the OS (10.21 pmol·cm− 2·s− 1) and the WT (0.09 pmol·cm− 2·s− 1). These results indicate that the OS plants absorbed Ca2+, K+ and H+ under low temperature stress, while the efflux of Na+ was observed, which maintains the stability of cell membrane function, and enhances the cold resistance of the plants.
Measurement of calcium deposition and H+-ATPase activity
We identified that many Ca2+ ions flowed in both genotypes under low and normal temperature. To further confirm this finding, we measured the deposition of Ca2+ in the root tips of WT and OS. In addition, we found that more Ca2+ was deposited in the OS compared with the WT under chilling stress, thus, indicating the involvement of Ca2+ in the cold response, while it is no difference in normal temperature (Fig. 4 A). The results of ion absorption in this study also indicated that OS plants could absorb large amounts of Ca2+ under low temperature stress. The root calcium deposition experiment confirmed that the calcium content of OS roots was significantly higher than that of the WT under low temperature. Moreover, the H+-ATPase activity was also measured in the roots of both genotypes. The OS exhibited higher H+-ATPase activity than the WT under low temperature, which increases the energy level for the influx and efflux of ions in the OS (Fig. 4B).
OTU cluster and species annotation
The soil bacterial community related to the defense of the WT and OS against cold was identified by utilizing deep 16s rDNA pyrosequencing. Across all the soil samples, 1,457,835 effective tags that contained species annotation were identified, with an average of 76,606 effective tags per sample. Among the total effective tags, 40,400 singletons that accounted for 2.9% of total tags were removed before further analysis (Figure S2). Rarefaction curves demonstrated the sequencing depth, and the number of observed species numbers is shown in Figure S3. At a 97% sequence similarity cut-off, we obtained a sum of 54,655 OTUs across the 18 samples. The OTUs numbers in the WT R, WT RS, WT N, OS R, OS RS and OS N plots were 3,132, 3,023, 2,905, 3,535, 3,411 and 2,212, respectively. On average, we were able to classify approximately 99.4%, 97.9%, 94.3%, 85.3%, 62.8% and 23.2% of effective sequences at the phylum, class, order, family, and genus and species level, respectively.
Alpha diversity indices
The alpha diversity was determined to further investigate the diversity of species within a sample. The Chao 1 and abundance-based coverage estimator ACE indices were calculated to estimate the abundance of each sample. Both the Shannon and Simpson indices were determined to estimate the diversity within each individual sample. In addition, the bacterial community analysis showed that a significantly higher bacterial diversity was observed in OS R and OS RS than WT R and WT RS. In contrast, less bacterial diversity was observed in the OS N than WT N (P ≤ 0.05). In addition, there were no significant differences in the bacterial species identified and the Shannon, Simpson, Chao 1 and ACE diversity indices between WT R and WT RS (P > 0.05). The richness indices, including the Shannon, Simpson, Chao 1 and ACE indices, in OS N were lower than those of the other treatments. Both the species observed and the Shannon, Simpson, Chao 1 and ACE diversity indices were significantly reduced in the change from OS R to OS N (P ≤ 0.05). However, only the Shannon and Simpson diversity indices were significantly reduced from WT R to WT N (Table 1).
Beta diversity indices
The beta diversity indices were determined by the differences in bacterial community composition and structure between the samples. In this study, the weighted UniFrac distance between the WT R and WT RS was a minimum of 0.074, but it was 0.151 in the OS R and OS RS. This indicated that the soil bacterial community between WT R and WT RS was more similar than that of OS R and OS RS. The weighted UniFrac distances between OS N vs. OS R (0.322) and OS N vs. OS RS (0.290) were greater than those of the WT N vs. WT R (0.268) and WT N vs. WT RS (0.239). This indicated that the soil bacterial community from the R to N in OS than WT had a greater difference. The weighted UniFrac distances between OS R vs. WT R, OS RS vs WT RS and OS N vs. WT N were 0.093, 0.108 and 0.231, respectively. This could explain why the highest difference in the soil bacterial community was observed in N compared with the other treatments (R and RS) as shown in Figure S4.
PCoA analysis
A PCoA was performed based on the weighted UniFrac distance. In addition, the PCoA analysis identified distinct differences in the soil bacterial community structure between different treatments. Nevertheless, the first two components (PC1 and PC2) of PCoA explained 60.17% and 18.34% of the total bacterial community variation, respectively, as shown in Fig. 5. Furthermore, the PCoA analysis showed that the bacterial communities of WT N and OS N were far from the other groups, and OS N was the most diverse among all the groups.
Analyses of bacterial communities
The cold significantly changed the bacterial communities in the rhizosphere compartments of OS and WT. We identified 52 phyla. However, more than 95% of the OTUs were members of 10 phyla, including Proteobacteria, Firmicutes, Bacteroidetes, Cyanobacteria, Acidobacteria, Chloroflexi, Verrucomicrobia, Actinobacteria, Planctomycetes and Gemmatimonadetes. The relative abundance of these phyla is shown in Figure S5. There were significantly fewer members of Proteobacteria in the WT N than in R and RS, but there were no significant differences identified between R and RS. In contrast, the number of Proteobacteria in the OS was higher in N and decreased significantly from RS to R. The numbers of Firmicutes and Cyanobacteria in the WT increased significantly from R to N. Nevertheless, the Firmicutes in the OS decreased significantly from R to N, while the Cyanobacteria increased from R to N as shown in Table S1.
Venn diagram of the genera identified in the OS compared with the WT
We not only identified 84 bacterial genera from R, RS and N but also used a Venn diagram to detect the exclusive and shared genera between six different soil samples (Fig. 6). The exclusive genera were 20, 18 and 30 in R, RS and N respectively. There were eight, one and four genera in common in R and RS, RS and N, R and N respectively, and three genera in common across R, RS and N.
Analysis of the dominant genera
In this study, 84 bacterial genera that were identified in six different treatments are shown in the heatmap in Fig. 7. We classified the 84 genera identified into 10 functional categories, including denitrification (7.14%), defense (5.95%), azotification (8.33%), photosynthesis (4.76%), chemotrophy (11.9), energy (1.19%), biodegradation (9.52%), carbon metabolism (4.76%), pathogenicity (26.19%) and unknown (20.24%). The distribution of microbial communities in R, RS and N differed significantly (Fig. 8 and Table S2).
The microorganisms for denitrification and biodegradation accumulated in WT N. In contrast, the microorganisms related to azotification and energy accumulated in OS N, while the microorganisms for defense and photosynthesis accumulated in OS R, RS and N. Moreover, the pathogenic microorganisms accumulated in WT RS and N. These results indicate that rice plants have a significant effect on shaping the composition and structure of microbial communities in the root environment. In contrast, the results of microbial community in the root environment also affect the growth and development of plants. The OS plants can select many beneficial microorganisms, such as those involved in defense, azotification, photosynthesis and energy, and block the invasion of harmful microorganisms under low temperature stress. These results indicate that rice plants have a significant effect on shaping the composition and structure of microbial communities in the root environment. Moreover, the results of microbial community in the root environment also affect the growth and development of plants.
The OS plants absorb Ca2+, K+, H+ and expel Na+, thus, better equipping the OS to maintain the stability of membrane function. Ion transport in the root system of rice is not only an important way to regulate the plant response to external low temperature stress but also an essential carrier material to mediate material exchange and energy flow between the root system and soil (Fig. 9). In brief, it can be considered as one of key factors that contributes to the microecological characteristics in different root environments of plants, and it could also be the basis for the establishment of plant-microorganism interactions.
Discussion
The increase in proline content enhanced the cold resistance of rice
Cold adversely effects the growth of plants and causes physiological dysfunction (Lukatkin et al. 2012; Kroemer et al. 2004). Plants may develop cold tolerance to some extent during the initial responses to cold (Fowler and Thomashow 2002). However, continued exposure to cold stress is disastrous for plant life. Silicon has been reported to help plants resist abiotic stresses, such as cold, drought and metal toxicities (Muneer et al. 2017). Previous studies have found that the overexpression of Lsi1 in rice resulted in significant resistance against cold Fang et al. 2017a, Azeem et al., 2016). Under stress, a large amount of ROS are produced in plants. ROS serve as important signaling molecules in plant growth and development and the responses to various stresses (Apel and Hirt 2004). When low temperature stress occurs, the ROS in rice starts to accumulate in large amounts, and the rice antioxidant system begins to function. Our previous study showed that the OS plants produce large amounts of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) (Azeem et al. 2016). It has been reported that Pro plays roles in protecting enzymes from denaturation and helps to stabilize the machinery of protein synthesis, regulate cytosolic acidity, enhance the capacity to bind water, and act as a reservoir of carbon and nitrogen (Liu et al. 2007). In this study, the Pro content of the OS plants was significantly higher than that of the WT plants, which protected the roots of rice system. In addition, it enhanced the activity of rice root protective enzymes, stabilized the function of the cell membrane, improved the storage of nutrients in the root system, effectively eliminated the damage of ROS to the plant, and improved the cold resistance of the plants.
The OS root ion dynamics and maintenance of the ion balance in response to cold
The cold severely damages the plant cell membrane, and as a result, the lipid membrane permeability increases, and solutes leak out of the cells, which causes water loss. This deleteriously affects the balance of exchange of plant cell material with the surrounding environment and thus, destroys it (Lyons and Raison 1970). Therefore, it is important to know how OS maintains the activity of plasma membrane proton pump and regulates the intracellular influx and efflux of important cold responsive ions, such as Na+, K+, Ca2+, and H+. Additionally, the balance of intracellular potassium and sodium depends on the “sodium potassium pump” on the cell membrane, namely the Na+-K+ATP enzyme. Moreover, an increase in the concentration of extracellular Ca2+ can inhibit the efflux of K+, reduce the intracellular Na+ concentration, and improve the activity of H+-ATPase on vacuolar membranes. In addition, studies have confirmed that the proton pump plays an important role in maintaining the K+/Na+ balance in plants (Niu et al. 1995). Higher proton pump activity can provide an H+ electrochemical potential gradient for the reversed transport of Na+/H+, reduce the depolarization of plasma membrane, and limit the efflux of K+ (Chen et al. 2010; Maathuis 2006). The results obtained during this study led us to hypothesize that under cold stress, OS maintains the mechanism of balancing the intracellular K+/Na+ by Na+ efflux, which helps to avoid the excessive accumulation of Na+ in the cytoplasm. Another way could be owing to the loss of inhibition of K+. Ca2+ plays an important role in the process of plant resistance, such as stress signal transduction, metabolic control and the protection of film stability (Polisensky and Braam 1996, Knight et al., 1997). The results of this study show that under cold stress, the Ca2+ in the OS rice roots tended to flow internally, which resulted in an increase in free Ca2+ in the roots. Therefore, it prevents the depolarization of plasma membrane H+-ATPase and limits the K+ efflux to maintain the balance of the intracellular Na+/K+. Nevertheless, the calcium deposits and H+-ATPase activity in the OS root were significantly higher than those in the WT, which confirms the ion dynamics data. Moreover, we found that the plasma membrane H+-ATPase activity was significantly higher than that of the WT, which indicates that the plasma membrane H + pump activity of the OS plants was significantly higher than that of the WT plants under low temperature stress. However, under chilling stress, the OS plants promote Na+ efflux and increase a small amount of K+ by increasing the activity of plasma membrane H+ pump, which negates the loss of excessive K+. This shows that the proton concentration gradient involves Na+ after improving the activity of plasma membrane H+ pump. Furthermore, the H+ transmembrane reverses transport or can also reduce the depolarization of plasma membrane and thus, limit K+ outflow. Numerous studies have indicated that exogenous Ca2+ can regulate the Na+/H+ reverse transport system of plant cells to maintain the intracellular K+/Na+ balance (Sun et al. 2010). The results suggest that that absorption of more Na + inhibited Ca2+in the WT. Membranes with an increasing ratio of Na+/Ca2+ ions lead to the damage of membrane integrity and functions, ultimately increasing the cell membrane permeability.
In brief, these results further confirm that Lsi1 mediates ion transport and enhances the chilling tolerance of plants.
The response of rice microbial communities to cold
The formation of cold resistance in plants is a complex biological process. Multiple factors interact and mutually influence the plant and its underground soil microbiome. Exploring the process of plant-microbe interactions and its regulatory mechanisms play an important role in studying the interrelationship between soil microbes and plant growth and development (Bais et al. 2004). In addition, studies have shown that different plants exhibit unique and representative rhizosphere microbial communities (Berendsen et al. 2012). The difference in the ion absorption of root leads to differences in plant growth and development, which, in turn, affect the composition of microbial community.
In Arabidopsis, characterization of the diversity of core root microbiome confirmed that the microbial population in the endosphere is less than those in the rhizosphere and rhizoplane microbiomes (Haas et al. 2011). The OS has a higher abundance of Proteobacteria in N than in R and RS, while its abundance in the WT was higher in R and RS and less in N. This could be one of the reasons that helped the OS to develop resistance against the cold. Furthermore, a PCoA analysis showed a distinct separation among all the soil samples and particularly those between the WT N and OS N samples. This suggests that there is a strong relationship between plant performance and the soil microbial community. In addition, the OS N had significantly higher microbial diversity than the WT N. A higher microbial diversity is thought to be responsible for the development and maintenance of disease suppressive soils (Qiu et al. 2012; Yuan et al. 2014, Bruggen and Semenov, 2000). The OS rice plants had significant effects on the microbial community composition and structure in different soil layers under low temperatures.
Moreover, we found significant variability in the patterns of relative abundance of genera between the soil compartments of WT and OS. The 84 bacterial genera identified have been classified in 10 functional classes. Among these soilborne microorganisms, some groups of microorganisms are known to benefit the plants. Examples include supplying key plant nutrients, such as nitrogen and phosphorus, protecting against soilborne diseases, and promoting resistance to abiotic stress (Berendsen et al. 2012). These beneficial microorganisms are collectively referred to as plant growth promoting rhizobacteria (PGPR). Clostridium is a pathogenic bacterium that produces exotoxins and invasive enzymes (Kasai et al. 2005). Flavobacterium is a genus of Gram-negative, non-motile and motile, rod-shaped bacteria that consists of 130 species that have been recognized as causing disease (Bernardet et al. 1996). The OS N accommodated a lower abundance of pathogenic bacteria, and most of them were found in the OS R compared to the WT. As a result of that, the OS plants effectively avoided the invasion of pathogenic bacteria and ensured the stability of organism functions. Lysobacter can not only colonize the rhizosphere of many plants but also secrete a variety of antibiotics (Md Tofazzal et al., 2005) and bioactive substances (Folman et al. 2003) that inhibit the growth of bacteria and control plant diseases (Jochum et al. 2006). It led to the large presence of pathogenic bacteria in OS R and RS. The bacteria in OS N that are associated with energy and azotification increase the nutrients and enhance the circulation of energy in rice roots.
In brief, OS N accommodates a lower abundance of pathogenic bacteria, and most were found in OS R compared with the WT. In contrast, OS N has higher proportions of microorganisms involved in photosynthesis, energy, azotification and defense than RS and R, which ultimately enhances its ability to tolerate cold. These results indicate that rice plants have a significant ability to shape the composition and structure of microbial communities in the root environment. In contrast, the results of microbial community in the root environment also affect the growth and development of plants. Based on microbial studies of R and N, it has been proposed that the plants might accommodate the microbes in two steps. The first is a general recruitment around the root, and the second step is on the genetic basis (species specific) entry of microbes inside the root. We suggest that the rhizoplane plays a critical role in regulating the transport of microbes that have adhered to the rhizosphere. Few microbes can bind to the rhizoplane, and only a fraction of them can enter and proliferate in the endosphere (Caporaso et al. 2010). Each of these events involves complex molecular signals from plant, possibly the root exudates, cell membrane proteins, plant metabolites or cell wall components (Lundberg et al. 2012).
Our study indicates that changes in the microbial community structure on the roots also have important effects on plant root absorption, energy flow, and information transfer, which, in turn, affect plant growth and development. However, the rhizocompartments showed the largest variation during the comparison of OS and WT, suggesting the involvement of genotypic factors for the recruitment of microbes. In summary, the shift in microbial diversity among each compartment indicates the two to three selective steps from the root to interior and response to multiple signals from the plant, which coordinate the assembly of the root microbiome.
Conclusion
Our results suggest that the Lsi1 gene plays a pivotal role in the response to chilling tolerance. The NMT analysis showed that the OS absorbed a significant amount of Ca2+ and H+ and a minute amount of K+. However, there was a large efflux of Na+ to maintain the balance of cell osmotic pressure. The calcium deposition method was used to locate the calcium content in the root of OS, and it was significantly higher than that of the WT, which confirmed our root ion absorption results of Ca2+. Additionally, the H+-ATPase activity on the root membrane in the OS was significantly higher than that of the WT, which indicated that the plant had stronger energy metabolism and helped it to resist the low temperatures of external environment. Further, the results of high-throughput sequencing showed that the number of microorganisms associated with photosynthesis, energy metabolism, nitrogen fixation and defense was significantly up-regulated at low temperature, while the number of microorganisms associated with pathogenicity was significantly down-regulated. Moreover, the overexpressed plants can respond to stress in a timely manner under low temperature, and the plant itself can tolerate adverse conditions through a series of reactions. In conclusion, the overexpression of the Lsi1 gene enhanced the cold tolerance of rice, and its mechanism was comprehensively analyzed, providing a theoretical basis for the production of rice under events of sudden temperature decrease.
References
Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55(1):373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Azeem S, Li Z, Zheng HP, Lin WW, Arafat Y, Zhang ZX, Lin XM, Lin WX (2016) Quantitative proteomics study on Lsi1 in regulation of rice (Oryza sativa L.) cold resistance. Plant growth regul 78(3):307–323. https://doi.org/10.1007/s10725-015-0094-2
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9(1):26–32
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17(8):478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Bernardet JF, Segers P, Vancanneyt MBerthe F, Kersters K, Vandamme (1996) Cutting a gordian knot - emended classification and description of the genus flavobacterium, emended description of the family flavobacteriaceae, and proposal of flavobacterium hydatis nom nov (basonym, cytophaga aquatilis strohl and tait 1978) [Revi. Int j system bacteriol 46(1):128–148
Bradford MM (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bruggen AHC, V, Semenov AM (2000) In search of biological indicators for soil health and disease suppression. Appl Soil Ecol 15(1):13–24. https://doi.org/10.1016/S0929-1393(00)00068-8
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P (2013) Structure and Functions of the Bacterial Microbiota of Plants. Annu Rev Plant Biol 64(1):807–838. https://doi.org/10.1146/annurev-arplant-050312-120106
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge B, Pirrung D, Reeder M, Sevinsky J, Tumbaugh JR, Walters PJ, Widmann WA, Yatsunenko J, Zaneveld T, Knight J R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H (2010) Nitric oxide enhances salt secretion and Na(+) sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H(+)-ATPase and Na(+)/H(+) antiporter under high salinity. Tree Physiol 30(12):1570–1585. https://doi.org/10.1093/treephys/tpq086
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998. https://doi.org/10.1038/nmeth.2604
Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V (2015) Structure, variation, and assembly of the root-associated microbiomes of rice. P Natl A Sci 112(8):E911–E920. https://doi.org/10.1073/pnas.1414592112
Fang C, Zhang P, Jian X, Chen W, Lin H, Li Y, Lin W (2017a) Overexpression of Lsi1 in cold-sensitive rice mediates transcriptional regulatory networks and enhances resistance to chilling stress. Plant Sci 262:115–126. https://doi.org/10.1016/j.plantsci.2017.06.002
Fang CX, Zhang PL, Jian X, Chen WS, Lin HM, Li YZ, Lin WX (2017b) Overexpression of Lsi1 in cold-sensitive rice mediates transcriptional regulatory networks and enhances resistance to chilling stress. Plant Sci 262:115–126. https://doi.org/10.1016/j.plantsci.2017.06.002
Folman LB, Postma J, Veen JA (2003) Inability to Find Consistent Bacterial Biocontrol Agents of Pythium aphanidermatum in Cucumber Using Screens Based on Ecophysiological Traits. Microb Ecol 45(1):72–87
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14(8):1675–1690
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methe B, DeSantis TZ, Human Microbiome Consortium, Petrosino JF, Knight R, Birren BW (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21(3):494–504. https://doi.org/10.1016/j.plantsci.2017.06.002
Jochum CC, Osborne LE, Yuen GY (2006) Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol Control 39(3):336–344. https://doi.org/10.1016/j.biocontrol.2006.05.004
Kasai Y, Kimura B, Kawasaki S, Fukaya T, Sakuma K, Fujii T (2005) Growth and toxin production by Clostridium botulinum in steamed rice aseptically packed under modified atmosphere. J Food Protect 68(5):1005–1011
Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12(5):1067–1078
Kroemer K, Reski R, Frank W (2004) Abiotic stress response in the moss Physcomitrella patens: evidence for an evolutionary alteration in signaling pathways in land plants. Plant Cell Rep 22(11):864–870
Li Q, LI B H, Kronzucker HJ, SHI WM (2010) Root growth inhibition by NH4 + in Arabidopsis is mediated by the root tip and is linked to NH4 + efflux and GMPase activity. Plant Cell Environ 33(9):1529–1542. https://doi.org/10.1111/j.1365-3040.2010.02162.x
Liu KM, Wang L, Xu YY, Chen N, Ma QB, Li F, Chong K (2007) Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 226(4):1007–1016
Lukatkin AS, Brazaityte A, Bobinas C, Duchovskis P (2012) Chilling injury in chilling-sensitive plants: a review. Zemdirbyste 99(2):111–124
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488(7409):86–. https://doi.org/10.1038/nature11237
Lyons JM, Raison JK (1970) Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol 45(4):386–389
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440(7084):688–691
Ma Y, Dai XY, Xu YY, Luo W, Zheng XM, Zeng DL, Pan YJ, Lin XL, Liu HH, Zhang DJ, Xiao J, Guo XY, Xu SJ, Niu YD, Jin JB, Zhang H, Xu X, Li LG, Wang W, Qian Q, Ge S, Chong K (2015) COLD1 Confers Chilling Tolerance in Rice. Cell 162(1):222–222. https://doi.org/10.1016/j.cell.2015.01.046
Maathuis FJ (2006) The role of monovalent cation transporters in plant responses to salinity. J Exp Bot 57(5):1137–1147
Mitani N, Ma JF, Iwashita T (2005) Identification of the silicon form in xylem sap of rice (Oryza sativa L.). Plant Cell Physiol 46(2):279–283
Mitani N, Yamaji N, Ma JF (2009) Identification of maize silicon influx transporters. Plant Cell Physiol 50(1):5–12. https://doi.org/10.1093/pcp/pcn110
Muneer S, Park YG, Kim S, Jeong BR (2017) Foliar or Subirrigation Silicon Supply Mitigates High Temperature Stress in Strawberry by Maintaining Photosynthetic and Stress-Responsive Proteins. J Plant Growth Regul 36(4):836–845. https://doi.org/10.1007/s00344-017-9687-5
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion Homeostasis in NaCl Stress Environments. Plant Physiol 109(3):735–742
Osborne Owen G et al (2018) Arbuscular mycorrhizal fungi promote coexistence and niche divergence of sympatric palm species on a remote oceanic island.[J]. New Phytol 217(3):1254–1266. https://doi.org/10.1111/nph.14850
Polisensky DH, Braam J (1996) Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol 111(4):1271–1279
Qin X, Wu H, Chen J, Wu L, Lin S, Khan MU, Boorboori MR, Lin W (2017) Transcriptome analysis of Pseudostellaria heterophylla in response to the infection of pathogenic Fusarium oxysporum. Bmc Plant Biol 17(1):155. https://doi.org/10.1186/s12870-017-1106-3
Qiu MH, Zhang RF, Xue C, Zhang SS, Li SQ, Zhang N, Shen QR (2012) Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol Fert Soils 48(7):807–816. https://doi.org/10.1007/s00374-012-0675-4
Shabala L, McMeekin T, Shabala S (2009) Osmotic adjustment and requirement for sodium in marine protist thraustochytrid. Environ Microbiol 11(7):1835–1843. https://doi.org/10.1111/j.1462-2920.2009.01908.x
Sun J, Li LS, Liu MQ, Wang MJ, Ding MQ, Deng SR, Lu CF, Zhou XY, Shen X, Zheng XJ, Chen SL (2010) Hydrogen peroxide and nitric oxide mediate K+/Na + homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting poplars. Plant Cell Tiss Org 103(2):205–215. https://doi.org/10.1007/s11240-010-9768-7
Xu, Jinjin et al (2017) A new perspective on studying burial environment before archaeological excavation: analyzing bacterial community distribution by high-throughput sequencing.[J]. Sci Rep 7(1):41691. https://doi.org/10.1038/srep41691
Xu WF, Shi WM, Jia LG, Liang JS, Zhang JH (2012) TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress. Plant Cell Environ 35(8):1393–1406. https://doi.org/10.1111/j.1365-3040.2012.02497.x
Yuan SF, Wang LL, Wu K, Shi JX, Wang MS, Yang XM, Shen QR, Shen BA (2014) Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol 75:86–94. https://doi.org/10.1016/j.apsoil.2013.11.004
Zhang YH, Sa G, Zhang YN, Zhu ZM, Deng SR, Sun J, Li NF, Li J, Yao J, Zhao N, Zhao R, Ma XJ, Polle A, Chen SL (2017) Paxillus involutus-Facilitated Cd2 + Influx through Plasma Membrane Ca2+-Permeable Channels Is Stimulated by H2O2 and H+-ATPase in Ectomycorrhizal Populus x canescens under Cadmium Stress. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.01975
Zhao R, Sun HM, Zhao N, Jing XS, Shen X, Chen SL (2015) The Arabidopsis Ca 2+-dependent protein kinase CPK27 is required for plant response to salt-stress. Gene 563(2):203–214. https://doi.org/10.1016/j.gene.2015.03.024
Acknowledgements
This work was supported by the National Key Research and Development program of China (2016YFD0300508), Fujian-Taiwan Joint Innovative Centre for Germplasm Resources and Cultivation of Crop (Fujian 2011 program, No. 2015-75) and the Natural Foundation of Fujian Higher Education Institutions for Young Scientists (Key Project) (JZ160435).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, Y., Waqas, M., Khan, M.U. et al. Overexpression of the rice gene Lsi1 (low silicon gene 1) enhances plant-microbe interactions that result in improved chilling tolerance. Plant Growth Regul 98, 525–538 (2022). https://doi.org/10.1007/s10725-022-00890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00890-1