Abstract
Although silicon (Si) has showed its potential role in mitigating abiotic stress-induced damages in many plant species its role in coordinated induction of antioxidant defense is yet to be elucidated. Therefore, we studied rapeseed (Brassica napus) seedlings applied with exogenous Si for changes occurring in antioxidant defense and glyoxalase systems. Seedlings (12-day-old) grown semi-hydroponically were exposed to Si (silicon dioxide, SiO2; 1 mM) solely and in combination with NaCl (100 and 200 mM) for 48 h. Salinity created oxidative damage by increasing H2O2 and malondialdehyde (MDA) contents resulting in disruption of antioxidant defense system and in arousing methylglyoxal (MG) toxicity by the down-regulation of glyoxalase enzyme activities. Exogenous Si treatment showed reduction of both H2O2 and MDA contents and up-regulation of antioxidant components including the activities of related enzymes (APX, MDHAR, DHAR, GR, GST, GPX and CAT) and the contents of AsA and GSH. Enhanced activities of glyoxalase I (Gly I) and glyoxalase II (Gly II) detoxified the toxic MG. Thus, this study clearly indicates that Si improved plant tolerance to salinity stress through enhancement of both antioxidant defense and glyoxalase systems that led to reduced oxidative damage and MG toxicity.
Zusammenfassung
Obwohl Silizium (Si) seine potenzielle Rolle bei der Abschwächung abiotischer stressinduzierter Schäden bei vielen Pflanzenarten gezeigt hat, ist seine Rolle bei der koordinierten Induktion der antioxidativen Abwehr noch zu klären. Daher untersuchten wir Rapskeimlinge (Brassica napus), die mit exogenem Si behandelt wurden, auf Veränderungen in der antioxidativen Abwehr und der Glyoxalase-Systeme. Keimlinge (12 Tage alt), die semi-hydroponisch gezüchtet wurden, wurden 48 h Si (Siliciumdioxid, SiO2; 1 mM) ausgesetzt – allein und in Kombination mit NaCl (100 und 200 mM). Die Salinität verursachte oxidative Schäden durch die Erhöhung des H2O2- und Malondialdehyd (MDA)-Gehalts, was zu einer Störung des antioxidativen Abwehrsystems und zur Stimulation der Methylglyoxal (MG)-Toxizität durch die Herunterregulierung der Glyoxalase-Enzymaktivitäten führte. Die exogene Si-Behandlung zeigte eine Reduktion der H2O2- und der MDA-Gehalte sowie eine Hochregulierung der antioxidativen Komponenten einschließlich der Aktivitäten verwandter Enzyme (APX, MDHAR, DHAR, GR, GST, GPX und CAT) und der AsA- und GSH-Gehalte. Verstärkte Aktivitäten von Glyoxalase I (Gly I) und Glyoxalase II (Gly II) entgifteten das toxische MG. Somit zeigt diese Studie deutlich, dass Si die Toleranz von Pflanzen gegenüber Salzstress durch die Steigerung der antioxidativen Abwehr und der Aktivität der Glyoxalase-Systeme verbesserte, was zu einer verringerten oxidativen Schädigung und MG-Toxizität führte.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stress has become the utmost threat for crop production in this age of climate change. Salinity, being a dreadful form of abiotic stress, has shown multidimensional effects on crop life including damaged growth and reduced yield. Initially, salinity affects the vascular transportation of solutes and electrochemical gradients which leads to osmotic imbalance (Manivannan et al. 2016) and also creates ionic imbalance and toxicity (Rahman et al. 2016). Higher amount of ions like Na+ and Cl− produced due to salt stress adds another dimension of injury to plant survival (Hasanuzzaman et al. 2012; Hussain et al. 2013; Assaha et al. 2015). The osmotic and ionic imbalance secondarily cause oxidative stress in plants which eventually results in detrimental effects on plant morphological, physiological and/or biochemical attributes (Mahmood et al. 2016).
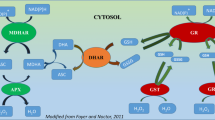
Singlet oxygen (1O2), superoxide (\(\text{O}_{2}^{\cdot -}\)), hydrogen peroxide (H2O2), hydroxyl radical (OH•) etc. are some of the reactive oxygen species (ROS) generated under as a response to saline condition. These ROS are considered responsible for the oxidation of lipids, amino acids and nucleic acids, damages at cellular level and even programmed cell death (Mahmud et al. 2017). To mitigate these injurious effects of ROS, an efficient antioxidant defense system is developed within plant cells (Apel and Hirt 2004). The components of this defense system act simultaneously to scavenge and/or detoxify these ROS and help to save plants from the damages caused by oxidative stress (Hasanuzzaman et al. 2014a). Methylglyoxal (MG), another compound produced under abiotic stress conditions including salinity, has been reported to be highly reactive and cytotoxic when produced in a higher amount (Yadav et al. 2008). Two thiol-dependent enzymes i. e. glyoxalase I (Gly I) and glyoxalase II (Gly II) form a well-organized glyoxalase system which detoxifies this toxic MG by some reactions in order (Rahman et al. 2016; Hasanuzzaman et al. 2017a, 2017b). Coordinated action and up-regulation of both the antioxidant defense and glyoxalase system components are needed to attain significant tolerance in plants against the oxidative stress and this was supported by several research findings (Nahar et al. 2015; Rahman et al. 2016; Mahmud et al. 2017).
Silicon (Si) ranks second in abundance within the earth crust and mostly exists as silica (SiO2) or silicate (\(\mathrm{SiO}_{4}^{4-}\)) as it shows strong affinity to oxygen (O2) (Hasanuzzaman et al. 2014b). Silicon has certain potential to improve plant growth and development under adverse conditions, yet it is considered as nonessential for plant production (Shahzad et al. 2017). Si-induced reduction of ROS in plants is the crucial effect of exogenous Si against abiotic stress which has been demonstrated by several lines of study (Hashemi et al. 2010; Hasanuzzaman et al. 2017c). In addition, decreased electrolytic leakage, malondialdehyde (MDA) content and toxic ions (e. g., Na+) accumulation was observed when Si applied exogenously under salt stress condition (Kim et al. 2017). Si-induced modifications in both apoplastic and symplastic components were also studied to evaluate the prospects to develop salinity tolerance in plants (Coskun et al. 2016). A number of experiments reported that the exogenous Si application mediated regulation of antioxidant defense system components in different crops (Torabi et al. 2015; Manivannan et al. 2016). However, Si-induced coordinated induction of antioxidant defense and other related systems have hardly been studied. Our hypothesis was that the Si-mediated activation of glutathione (GSH) synthesis may have played an important role in the MG detoxification process by the up-regulation of Gly I and Gly II activities. Based on the above discussed notions, we designed this experiment to investigate the effect of Si applied exogenously on Brassica napus seedlings influencing the contents of non-enzymatic and enzymatic components of the antioxidant defense and glyoxalase systems under salinity condition.
Materials and Methods
Plant Materials and Stress Treatments
Uniform rapeseed (Brassica napus L. cv. BINA sharisha 3) seeds were washed for several times to obtain clean seeds. Six layers of filter papers were lined on Petri plates (9 cm) which were used for sowing seeds. Initially 60 seeds were sown from where 40 seedlings were kept for further analysis. Seedlings were kept in growth chambers maintaining controlled conditions with 100 μmol photon m−2 s−1 light, 25 ± 2 °C temp and 65–70% RH. The growing medium of seeds was semi-hydroponic containing Hyponex solution (10,000-fold diluted) which was added when necessary. After 12 days of sowing, silicon dioxide, SiO2 (1 mM) and NaCl (100 and 200 mM) were added in the solution; alone or in combination. The first leaves were harvested and used for quantifying different parameters after 48 h of treatment exposure. Only Hyponex solution was used for control plants to grow in. The experiment was laid out following completely randomized design (CRD) and the same experiments were replicated for three times.
Measurement of H2O2 Content
The procedure used by Yu et al. (2003) was followed to estimate the levels of H2O2. After homogenizing and extracting the harvested leaves in K‑P buffer (50 mM, pH 6.5), the supernatant was mixed with TiCl4 (0.1%) in H2SO4 (20%). This solution was allowed to set for 10 min in normal temperature and centrifuged again before reading the absorbance at 410 nm. The unit used for expressing the result is nmol H2O2 g−1 fresh weight.
Measurement of MDA Content
The method of Heath and Packer (1968) was followed to determine the MDA content (an indicator of lipid peroxidation). Trichloroacetic acid (TCA) (5%) was used as extraction buffer and 0.5% thiobarbituric acid (TBA) (in 20% TCA) was used as reaction mixture. After the extraction and occurrence of reaction, the solution was exposed to boiling at 95 °C temp. It was boiled for 30 min and then cooled on ice, before reading the absorbance at both 532 and 600 nm. The later absorbance was deducted from the former one to attain the precision of non-specific turbidity. The unit used for expressing the result is nmol MDA g−1 fresh weight.
Determination of AsA and GSH
Ascorbate (AsA) and GSH contents were measured by homogenizing 0.5 g fresh rapeseed leaves in 3 ml of meta-phosphoric acid solution (5%) containing ethylenediaminetetraacetic acid (EDTA; 1 mM). After centrifuging this supernatant at 11,500 × g (for 15 min) the AsA content was estimated spectrophotometrically at 265 nm using different reacting solutions (Huang et al. 2005). Neutralization of supernatant was done with potassium-phosphate (K-P) buffer (0.5 M, pH 7.0), and then assayed with ascorbate oxidase (AO; 0.5 unit) in K‑P buffer (100 mM, pH 7.0). The content of GSH pool was quantified at 412 nm following the procedure described by Yu et al. (2003) with some changes (Paradiso et al. 2008). Neutralization of supernatant with K‑P buffer (0.5 M, pH 7.0) was done, and then mixed with 5,5′-dithio-bis 2-nitrobenzoic acid (DTNB), nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione reductase (GR) and then assayed for total GSH content. Oxidized glutathione (GSSG) content was measured by the reaction of 2‑vinylpyridine to remove the GSH. Final calculation of AsA, GSH and GSSG was done with the help of standard curves plotted from concentrations of AsA, GSH and GSSG, which were known. The content of GSSG was deducted from total GSH content, and the result was considered as the reduced GSH content.
Enzyme Extraction
Rapeseed leaves (0.5 g fresh weight) were homogenized, extracted and centrifuged according to Bradford (1976) and accordingly bovine serum albumin (BSA) was used as a protein standard for the determination of protein content. This supernatant was also used for the enzyme assays (Ascorbate peroxidase, APX; monodehydroascorbate reductase, MDHAR; dehydroascorbate reductase, DHAR; GR; glutathione peroxidase, GPX; glutathione S-transferase, GST and catalase, CAT).
Enzyme Assay
The activity of APX was quantified following Nakano and Asada (1981) explained method using K‑P buffer (pH 7.0) and AsA, at a concentration of 50 and 0.5 mM, respectively along with H2O2 and EDTA of 0.1 mM concentration.
The activity of MDHAR was measured following Hossain et al. (1984) explained procedure using Tris–HCl buffer (pH 7.5), NADPH and AsA at a concentration of 50 mM, 0.2 mM and 2.5 mM, respectively along with AO of 0.5 unit.
The activity of DHAR was estimated following Nakano and Asada (1981) described method using K‑P buffer (pH 7.0), GSH and dehydroascorbate (DHA) at a concentration of 50 mM, 2.5 mM and 0.1 mM, respectively.
The activity of GR was determined following Cakmak et al. (1993) described procedure using K‑P buffer (pH 7.8) and NADPH at a concentration of 0.1 M and 0.2 mM, respectively along with EDTA and GSSG of 1.0 mM concentration.
The activity of GST was estimated following Booth et al. (1961) described procedure with inputs from Hossain et al. (2009) using Tris–HCl buffer (pH 6.5), GSH and 1‑chloro-2,4-dinitrobenzene (CDNB) at a concentration of 100 mM, 1.5 mM and 1.0 mM, respectively.
The activity of GPX was quantified following the procedure mentioned in Elia et al. (2003) using EDTA and NaN3 of 1 mM concentration, NADPH, GSH and H2O2 at a concentration of 0.12 mM, 2.0 mM and 0.6 mM, respectively and single unit of GR. All of these were mixed in 100 mM of K‑P buffer (pH 7.0) as an input from Hasanuzzaman and Fujita (2013) explained method.
The activity of CAT was determined following the method mentioned in Hossain et al. (2009) using K‑P buffer (pH 7.0) and H2O2 at a concentration of 50 and 15 mM, respectively.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed. Mean differences were compared and tested by Fisher’s Least Significant Difference (LSD) using CoStat software (CoHort Software, Monterey, CA) from three replications. Differences at P ≤ 0.05 were considered significant.
Results
Accumulation of ROS and Oxidative Damage
Results revealed that salt stress increased the H2O2 content in salt stressed rapeseed plants. Lipid peroxidation level (denoted by MDA content) markedly increased in plants exposed to salt. These are the signs for oxidative damage in rapeseed seedlings caused by salt stress. Gradual rise of H2O2 (63 to 98%) and MDA (60 to 129%) levels with the increased dose (100 to 200 mM NaCl) of salinity shows that high salt dose privileged more oxidative damage, compared to control. Silicon addition with salt stress reversed the adverse consequences of salt stress by decreasing levels of both MDA and H2O2 (compared to seedlings exposed to salt stress only) (Fig. 1).
AsA and GSH Levels
The vital antioxidant AsA dropped by 17% due to mild salt stress which decreased by 44% under severe salt stress. Co-application of Si with salt treatments arouses the content of AsA, compared to salt treatment alone (Fig. 2a). GSH level was found to increase with salt stress. Its level further up-regulated in Si co-treated salt stresses. The level of GSH increased after supplemental Si in mild and severe salt treated rapeseed seedlings which were 14 and 21% higher, compared to mild and severe salt treated seedlings without Si, respectively. The oxidized form of GSH—GSSG—mounted noticeably in salt stressed seedlings. The content of GSSG increased by 54 and 116% due to mild and severe levels of stress, respectively, compared to control plants. Salt stressed seedlings provided with additional Si showed significant decrease of GSSG level, compared to salt stress alone (Fig. 2b).
Enzymes of AsA-GSH Cycle
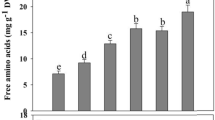
Ascorbate-glutathione cycle enzyme activities were noticed to respond differentially under saline condition. Ascorbate peroxidase activity increased but the MDHAR, DHAR and GR activities reduced due to salt imposition, compared to control. Silicon supplementation showed its additive effects by increasing the activities of these enzymes in both levels of stress (except the APX activity as Si increased its activity under mild salt stress only), compared to seedlings exposed to salt stress only. Addition of Si increased APX activity by 16 and 11% under mild salt stress, compared to salt treatment without Si (Fig. 3a). Silicon co-treatment with mild and severe salt stress increased MDHAR activity by 49 and 52%, and DHAR activity by 34 and 43%, respectively, compared to salt stress alone (Fig. 3b, c). Si addition with mild salt stress increased GR activity by 8%, compared to salt stress alone (Fig. 3d).
Activities of APX (a), MDHAR (b), DHAR (c) and GR (d) in rapeseed leaves induced by Si under salt stress. N1, N2, Si, Si+N1 and Si+N2 indicate 100 mM NaCl, 200 mM NaCl, 1 mM SiO2, 100 mM NaCl+SiO2, 200 mM NaCl+SiO2, respectively. Values sharing the same letter do not differ significantly at P ≤ 0.05
Thiol-Dependent Antioxidant Enzymes, GPX and GST
The thiol-dependent antioxidant enzyme GPX showed slightly increased activity under mild salt stress whereas the activity decreased under severe salt stress, if compared with control. Another thiol-dependent enzyme, GST, showed increased activity due to both mild and severe salt stress levels, compared to control. When adding Si to stress treatments the activity of GST increased by 13 and 31% in mild and severe stress, respectively, compared to only salt treatments (Fig. 4a). Glutathione peroxidase activity increased only in mild stress after Si addition which was 23% higher, compared to salt stress alone (Fig. 4b).
Activity of CAT
Activity of the vital H2O2 scavenging enzyme, CAT, dropped significantly due to salt imposition. Mild and severe salt stress decreased its activity by 32 and 41%, respectively, compared to control. Exogenous Si supplementation restored and increased CAT activity to a great extent which was 53 and 75% higher than its activity in mild and severe salt stress, respectively, compared to only salt treatments (Fig. 4c).
Enzymes of Glyoxalase System
Saline stress disrupted the MG detoxification system. The activity of glyoxalase enzymes decreased in salt imposed rapeseed seedlings, compared to control seedlings. Glyoxalase I activity upheld by 47 and 72% after applying Si in mild and severe salt treatments, respectively, compared to only salt stress treatments (Fig. 5a). Silicon supplementation also increased the activity of Gly II by 20 and 36% in mild and severe salt stress, respectively, when compared to seedlings exposed to salt stress only (Fig. 5b).
Activities of glyoxalase system enzymes, Gly I (a) and Gly II (b) in rapeseed leaves induced by Si under salt stress. N1, N2, Si, Si+N1 and Si+N2 indicate 100 mM NaCl, 200 mM NaCl, 1 mM SiO2, 100 mM NaCl+SiO2, 200 mM NaCl+SiO2, respectively. Values sharing the same letter do not differ significantly at P ≤ 0.05
Discussion
High salt concentration increases ionic toxicity and osmotic or physiological drought stress. Both of these interrupt normal movement of stomata, decreases CO2 concentration to the fixation site of Calvin cycle and due imbalanced electron transport ROS are overproduced (Hasanuzzaman et al. 2013). Salt stress reduces efficiency of antioxidant defense enzymes which further increases ROS accumulation in plant cell (Rahman et al. 2016). In our experiment, when exposed to salt, the seedlings of B. napus showed an excessive H2O2 production which was responsible for the disruption of the membrane properties as designated by high rise of MDA level, a product of lipid peroxidation. The membrane damage indicates the oxidative stress created by salt stress. Related advantageous role of Si is evident in our study. Exogenous Si co-treatment prevented production of excess ROS and the oxidative damage due to adverse effects of salt stress. Exogenous Si also enhanced antioxidant system that also prevented ROS accumulation and oxidative damage in rapeseed seedlings. These results are supported by previously published results with exogenous Si application in cucumber plants under salt stress (Khoshgoftarmanesh et al. 2014).
Production of ROS is a spontaneous and inevitable occurrence in plant cell. An efficient antioxidant defense system can scavenge and keep a balance state of ROS accumulation and in contrast, declining the efficiency of this system may cause irreparable damage of components of plant cell and even cause death. Therefore, enhanced antioxidant defense system i. e. increased levels of non-enzymatic antioxidants and enhanced activities of enzymes are pivotal for an improved oxidative stress tolerance under different abiotic stresses (Hasanuzzaman et al. 2017a).
Ascorbate, which is one of the simplest, most copious and effective primary antioxidant protecting cells against oxidative stress works in AsA-GSH cycle together with GSH (Hasanuzzaman et al. 2017a). After sudden salt imposition to the growth medium of rapeseed plants their AsA levels dropped down irrespective of the dose that is comparable with the findings of other research (Nahar et al. 2016). This dropdown of AsA was responsible for raising the H2O2 and the oxidative damage of cell. Ascorbate is disproportionate to DHA and monodehydroascorbate (MDHA) while scavenging ROS. Due to the activity of DHAR and MDHAR enzymes, respectively, AsA can be regenerated through the AsA-GSH cycle (Hasanuzzaman et al. 2017b). Then, when Si was added to the salt solution of rapeseed seedlings it alleviated the stress effects raising the AsA level and reducing the oxidative injury. The raise of AsA in Si-added salt treatment was associated with enhanced MDHAR and DHAR activities those proficiently recycled back AsA.
Glutathione is a low molecular and water soluble tripeptide γ‑glutamyl cysteinyl glycine (γ-Glu-Cys-Gly). Glutathione reduces oxidative stress scavenging ROS of various forms including H2O2, 1O2, \(\text{O}_{2}^{\cdot -}\) and OH. Without acting as direct scavenger of ROS, the functions of GSH in retaining cellular redox balance, and stress signal transfer and adaptation are imperative in plants (Foyer and Noctor 2005; Hasanuzzaman et al. 2017b). Salt-induced rise in GSH levels of the rapeseed seedlings of the present study is comparable to the findings of earlier study (Nahar et al. 2016). After participating in ROS detoxification process, GSH is transformed into GSSG for which the level of GSSG also increased under salt stress that is an indicator of stress. Nonetheless, a stress tolerant plant efficiently recycles GSSG into GSH immediately so that plants become instantly ready to detoxify ROS again. After inclusion of exogenous Si to the salt media the GSH level became much improved (with a noticeable decrease of GSSG), compared to the only salt treatment. The reason behind this increased GSH level was the enhanced activity of GR (a GSH recycling enzyme) by Si addition.
The modes of alteration of AsA and GSH pool as well as the functioning of enzymes involved in AsA-GSH cycle are in the same line with the observation and findings of previous research. Exogenous Si addition rose AsA and GSH level in salt affected sunflower plant which relaxed the oxidative stress from that plant (Ali et al. 2013). High silica uptake capacity of rice considerably enhanced the efficiency of antioxidant enzymes including the activity of APX under salt stress (Farooq et al. 2016). Likewise, Si use increased GSH content, APX activities in sorghum grown under saline condition (Kafi et al. 2011). Improved activities of three AsA-GSH cycle enzymes i. e., APX, MDHAR and GR with enhanced levels of AsA and GSH with diminishing oxidative stress were noticed in Si added cucumber suffered from chill-induced oxidative damage (Jiao-jing et al. 2009).
Glutathione peroxidase catalyzes H2O2 and organic hydroperoxides detoxification reaction that is dependent on GSH. The thiol/disulfide or NADPH/NADP+ balance is maintained by GPX that plays roles in regulation of redox homeostasis which protects cells from oxidative damage. Glutathione peroxidase shows its activity during growth and development (Bela et al. 2015). The activity of GPX of rapeseed plants slightly increased with mild salt stress but declined with severe stress. Exogenous Si application with mild salt stress increased its activity whereas in severe stress, Si failed to up-regulate its activity. In contrary, in salt stressed rice plant, high Si uptake capacity was associated with an increased GPX activity and declined oxidative stress (Farooq et al. 2016). Plant GSTs are multifunctional enzymes catalyzing conjugation of xenobiotic substrates with GSH (Dixon et al. 2010). The peroxidase activity of GST reduces damage caused by oxidative stress (Gill and Tuteja 2010). Compared to control treatment, GST activity was up-regulated in B. napus seedlings when exposed to salt stress. It’s activity further upheld again by exogenous Si addition with salt stress. Debona et al. (2014) showed similar pattern of Si induced enhancement of GST activity in wheat leaves.
Catalase plays important role in signal perception, metabolism and resistance development in plants (Shugaev et al. 2011; Su et al. 2014). Salt stress declined the activity of CAT that increased H2O2 accumulation. When Si was supplemented with NaCl the CAT activity increased which dismutated H2O2 to H2O and relaxed the cellular environment from oxidative stress. Exogenous Si addition increased CAT activity that was observed in salt affected sunflower (Ali et al. 2013), sorghum (Kafi et al. 2011) and in lettuce (Milne et al. 2012).
The high reactive nature of MG damages plant cell under salt stress. Methylglyoxal is also accountable for increasing ROS (Hasanuzzaman et al. 2017a). Its cytotoxic effects have been reported through breaking down the cellular proteins and DNA (Yadav et al. 2005). The enzymes Gly I and Gly II are the components of glyoxalase system and these enzymes catalyze the conversion of highly active MG to a safe form d-lactate, where GSH is performing its function as a co-factor (Yadav et al. 2005; El-Shabrawi et al. 2010). In this experiment, the Gly I and Gly II activities of salt distressed rapeseed seedlings decreased with the rise of salt doses pointing toward the disrupted MG detoxification system and increased MG-induced damage (Nahar et al. 2016). When those salt affected plants were treated with Si, the leaf RWC was retrieved and improved more than that of salt treated plants only. NaCl treatment demonstrated the clear picture that Si proficiently up-regulated the Gly I and Gly II activities with a concomitant increase of GSH level which are accountable for detoxifying cellular MG.
Conclusion
Although it is not essential for the life of plants in general, Si has many important roles in the physiological process of many crop plants. In recent studies, Si was found to play vital role in plant’s defense against abiotic stress. In our study, we found out that Si effectively mitigated the oxidative stress produced due to salinity by up-regulating the antioxidant enzyme activities and maintaining the higher amount of non-enzymatic antioxidant. Moreover, Si also enhanced the glyoxalase system enzyme activities. Further research should be focused on the transport and regulation of Si in different plant species facing abiotic stress.
References
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Assaha DVM, Liu L, Mekawy AMM, Ueda A, Nagaoka T, Saneoka H (2015) Effect of salt stress on Na accumulation, antioxidant enzyme activities and activity of cell wall peroxidase of huckleberry (Solanum ) and eggplant (Solanum melongena). Int J Agric Biol 17(6):1149–1156
Bela K, Horváth E, Gallé A, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201
Booth J, Boyland E, Sims P (1961) An enzyme from rat liver catalyzing conjugation. Biochem J 79(3):516–524
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogenperoxide scavenging enzymes in germinating wheat seeds. J Exp Bot 44(1):127–132
Coskun D, Britto DT, Huynh WQ, Kronzucker HJ (2016) The role of silicon in higher plants under salinity and drought stress. Front Plant Sci 7:1072
Debona D, Rodrigues FA, Rios JA, Nascimento KJT, Silva LC (2014) The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol 63:581–589
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71(4):338–350
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245(1–4):85–96
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55(2):162–167
Farooq MA, Saqib ZA, Akhtar J, Bakhat JF, Pasala RK, Dietz KJ (2016) Protective role of silicon (Si) against combined stress of salinity and boron (B) toxicity by improving antioxidant enzymes activity in rice. Silicon. https://doi.org/10.1007/s12633-015-9346-z
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22(3):584–596
Hasanuzzaman M, Hossain MA, Teixeira da Silva JA, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateshwarulu B, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–316
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014a) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int. https://doi.org/10.1155/2014/757219
Hasanuzzaman M, Nahar K, Fujita M (2014b) Silicon and selenium: two vital trace elements that confer abiotic stress tolerance to plants. In: Ahmed P, Rasool S (eds) Emerging technologies and management of crop stress tolerance. Academic Press, New York, pp 377–422
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M (2017a) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18(1):200
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017b) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23(2):249–268
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017c) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci 8:1061
Hashemi A, Abdolzadeh A, Sadeghipour HR (2010) Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L. plants. Soil Sci Plant Nutr 56(2):244–253
Heath RL, Packer L (1968) Photo peroxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25(3):385–395
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3(2):53–64
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J Exp Bot 56(422):3041–3049
Hussain M, Park HW, Farooq M, Jabran K, Lee DJ (2013) Morphological and physiological basis of salt resistance in different rice genotypes. Int J Agric Biol 15:113–118
Jiao-jing L, Shao-hang L, Pei-lei X, Xiu-juan W, Ji-gang B (2009) Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric Sci China 8:1075–1086
Kafi M, Nabati J, Zare Mehrjerdi M (2011) Effect of salinity and silicon application on oxidative damage of sorghum [Sorghum bicolor (L.) Moench]. Pak J Bot 43(5):2457–2462
Khoshgoftarmanesh AH, Khodarahmi S, Haghighi M (2014) Effect of silicon nutrition on lipid peroxidation and antioxidant response of cucumber plants exposed to salinity stress. Arch Agron Soil Sci 60(5):639–653
Kim Y‑H, Khan AL, Waqas M, Lee I‑J (2017) Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front Plant Sci 8:510
Mahmood S, Daur I, Al-Solaimani SG, Ahmad S, Madkour MH, Yasir M, Hirt H, Ali S, Ali Z (2016) Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front Plant Sci 7:876
Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M (2017) γ‑aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology 26(5):675–690
Manivannan A, Soundararajan P, Muneer S, Ko CH, Jeong BR (2016) Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang. Biomed Res Int. https://doi.org/10.1155/2016/3076357
Milne CJ, Laubscher CP, Ndakidemi PA, Marnewick JL, Rautenbach F (2012) Salinity induced changes in oxidative stress and antioxidant status as affected by applications of silicon in lettuce (Lactuca sativa). Int J Agric Biol 14(5):763–768
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015) Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant 59(4):745–756
Nahar K, Hasanuzzaman M, Rahman A, Alam MM, Mahmud JA, Suzuki T, Fujita M (2016) Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci 7:1104
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Paradiso A, Berardino R, de Pinto M, di Toppi LS, Storelli FT, de Gara L (2008) Increase in ascorbate–glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49(3):362–374
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 7:609
Shahzad M, Qadir A, Masood S (2017) Silicon and alleviation of salt stress in crop genotypes differing in salt tolerance. In: Tripathi DK, Singh VP, Ahmad P, Chauhan DK, Prasad SM (eds) Silicon in plants: advances and future prospects. CRC, Boca Raton, pp 133–148
Shugaev AG, Lashtabega DA, Shugaeva NA, Vyskrebentseva EI (2011) Activities of antioxidant enzymes in mitochondria of growing and dormant sugar beet roots. Russ J Plant Physiol 58(3):387–393
Su Y, Guo J, Ling H, Chen S, Wang S, Xu L, Allan AC, Que Y (2014) Isolation of a novel peroxisomal catalase gene from sugarcane, which is responsive to biotic and abiotic stresses. PLoS ONE. https://doi.org/10.1371/journal.pone.0084426
Torabi F, Majd A, Enteshari S (2015) The effect of silicon on alleviation of salt stress in borage (Borago officinalis L.). Soil Sci Plant Nutr 61(5):788–798
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337(1):61–67
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2008) An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact 23(1–2):51–68
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Acknowledgements
We acknowledge Ms. Khursheda Parvin, Assistant Professor, Department of Horticulture, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh for the critical reading and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Hasanuzzaman, K. Nahar, M.M. Rohman, T.I. Anee, Y. Huang and M. Fujita declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hasanuzzaman, M., Nahar, K., Rohman, M.M. et al. Exogenous Silicon Protects Brassica napus Plants from Salinity-Induced Oxidative Stress Through the Modulation of AsA-GSH Pathway, Thiol-Dependent Antioxidant Enzymes and Glyoxalase Systems. Gesunde Pflanzen 70, 185–194 (2018). https://doi.org/10.1007/s10343-018-0430-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-018-0430-3