Abstract
Different biological variables of tadpoles, including survival, development and growth rates, and biomarkers [cholinesterases, glutathione-S-transferases (GST), and blood cell morphology] were evaluated in two anuran species, Scinax squalirostris (Hylidae) and Leptodactylus mystacinus (Leptodactylidae), using in situ experimental chambers in a rice field (RF) sprayed with insecticide Lambda-cyhalothrin (LTC) by aircraft in Santa Fe Province, Argentina. We found a significant decrease in body weight (0.62 ± 0.04 g) of L. mystacinus and an increased development rate of S. squalirostris in individuals from RF (41 ± 1; Gosner) with respect to individuals from the reference site (RS: 0.93 ± 0.04 g and 37 ± 0; respectively). In S. squalirostris, individuals from RF mean values of butyrylcholinesterase activities decreased at 48 (4.09 ± 0.32 nmol min-1 mg-1 of TP) and 96 h (3.74 ± 0.20 nmol min-1 mg-1 of TP), whereas inhibition of acetylcholinesterase was observed at 96 h (47.44 ± 2.78 nmol min-1 mg-1 of TP). In L. mystacinus from RF, an induction of acetylcholinesterase activity was observed at 96 h (36.01 ± 1.09 nmol min-1 mg-1 of TP). Glutathione-S-transferase levels varied between species, being higher in L. mystacinus individuals but lower in S. squalirostris from RF at 48 (272.29 ±11.78 and 71.87 ± 1.70 nmol min-1 mg-1 of TP; respectively) and 96 h (279.25 ± 13.06 and 57.62 ± 4.58 nmol min-1 mg-1 of TP, respectively). Blood cell parameters revealed a lower number of mitotic cells (MC: 0.36 ± 0.31%o for S. squalirostris and 0.08 ± 0.05 %o for L. mystacinus) and higher number of eosinophils (E: 3.45 ± 1.75 %o for S. squalirostris and 7.64 ± 0.98 %o for L. mystacinus) in individuals from the RF than in individuals from the RS (MC: 2.55 ± 0.74 %o for S. squalirostris and 1.87 ± 0.72%o for L. mystacinus; and E: 0.13 ± 0.09 for S. squalirostris and 3.20 ± 0.80 for L. mystacinus). Overall, our results demonstrate the existence of apparent differences in sensitivity between species in a series of sublethal responses to short-term exposure in RF after the application of Lambda-cyhalothrin. We suggest that the integral use of biological endpoints (development and growth) together with biomarkers (cholinesterase, GST, and blood cell parameters) may be a promising integral procedure for investigating pesticide exposure in wild frog populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice field (RF) expansion is one of the activities associated with the disappearance of wetlands throughout the world (Juliano 1993; Millennium Ecosystem Assessment 2005; Machado and Maltchik 2010). Rice cultivation demands the application of increasing amounts of agrochemicals (synthetic pesticides and fertilizers), which leads to increased environmental degradation and reliance on high capital costs and production inputs (Tilman et al. 2002). RFs, together with their adjacent aquatic habitats and dryland, comprise a rich mosaic of fast-changing ecotones that harbor biological diversity, which is maintained by rapid colonization, and reproduction and growth of organisms (Fernando 1995; 1996). Hence, rice agriculture has been recognized as having considerable potential value for many aquatic organisms such as fish, amphibians, and birds (Czech and Parsons 2002; Elphick and Oring 2003; Bambaradeniya et al. 2004). Conversely, the health status and ecology of amphibians living in RFs, and their intraspecific or interspecific biological responses have not been sufficiently characterized worldwide (Hirai and Matsui 1999; Hirai 2004; Attademo et al. 2011).
Exposure to pesticides can be particularly deleterious to amphibian species and may become an important factor in population declines in agricultural landscapes (Mann et al. 2009). Amphibians may be sensitive to water pollutants principally during the aquatic embryonic and larval stages (Gibbs et al. 2005; García-Muñoz et al. 2010); however, this sensitivity varies at intraspecific and interspecific levels (e.g., Christin et al. 2004; Navas and Otani 2007; Jones et al. 2009; Relyea and Jones 2009).
Organisms that possess adaptive plastic traits have the ability to alter their biological traits in response to environmental cues to survive under new environmental conditions (DeWitt and Scheiner 2004). The ability to withstand toxic chemicals and oxidative stress is crucial for the survival of all organisms. In vivo inhibition or induction of biomarkers can be considered an appropriate tool to evaluate exposure to xenobiotics and their potential effects on organisms (McCarthy and Shugart 1990; Walker 1998; Sánchez-Hernández 2007). The ChEs enzymes acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8) have been recommended as useful indicators of amphibian exposure to anti-cholinesterase chemicals (Attademo et al. 2007; Attademo et al. 2011; Brodeur et al. 2011; Lajmanovich et al. 2009; Lajmanovich et al. 2010; Lajmanovich et al. 2011). Moreover, glutathione-S-transferases (GST, EC 2.5.1.18) are a multigene family of cytosolic enzymes involved in the conjugation of electrophilic metabolites with the tripeptide glutathione to yield a water-soluble conjugated metabolite. GST activity is also used as a biomarker in ecological risk assessment of a pesticide-contaminated environment (Greulich and Pflugmacher 2004; Attademo et al. 2007). Moreover, blood parameters are other biomarkers that are being increasingly used in herpetofaunal studies (Barni et al. 2007). Amphibian blood is a very plastic tissue (Barni et al. 2007); variations in the morphology of several blood cells in anurans have been reported as a response to stress in agroecosystems (Stansley and Roscoe 1996; Cabagna et al. 2005). Significant relationships between enzyme activity and haematological parameters of amphibians, and application of pesticides have been recently demonstrated in anurans from Argentina (Attademo et al. 2011; Brodeur et al. 2011).
Different tadpole biological variables, including survival, development, and growth rates, and biomarkers (cholinesterase, GST, and blood cell morphology) in two anuran species, Scinax squalirostris (Hylidae) and Leptodactylus mystacinus (Leptodactylidae) were evaluated using in situ experimental chambers in RF from Santa Fe Province, Argentina. This work provides a basic scheme using related biological parameters as endpoints for monitoring the health status of anurans present in agricultural aquatic systems.
Material and methods
Study area
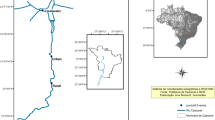
The study area was situated in the Mid-eastern region of Argentina, precisely in Santa Fe Province. The area is primarily devoted to irrigated transgenic rice production, with a rainy season from October to March and a dry season from April to September. Expansion of rice production (229,711 ha in 2010), particularly in Santa Fe, Entre Ríos, and Corrientes provinces, involves deforestation and destruction of native forests. In Santa Fe province, rice is produced mainly on the floodplains of the Paraná River (Alvisio 1998). Rice fields are surrounded by several forest fragments characterized by native vegetation of the deltas and islands of Paraná River and Espinal ecoregions (Burkart et al. 1999).
We selected two sites (Fig. 1), a reference site (RS) and a RF. RS was located within a private native reserve of the Paraná River floodplain in the Capital department of the province (RS 31°39′34.7″S–60°35′31.1″W). We considered the RS site free of anticholinesterase chemicals because no agricultural activities or pesticide use have been observed in the nearby areas (Lajmanovich et al. 2010). This site is dominated by different trees such as Salix humboldtiana, Tessaria integrifolia, Enterolobium contortisiliquum, and Acacia caven. The ephemeral ponds and marshes present Eichhornia crassipes, Pontederia cordata, Saggitaria montevidensis, Typha latifolia, Cortaderia selloana, Cyperus corymbosus, Salvinia biloba, and Pistia stratiotes. RF was a transgenic rice plantation (Oryza sativa) located in San Javier department (30°05′13.56″S–59° 53′19.98″W). On 30 January 2011, we located the chambers in the ditches and paths. The next day (31 January) the insecticide Lambda-cyhalothrin (LTC; trade name CILAMBDA AGROS) was applied at a proportion of 100 cm3 in 20 L of water by aircraft. LTC is a synthetic type II pyrethroid insecticide (Meister 1992) that is widely used in agricultural formulations to control numerous rice pests (CASAFE 2005) such as the hemipterans Tibraca limbativentris and Tibraca obscurata. LTC is moderately persistent and relatively photostable under natural irradiation with a half-life > 3 week (He et al. 2008).
At each site, pH, temperature (°C), conductivity (μS s-1), and dissolved oxygen (mg L-1), ammonia (NH4+, mg L-1), nitrate (NO3-, mg L-1), and orthophosphate (PO4 mg L-1) concentrations were recorded daily with digital instruments and standard Aquamerk® kits. All water chemistry measurements were taken in the morning.
Test organism and experimental design
Tadpoles of S. squalirostris and L. mystacinus were selected for this study because of their extensive Neotropical distribution (Argentina, Paraguay, Uruguay, Bolivia, and Brazil). These anurans are common in forests, wetlands, riparian and urban areas, and agricultural lands; in the latter areas, these species are likely exposed to xenobiotics during their breeding season and early development (Peltzer et al. 2006). These two tadpole species differ in ecological habits, S. squalirostris being nektonic and L. mystacinus being benthonic-nektonic (Peltzer and Lajmanovich 2007).
Prometamorphic larvae (Gosner stage 26–29) of both species were collected from temporary ponds in Paraná River floodplain in January 2011. Despite the wide distribution of rice agroecosystems in the area, all the tadpoles were collected from nonagricultural sites, so we assumed that exposure to pesticides was minimal (Lajmanovich et al. 2010). All tadpoles were collected with authorization of the Ministerio de Aguas, Servicios Públicos y Medio Ambiente, and Santa Fe province, Argentina. The tadpoles were acclimatized until reaching Gosner stage 36–37 (Peltzer et al. 2008) at a 12 h light/dark cycle with dechlorinated tap water (DTW) of pH 7.4 ± 0.05; conductivity, 165 ± 12.5 μmhos m-1; dissolved oxygen concentration, 6.5 ± 1.5 mg L-1 hardness, 50.6 mg L-1 of CaCO3 at 22 ± 2 °C.
Because these two anuran species differ in spatial habits, we constructed two experimental chambers based on those traits. The experimental chambers used for S. squalirostris consisted in 5-L plastic cylinders and transparent bottles (35 × 17 cm; Fig. 2a). The chamber used for L. mystacinus consisted in a 10-L bucket perforated irregularly to allow water exchange. The buckets were buried into the sediment with two iron supports (Fig 2b). Initial body length (BL) of each species was recorded at Gosner stage 36–37 with a digital caliper (to the nearest 0.01 mm). We also recorded the weight (W) of each tadpole with a digital balance (to the nearest 0.1 g) to determine initial wet weight. A number of 10 tadpoles of similar Gosner development stage (Gosner stage 36–37), body length (24.78 ± 1.53 mm for S. squalirostris and 39.15 ± 0.03 mm for L. mystacinus), and wet weight (0.33 ± 0.03 g for S. squalirostris and 0.93 ± 0.04 g for L. mystacinus) were then caged in each in situ chamber and provided boiled lettuce as food source. Three replicates of each in situ chamber were located in each field, totalling 90 tadpoles per site and species.
Survival, development, and growth rates
Survival of tadpoles was recorded at each site after 168 h of in situ exposure (Peltzer et al. 2008; Attademo et al. 2011); the surviving tadpoles were immediately transported to the laboratory to determine biological variables, using plastic buckets containing water from each sampling site. We determined the developmental stages of tadpoles according to Gosner (1960) table. Growth parameters (body length and wet weight) were also recorded. Development rates were estimated according to the equation [development rates = (final stage-initial stage)/number of days of experimentation; Teplitsky et al. 2003]. To minimize stress, tadpoles were submerged in water during staging and measurement, except for measurement of wet weight.
Enzymatic assays
For biomarker assessments, a subsample of tadpoles of each species and from each site was taken at 48 and 96 h after LTC application; tadpoles were euthanized according to the criteria of ASIH and SSAR (2001) and Biochemistry and Biological Sciences (UNL) animal ethics committee. Whole tadpoles were homogenized on ice in 0.1 % t-octylphenoxypolyethoxy ethanol (triton X-100) in 25 mM tris (hydroxymethyl) aminomethane hydrochloride (pH 8.0) using a polytron. The homogenates were centrifuged at 10,000 rpm at 4 °C for 15 min, and the supernatant was collected and frozen at -80 °C until assayed for enzymatic determination. Total protein (TP) concentrations in the supernatants were determined according to the Biuret method (Kingbley 1942). When sample volume was enough, enzyme kinetics assays were performed in duplicate. AChE and BChE activities were measured following Ellman et al. (1961). The reaction mixture consisted of 0.01 mL extract, 2 mM dithio bis 2-nitrobenzoic acid, 20 mM acetylthiocholine, and butyrylthiocholine iodide (AcSCh and BuSCh, respectively); 25 mM Tris–HCl; and 1 mM CaCl2 (pH 7.6). Assays were conducted at 25 °C. The variation in optical density was recorded at 410 nm at 25 °C for 1 min using a Jenway 6405 UV–VIS spectrophotometer. AChE and BChE activities were expressed as nmol min-1 mg-1 of TP using a molar extinction coefficient of 13.6 × 103 M-1 cm-1.
GST activity was determined spectrophotometrically by the method described by Habig et al. (1974) and adapted by Habdous et al. (2002) for mammal serum GST activity. The enzyme assay was performed at 340 nm in 100 mM Na–phosphate buffer (pH 6.5), 2 mM 1-chloro-2, 4-dinitrobenzene and 5 mM reduced glutathione. Enzyme kinetics assays were performed at 25 °C and whole GST activity was expressed as μmol min-1 mg-1 of TP using a molar extinction coefficient of 9.6 × 103 M-1 cm-1
Blood cell morphology
Blood samples were taken from surviving tadpoles after 168 h of in situ exposure by cardiac puncture and one smear was prepared on clean slides, fixed and stained by the May–Grünwald–Giemsa method (Dacie and Lewis 1984; Attademo et al. 2011). Erythrocyte cell morphology was evaluated for evidence of immature erythrocyte (IE), mitotic cells (MC), and micronuclei (MN) frequencies. Genotoxicity was tested using the presence of erythrocyte nuclear abnormalities (ENA), carried out in mature peripheral erythrocytes according to the procedures of Guilherme et al. (2008), by determining the frequency of the following nuclear lesions: MN, lobed nuclei (L), binucleates or segmented nuclei (S), kidney shaped nuclei (K), and notched nuclei (N). The results were expressed as ENA frequency, the mean value (%) of the sum (MN + L + S + K + N) for all the lesions observed. In addition, the appearance of different leukocytes was assessed before performing a differential eosinophils count.
Data analysis
Data of enzymatic activity were expressed as the mean and standard error of the mean (x ± SEM). In all experiments, replicates were tested for differences using ANOVA (Hurlbert 1984). No significant differences were found among replicates (p > 0.05); thus, no experimental chamber effect was identified, and replicates were pooled. The influence of sampling sites (RF and RS) and sampling period (48 and 96 h) on cholinesterase and GST enzyme activities was analyzed with two-way ANOVA using general linear models (GLMs) and by Student’s t-test for comparison between RS and RF at 48 and 96 h. Differences in wet weight and body length were analyzed using ANCOVA, followed by Fisher’s pairwise LSD. Since tadpole size could also be a function of developmental stage, we used the latter parameter as a covariate for weight and length (Hogan et al. 2008). We also used Student’s t-test for comparison of development rates. Data were tested for homogeneity and normality of variance (Kolmogorov–Smirnov test and Levene test). Statistical analyses were performed using INFOSTAT/ P 1.1 for Windows software (Grupo InfoStat Professional, FCA, Universidad Nacional de Córdoba, Argentina). Blood cell morphology between anurans of the two sites was evaluated with a binomial proportion test (Margolin et al. 1983) with BioEstat software 5.0 (Ayres et al. 2008). The criterion for significance was p < 0.05.
Results
Survival, development, and growth rates
Habitat variables and biological endpoints of each tadpole species from each sampling site (RS and RF) are summarized in Table 1. Concentrations of nitrate and orthophosphate were higher in the RF than in the RS. Levels of pH, dissolved oxygen, and conductivity were lower in RF, than in RS. No mortality of S. squalirostris and L. mystacinus tadpoles was recorded in RS (Table 1). By contrast, dead tadpoles were detected in RF (33 % in L. mystacinus and 15 % in S. squalirostris). S. squalirostris tadpoles raised at RS increased in body length after 7 days of in situ exposure with respect to those present at RF site, showing statistically significant differences (F = 23.48, p < 0.05), whereas wet weight did not vary significantly between sites (F = 0.50, p > 0.05). Conversely, tadpoles from RF showed differences in wet weight (p < 0.05) before and after caging. Development rates increased only in tadpoles from RF (t = 3.33, p < 0.05).
L. mystacinus tadpoles exposed in RF presented significant differences in body length and wet weight (F = 6.31, p < 0.05 and F = 14.23, p < 0.05; respectively; Table 2), whereas development did not vary significantly (t = 2.33, p > 0.05). By contrast, tadpoles from RS did not show differences in body length (F = 1.22, p > 0.05) or wet weight (F = 1.91, p > 0.05) before and after caging. Development rates did not differ significantly between sites (t = 0.20, p > 0.05).
Cholinesterase and GST
Responses of cholinesterases (AChE and BChE) to site treatments and sampling periods (48 and 96) are summarized in Table 2. In S. squalirostris, AChE activity varied significantly between sampling periods (p < 0.05, Table 2), but not between treatment sites (p > 0.05) or in the interaction between treatment sites and sampling periods (p > 0.05). AChE activity was lower for tadpoles collected from RF than for those from RS at 96 h (Fig. 3a). Reference BChE activities were 5.95 ± 0.69 nmol min-1 mg-1 of TP at 48 h and 6.78 ± 0.80 nmol min-1 mg-1 of PT at 96 h. BChE activity varied significantly between treatment sites (p < 0.0001; Table 2); however, variation was not significant between sampling periods (p > 0.05) or in the interaction between factors (p > 0.05). BChE activity was significantly lower (Fig. 3b) in RF than in RS at 48 and 96 h. GST activitiy in RS tadpoles was 129.17 ± 14.18 μmol min-1 mg-1 of TP at 48 h and 105.34 ± 19.36 nmol min-1 mg-1 of TP at 96 h. GST activity was significantly influenced by treatment sites (p < 0.0001, Table 1), with lower values in tadpoles collected from RF than in those from RS at 48 and 96 h (Fig. 3c).
In L. mystacinus tadpoles, the mean values of the AChE activity were 32.28 ± 1.88 nmol min-1 mg-1 of TP at 48 h and 31.54 ± 1.37 nmol min-1 mg-1 of TP at 96 h in RS. AChE varied significantly between sampling periods (p < 0.05) and in the interaction between treatment sites and sampling periods (p < 0.05); however, AChE did not vary between treatment sites (p > 0.05). AChE activity was statistically higher for tadpoles collected from RF at 96 h than for RS tadpoles (Fig. 4a). Control BChE activity in RS was 10.36 ± 0.93 nmol min-1 mg-1 of TP at 48 h and 11.50 ± 1.00 nmol min-1 mg-1 of TP at 96 h. Enzyme activity was not significantly influenced by sampling site (p > 0.05), sampling period (F = 0.64, p > 0.05), or the interaction between those factors (p > 0.05). BChE activity was similar among tadpoles from RS and RF (Fig. 4b). GST activity in the tadpoles from RF was 176.14 ± 26.53 nmol min-1 mg-1 of TP at 48 h and 190.28 ± 36.99 nmol min-1 mg-1 of TP at 96 h. GST activity was only significantly influenced by treatment sites (p < 0.0001). GST activities were statistically higher for tadpoles collected from RF than for those from RS at 48 and 96 h (Fig. 4c).
Blood cell morphology
The blood cell parameters of S. squalirostris and L. mystacinus from agricultural site (RF) are similar from those of RS (p > 0.05, Table 3), with the exception of MC and eosinophils tadpoles (Fig. 5)
Discussion
The results of this study show different adverse effects on survival and health status of S. squalirostris and L. mystacinus in relatively short in situ exposure in RF. These results highlight the importance of testing multiple species of amphibians because the sensitivity of each species within the community can be markedly different (Relyea 2009)
In RF, inorganic phosphate concentrations are within the range considered toxic for amphibians (Hamer et al. 2004). Inorganic phosphate is a product from fertilizer applications that generally runoff from agricultural fields (Andraski and Bundy 2003); high concentrations in water bodies are related to tadpole impairments (Earl and Whiteman 2010). Many investigations have clearly shown that amphibian populations can be impacted by the input of nutrients in water bodies (Hatch and Blaustein 2000; Nebeker and Schuytema 2000) that generally interact with pesticides and increase toxic effects (Sullivan and Spence 2003). For example, Bishop et al. (1999) found that inorganic phosphate was responsible for reduced diversity, density, and reproductive success in two anuran species (Anaxyrus americanus and Lithobates clamitans) in Ontario, Canada. Likewise, Hamer et al. (2004) reported that the presence of phosphate affected each species differently, with survival being increased in Limnodynastes peronii but decreased in Litoria aurea at phosphate concentrations ≥ 15 mg L-1. Moreover, nitrate concentration was higher (2 mg L-1) in RF than in RS. According to this value and nitrate levels permitted for the protection of aquatic biota in Argentina (<10 mg L-1) and literature (Rousse et al. 1999), the concentration found in RF have not lethal effects on two amphibian species. Moreover, ammonia was found at 1.5 mg L-1 in RF during our study. Jofre and Karasov (1999) reported a decrease in growth and development and an increase in malformations in green frog larvae exposed to ≥0.5 mg L-1 of ammonia. Clearly, more studies are necessary on the effects of those nutrients on different species of amphibian populations in RF.
Our results demonstrated a decrease in body weight of L. mystacinus in RF where LCT was applied; this observation is consistent with some evidences that LCT contribute to decrease the body weight of adult rats (Fetoui et al. 2009). Moreover, the high metabolic rate of animals exposed to pesticides may result in reduced body weight (Rowe et al. 1998; Zaya et al. 2011). However, S. squalirostris development rate increased in RF. An explanation of the last observation may be that faster metamorphosis have been an outcome of ‘stress’ condition, a common expression applied to any factor that increases corticosterone levels and thus increases metamorphic rate (Hayes 1995). Orton and Routledge (2011) reported a faster development in the toad Bufo bufo tadpoles in agricultural sites than in the RS. This variation in growth rates and time to metamorphosis in two species to different water chemistry has important implications for both field exposure studies and laboratory toxicity testing, and could be reflect genetic effects in water chemistry tolerance (Parichy and Kaplan 1992).
Similarly to development and growth effects, we found variation in cholinesterase (AChE and BChE) and GST enzyme activities between RF and RS. S. squalirostris was much more sensitive to the effects of LCT on activities of both cholinesterase (AChE and BChE) than L. mystacinus. AChE and BChE of S. squalirostris tadpoles were significantly inhibited in RF compared to enzyme activities of individuals from RS at 48 and 96 h. Several studies have evaluated BChE and AChE inhibition by pesticides in native anuran tadpoles under laboratory conditions (e.g., Lajmanovich et al. 2009; 2010; 2011); however, similar findings were observed in adult anurans in the field. A significant reduction of BChE activity in RFs with respect to the RS was determined in other native adult amphibian species (Rhinella schneideri and Leptodactylus chaquensis, Attademo et al. 2007, 2011). In the present work, reduction of BChE and AChE activities in S. squalirostris from RF may be attributed to LTC. This observation is in agreement with findings of Khan et al. (2003), who determined that this pesticide inhibits BChE activity of the anuran adults Euphlyctis cyanophlyctis. AChE inhibition would typically reduce normal neuron firing, which would inhibit tadpole movement (Sparling and Fellers 2007). This lower activity may have reduced feeding rates and might account for the decrease in body weight of S. squalirostris larvae exposed to LCT. This pesticide has restricted uses in the USA and European countries due to the threat that their formulations pose to mammals, birds, and aquatic organisms (He et al. 2008). A half-life of > 3 weeks has been reported under natural irradiation (He et al. 2008). Based on this half-life, we can assume that Lambda-cyhalothrin residue may be a potential risk for aquatic life during a prolonged period. For instance, Hill et al. (1994) demonstrated that this chemical may accumulate in fish. By contrast, we found higher AChE activity in L. mystacinus collected from RF at 96 h than in enzyme activity of RF individuals. Cong et al. (2008) reported an influence of time on inhibition on AChE activity followed by a gradual recovery up to the end of experiment in the snakehead fish (Channa striata) after pesticide spray application in RF. Reactivation of the mediated anti-cholinesterase effect or even an enzyme superproduction has been reported as a response to inhibition, usually associated with the end of the treatment (Hernández-Moreno et al. 2010). Farchi et al. (2003) confirmed that overexpression of AChE activity might induce malfunctioning beyond neuromuscular transmission in mouse muscle. However, this assumption needs to be tested in amphibian species.
Moreover, GST activity increased in L. mystacinus from RF at 48 and 96 h of exposure to LCT. This enzyme may be generally involved in detoxication of environmental toxicants including polyaromatic hydrocarbons, pesticides, and other biochemical reactions (Ognjanovic’ et al. 2003; Ferrari et al. 2011; Lajmanovich et al. 2011). GST activity is involved in the I-phase of xenobiotic biotransformation together with CYP450-dependent monooxygenases. The fact of finding an induction in this enzyme activity suggests that there might be a higher detoxification of contaminants (or endogenous compounds) susceptible of interacting with GST; however, this assumption must be taken as merely speculative. Similarly, we reported an increase in GST activity in plasma samples of the adult toad R. schneideri from an agricultural area (Attademo et al. 2007). By contrast, inhibition of GST activity has also been reported in other species treated with various pesticides. Accordingly, a lower level of GST activity in S. squalirostris from RF was observed. These results are in agreement with studies of Frasco and Guilhermino (2002) and Lajmanovich et al. (2011), who demonstrated that dimethoate and glyphosate inhibit GST activity in fish (Poecilia reticulata) and toad tadpoles (Rhinella arenarum). The interspecific enzymatic responses reinforce the assumption of Greulich and Pflugmacher (2004) and Martínez-Alvarez et al. (2005) that GST expression and activity may be an adaptive response to toxic stress in an organism.
On the other hand, variations of several haematological parameters and their relationships with natural or human-induced changes in the environment have been described in amphibians (Stansley and Roscoe 1996; Cabagna et al. 2005; Barni et al. 2007). We observed a decrease of mitotic erythrocytes in S. squalirostris and L. mystacinus collected from RF. This decrease in mitotic index could lead to a false reduction of MN and nuclear alterations, because the erythrocytes must be on division. Beyond this consideration, we did not observe increase in MN and ENA during the application of LCT in S. squalirostris and L mystacinus. We also found an increase in the number eosinophils in the RF of the two amphibian species studied. These results are consistent with previous studies of other stress-inducing conditions on amphibian leukocyte parameters (Barni et al. 2007). The relative increase of eosinophils was observed in adults of Rana sp. in a transformed urban environment in Russia (Romanova and Egorikhina 2006). Thus, the increased number of eosinophils in these frogs might be considered a sign of immunological response to pesticide exposure, as previously observed (Chernyshova and Starostin 1994; Kiesecker 2002). However, our results are not concluding and we cannot establish a relationship between this hematological biomarker response and LCT toxicity.
Conclusion
Overall, our results demonstrate (1) the existence of apparent differences in sensitivity between species in a series of sublethal responses to short-term exposure in RFs with application of Lambda-cyhalothrin; (2) inhibition of BChE at 48 and 96 h and AChE at 96 h in S. squalirostris in the RF, and induction of AChE activity at 96 h in L. mystacinus; (3) induction of GST activity in individuals L. mystacinus but inhibition in S. squalirostris from the RF at 48 and 96 h; (4) lower number of MC and higher number of eosinophils in individuals of the two species from the RF than in individuals from the RS. Interspecific differences observed in cholinesterase, GST, and blood cell parameters between S. squalirostris and L. mystacinus may be attributing to differences in tolerance to LCT, either in rates of absorption through the skin or in variation in the ability to detoxify chemicals, as indicated for other amphibians (Marco and Blaustein 1999; Semlitsch et al. 2000). Although these results would have been more valuable if we had measured pesticide residues in environmental matrices (soil, water), we suggest that integral use of several biological endpoints (development and growth) together with biomarkers (cholinesterase, GST, and blood cell parameters) may be promising integral procedures for investigating pesticide exposure of wild frog populations. Thus, it might also be possible to use the enzymatic and hematological parameters under field conditions on adult amphibians with a nondestructive method to know real health status and provide valuable information for ecological risk assessments and remediation programs (Attademo et al. 2011; Ilizaliturri-Hernández et al. 2013).
References
Alvisio, A. (1998). Arroz. Modelos zonales de producción en el movimiento CREA: región litoral Norte. Cuaderno Actual Técnico, 61, 141–145.
Andraski, T. W., & Bundy, L. G. (2003). Relationships between phosphorus levels in soil and in runoff from corn production systems. Journal of Environmental Quality, 32, 310–316.
ASIH, HL, & SSAR (2001). Guidelines for use of live amphibians and reptiles in field research. http://www.utexas.edu/depts/asih/herpcoll.htlm. Accessed on 13/06/01.
Attademo, A. M., Peltzer, P. M., Lajmanovich, R. C., Cabagna, M., & Fiorenza, G. (2007). Plasma B-esterases and glutathione S-transferase activities in the toad chaunus schneideri (amphibia, anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology, 16, 533–539.
Attademo, A. M., Cabagna-Zenklusen, M., Lajmanovich, R. C., Peltzer, P. M., Junges, C., & Basso, A. (2011). B-esterase activities and blood cell morphology in the frog leptodactylus chaquensis (amphibia: leptodactylidae) on rice agroecosystems from Santa Fe province (Argentina). Ecotoxicology, 20, 274–282.
Ayres, M., Jr., Ayres, D., & Santos, A. (2008). BioEstat Versão5.0. Belém, Pará, Brazil: Sociedade Civil Mamirauá, MCT-CNPq.
Bambaradeniya, C. N. B., Edirisinghe, J. P., De Silva, D. N., Gunatilleke, C. V. S., Ranawana, K. B., & Wijekoon, S. (2004). Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodiversity and Conservation, 13, 1715–1753.
Barni, S., Boncompagni, E., Grosso, A., Bertone, V., Freitas, I., Fasola, M., et al. (2007). Evaluation of rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquatic Toxicology, 81, 45–54.
Bishop, C. A., Mahony, N. A., Struger, J. N. P., & Pettit, K. E. (1999). Anuran development, density and diversity in relation to agricultural activities in the Holland river watershed, Ontario, Canada (1990–1992). Environmental Monitoring and Assessment, 57, 21–43.
Brodeur, J. C., Suarez, R. P., Natale, G. S., Ronco, A. E., & Zaccagnini, M. E. (2011). Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicology and Environmental Safety, 74, 1370–1380.
Burkart, R., Barbaro, N. O., Sánchez, R. O., & Gómez, D. A. (1999). Eco-regiones de la Argentina. Buenos Aires, Argentina: PRODIA.
Cabagna, M., Lajmanovich, R. C., Stringhini, G., & Peltzer, P. M. (2005). Hematological studies in the common toad (bufo arenarum) in agrosystems of Argentina. Applied Herpetology, 2, 373–380.
CASAFE. (2005). Cámara de sanidad agropecuaria y fertilizantes de la república Argentina. Buenos Aires: Guía de Productos Fitosanitarios para la República Argentina.
Chernyshova, E. V., & Starostin, V. I. (1994). The peripheral blood of frogs in the genus rana as a test system for assessing environmental pollution. Izvestiya Rossiiskoi Akademii Nauk – Seriya Biologicheskaya, 4, 656–660.
Christin, M. S., Menard, L., Gendron, A. D., Ruby, S., Cyr, D., Marcogliese, D. J., et al. (2004). Effects of agricultural pesticides on the immune system of xenopus laevis and rana pipiens. Aquatic Toxicology, 67, 33–43.
Cong, N. V., Phuong, N. T., & Bayley, M. (2008). Brain cholinesterase response in snakehead fish (channa striata) after field exposure to diazinon. Ecotoxicology and Environmental Safety, 71, 314–318.
Czech, H. A., & Parsons, K. C. (2002). Agricultural wetlands and waterbirds: a rewiew. Waterbirds, 25, 56–63.
Dacie, J. V., & Lewis, S. M. (1984). Practical hematology. New York: Churchill Livingstone.
DeWitt, T. J., & Scheiner, S. M. (2004). Phenotypic plasticity: functional and conceptual approaches. New York: Oxford University Press.
Earl, J. E., & Whiteman, H. H. (2010). Evaluation of phosphate toxicity in Cope’s gray treefrog (hyla chrysoscelis) tadpoles. Journal of Herpetology, 44, 201–208.
Ellman, G. L., Courtney, K. D., Andreas, V., Jr., & Featherstone, R. M. (1961). A new and rapid calorimetric determination of cholinesterase activity. Biochemical Pharmacology, 7, 88–95.
Elphick, C. S., & Oring, L. W. (2003). Conservation implications of flooding rice fields on winter waterbird communities. Agriculture, Ecosystems and Environmental, 94, 17–29.
Farchi, N., Soreq, H., & Hochner, B. (2003). Chronic acetylcholinesterase overexpression induces multilevelled aberrations in mouse neuromuscular physiology. The Journal of Phisiology, 546, 165–173.
Fernando, C. H. (1995). Rice fields are aquatic, semi-aquatic, terrestrial, and agricultural: A complex and questionable limnology. In K. H. Timotius and F. Goltenboth (Eds.), Tropical limnology (vol. 1, pp. 121–148).
Fernando, C. H. (1996). Ecology of rice fields and its bearing on fisheries and fish culture. In S. S. de Silva, (Ed.), Perspectives in Asian fisheries (pp. 217–237).
Ferrari, A., Lascano, C., Pechen de D’Angelo, A. M., & Venturino, A. (2011). Effects of azinphos methyl and carbaryl on rhinella arenarum larvae esterases and antioxidant enzymes. Comparative Biochemistry and Physiology, 153, 34–39.
Fetoui, H., El Mouldi, G., & Zeghal, N. (2009). Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. Experimental Toxicology and Pathology, 61, 189–196.
Frasco, M. F., & Guilhermino, L. (2002). Effects of dimethoate and beta-naphthoflavone on selected biomarkers of poecilia reticulata. Fish Physiology and Biochemistry, 26, 149–156.
García-Muñoz, E., Gilbert, J. D., Parra, G., & Guerrero, F. (2010). Wetlands classification for amphibian conservation in Mediterranean landscapes. Biodiversity and Conservation, 19, 901–911.
Gibbs, J. P., Whiteleather, K. K., & Schueler, F. W. (2005). Changes in frog and toad populations over 30 years in New York state. Ecology Application, 15, 1148–1157.
Gosner, K. L. (1960). A simplified table for staging anuran embryos and larvae with note on identification. Herpetologica, 16, 183–190.
Greulich, K., & Pflugmacher, S. (2004). Uptake and effects on detoxication enzymes of cypermethrin in embryos and tadpoles of amphibians. Archives of Environmental Contamination and Toxicology, 47, 489–495.
Guilherme, S., Válega, M., Pereira, M. E., Santos, M. A., & Pacheco, M. (2008). Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicology and Environmental Safety, 70, 411–421.
Habdous, M., Visvikis, S., & Visvikis, S. (2002). Rapid spectrophotometric method for serum glutathione S-transferases activity. Clinical Chemistry Acta, 326, 131–142.
Habig, W. H., Pabst, M. J., & Jakoby, W. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Hamer, A. J., Makings, J. A., Lane, S. J., & Mahony, M. J. (2004). Amphibian decline and fertilizer used on agricultural land in south-eastern Australia. Agriculture Ecosystems and Environmental, 102, 299–305.
Hatch, A. C., & Blaustein, A. R. (2000). Combined effects of UV-B, nitrate, and low pH reduce the survival and activity level of larval cascades frogs (rana cascadae). Archives of Environmental Contamination and Toxicology, 39, 494–499.
Hayes, T. B. (1995). Interdependance of corticosterone-hormones and thyroid-hormones in larval toads (bufo Boreas). I thyroid hormone-dependent and hormone-independent effects of corticosterone on growth and development. Journal of Experimental Zoology, 271(2), 95–102.
He, L. M., Troiano, J. K. S., & Goh, A. (2008). Wang environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. Review of Environmental Contamination and Toxicology, 195, 71–91.
Hernández-Moreno, D., Soler, F., Míguez, M. P., & Pérez-López, M. (2010). Brain acetylcholinesterase, malondialdehyde and reduced glutathione as biomarkers of continuous exposure of tench, tinca tinca, to carbofuran or deltamethrin. Science of the Total Environmental, 408, 4976–4983.
Hill, I. R., Runnalls, J. K., Kennedy, J. H., & Ekoniak, P. (1994). In I. R. Hill, F. Heimbach, P. Leeuwangh, & P. Matthiessen (Eds.), Effects of lambda-cyhalothrin on aquatic organisms in large-scale mesocosms (pp. 345–360). London, UK: Lewis: Freshwater field tests for hazard assessment of chemicals.
Hirai, T. (2004). Diet composition of the Indian rice frog, rana limnocharis in the floodplain of the kizu river, Japan. Herpetological Journal, 14, 149–152.
Hirai, T., & Matsui, M. (1999). Feeding habits of the pond frog, rana nigromaculata, inhabiting rice fields in Kyoto, Japan. Copeia, 1999, 940–947.
Hogan, N. S., Duarte, P., Wade, M. G., Lean, D. R. S., & Trudeau, V. L. (2008). Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (rana pipiens): identifying critically vulnerable periods of development. General and Comparative Endocrinology, 156, 515–523.
Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs, 53, 187–211.
Ilizaliturri-Hernández, C. A., González-Mille, D. J., Mejía-Saavedra, J., Espinosa-Reyes, G., Torres-Dosal, A., & Pérez-Maldonado, I. (2013). Blood lead levels, δ-ALAD inhibition, and hemoglobin content in blood of giant toad (rhinella marina) to asses lead exposure in three areas surrounding an industrial complex in Coatzacoalcos, Veracruz, Mexico. Environmental Monitoring and Assessment, 185, 1685–1698.
Jofre, M. B., & Karasov, W. H. (1999). The direct effect of ammonia on three species of north American anuran amphibians. Environmental Toxicology and Chemistry, 18, 1806–1812.
Jones, D. K., Hammond, J. I., & Relyea, R. A. (2009). Very highly toxic effects of endosulfan across nine species of tadpoles: Lag effects and family-level sensitivity. Environmental Toxicology and Chemistry, 28, 1939–1945.
Juliano, B. O. (1993). Rice in human nutrition. Manila, Philippines: Food and Agriculture Organization (FAO) and International Rice Research Institute (IRRI).
Khan, M. Z., Tabassum, R., Naqvi, S. N. H., Shah, E. Z., Tabassum, F., Ahmad, I., et al. (2003). Effect of cypermethrin and permethrin on cholinesterase activity and protein contents in rana tigrina (amphibia). Turkish Journal of Zoology, 27, 243–246.
Kiesecker, J. M. (2002). Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? Proceedings of the National Academic of Sciences of the United States of America, 99, 9900–9904.
Kingbley, G. R. (1942). The direct biuret method for the determination of serum proteins as applied to photoelectric and visual calorimetry. Journal of Laboratory and Clinical Medicine, 27, 840–845.
Lajmanovich, R. C., Attademo, A. M., Peltzer, P. M., & Junges, C. M. (2009). Inhibition and recovery of brain and tail cholinesterases of odontophrynus americanus tadpoles (amphibia: cycloramphidae) exposed to fenitrothion. Journal of Environmental Biology, 30, 923–992.
Lajmanovich, R. C., Peltzer, P. M., Junges, C. M., Attademo, A. M., Sanchez, L. C., & Basso, A. (2010). Activity levels of B-esterases in the tadpoles of 11 species of frogs in the middle Paraná river floodplain: implication for ecological risk assessment of soybean crops. Ecotoxicology and Environmental Safety, 73, 1517–1524.
Lajmanovich, R. C., Attademo, A. M., Peltzer, P. M., Junges, C. M., & Cabagna, M. C. (2011). Toxicity of four glyphosate formulations on rhinella arenarum (anura: bufonidae) tadpoles: B-esterases and glutathione S-transferase inhibitions. Archives of Environmental Contamination and Toxicology, 60, 681–689.
Machado, I. F., & Maltchik, L. (2010). Can management practices in rice fields contribute to amphibian conservation in southern Brazilian wetlands? Aquatic Conservation: Marine and Freshwater Ecosystems, 20, 39–46.
Mann, R. M., Hyne, R. V., Choung, C. B., & Wilson, S. P. (2009). Amphibians and agricultural chemicals: review of the risks in a complex environment. Environmental Pollution, 157, 2903–2927.
Marco, A., & Blaustein, A. R. (1999). The effects of nitrite on behavior and metamorphosis in cascades frogs (rana cascadae). Environmental and Toxicology Chemistry, 18, 949–949.
Margolin, B. H., Collings, B. J., & Mason, J. M. (1983). Statistical analysis and sample size determinations for Mutagenicity experiments with binomial responses. Environmental Mutagenesis, 5, 705–716.
Martínez-Alvarez, R. M., Morales, A. E., & Sanz, A. (2005). Antioxidant defenses in fish: biotic and abiotic factors. Review in Fish Biology and Fisheries, 15, 75–88.
McCarthy, J., & Shugart, L. (1990). In J. McCarthyand & L. Shugart (Eds.), Biological markers of environmental contamination (pp. 3–14). Boca Raton, FL: Lewis: Biomarkers of environmental contamination.
Meister, R. T. (1992). Farm chemicals handbook 92. Willough-by, OH: Meister Publishing.
Millennium Ecosystem Assessment. (2005). Ecosystems and human well-being: wetlands and water. Washington, DC: World Resources Institute.
Navas, C. A., & Otani, L. (2007). Physiology, environmental change, and anuran conservation. Phyllomedusa, 6, 83–102.
Nebeker, A. V., & Schuytema, G. S. (2000). Effects of ammonium sulfate on growth of larval northwestern salamanders, red-legged and pacific treefrog tadpoles and juvenile fathead minnows. Bulletin of Environmental Contamination and Toxicology, 64, 271–278.
Ognjanovic’, B. I., et al. (2003). Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiology Research, 52, 563–570.
Orton, F., & Routledge, E. (2011). Agricultural intensity in ovo affects growth, metamorphic development and sexual differentiation in the common toad (bufo bufo). Ecotoxicology, 20, 901–911.
Parichy, D. M., & Kaplan, R. H. (1992). Maternal effects on offspring growth and development depend on environmental quality in the frog bombina orientalis. Oecologia, 91, 579–586.
Peltzer, P. M., & Lajmanovich, R. C. (2007). Amphibians. In M. H. Iriondo, J. C. Paggi, & M. J. Parma (Eds.), The middle Parana river: limnology of a subtropical wetland (pp. 327–340). Heidelberg: Springer Berlin.
Peltzer, P. M., Lajmanovich, R. C., Attademo, A. M., & Beltzer, A. H. (2006). Anuran diversity across agricultural pond in Argentina. Biodiversity and Conservation, 15, 3499–3513.
Peltzer, P. M., Lajmanovich, R. C., Sánchez- Hernández, J. C., Cabagna, M. C., Attademo, A. M., & Bassó, A. (2008). Assessment of agricultural pond eutrophication on survival and health status of the scinax nasicus tadpoles. Environmental and Ecotoxicology Safety, 70, 185–197.
Relyea, R. A. (2009). A cocktail of contaminants: How pesticide mixtures at low concentrations affect aquatic communities. Oecologia, 159, 363–376.
Relyea, R. A., & Jones, D. K. (2009). The toxicity of roundup original MAX® to 13 species of larval amphibians. Environmental Toxicology and Chemistry, 28, 2004–2008.
Romanova, E. B., & Egorikhina, M. N. (2006). Changes in haematological parameters of rana frogs in a transformed urban environment. Russian Journal of Ecology, 37, 208–213.
Rousse, J. D., Bishop, C. A., & Struger, J. (1999). Nitrogen pollution: an assessment of its threat to amphibian survival. Environmental Health Perspectectives, 107, 799–803.
Rowe, C. L., Kinney, O. M., Nagle, R. D., & Congdon, J. C. (1998). Elevated maintenance costs in an anura exposed to a mixture of trace elements during the embryonic and early larval periods. Physiology Zoology, 71, 27–35.
Sánchez-Hernández, J. C. (2007). Ecotoxicological perspectives of B-esterases in the assessment of pesticide contamination. In R. H. Plattenberg (Ed.), Environmental pollution: New research (pp. 1–45). New York: Nova Science Publishers.
Semlitsch, R. D., Bridges, C. M., & Welch, A. M. (2000). Genetic variation and a fitness trade off in the tolerance of gray treefrog (hyla versicolor) tadpoles to the insecticide carbaryl. Oecologica, 125, 179–185.
Sparling, D. W., & Fellers, G. M. (2007). Comparative toxicity of chlorpyrifos, diazinon, to larval rana boylii. Environmetal Pollution, 147, 535–539.
Stansley, W., & Roscoe, D. E. (1996). The uptake and effects of lead in small mammals and frogs at a trap and skeet range. Archives of Environmental Contamination and Toxicology, 30, 220–226.
Sullivan, K. B., & Spence, K. M. (2003). Effects of sublethal concentrations of atrazine and nitrate on metamorphosis of the African clawed frog. Environmental Toxicology and Chemistry, 33, 627–635.
Teplitsky, C., Plénet, S., & Joly, P. (2003). Tadpoles responses to risk of fish introduction. Oecologia, 134, 270–277.
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R., & Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature, 418, 671–677.
Walker, C. H. (1998). The use of biomarkers to measure the interactive effects of chemicals. Ecotoxicology and Environmental Safety, 40, 65–70.
Zaya, R. M., Amini, Z., Whitaker, A. S., Kohler, S. L., & Ide, C. F. (2011). Atrazine exposure affects growth, body condition and liver health in xenopus laevis tadpoles. Aquatic Toxicology, 104, 243–253.
Acknowledgments
We thank the owner of the field for allowing us to conduct the study. We thank the members of the Department of Mathematics, Faculty of Biochemistry and Biological Sciences, UNL for their statistical suggestions. This study was supported in part by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANCyT) and Curso de Acción para la Investigación y Desarrollo (CAI + D-UNL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attademo, A.M., Peltzer, P.M., Lajmanovich, R.C. et al. Biological endpoints, enzyme activities, and blood cell parameters in two anuran tadpole species in rice agroecosystems of mid-eastern Argentina. Environ Monit Assess 186, 635–649 (2014). https://doi.org/10.1007/s10661-013-3404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3404-z