Abstract
In this chapter, we show the results obtained after exposing the freshwater gastropod Chilina gibbosa 7 and 14 days to the organophosphate azinphos-methyl and the carbamate carbaryl. These insecticides are applied in Argentina and found in water bodies. Chilina gibbosa is naturally distributed in our country, and the family (Chilinidae) is considered vulnerable. It has an important role in the ecosystem being food of fishes and birds. After exposure to the same molar concentration of these insecticides, which corresponds to an environmental concentration in both cases, we registered lethality, neurotoxicity, and cholinesterase and carboxylesterase activities.
Our results show that the subchronic exposure to an environmental concentration of azinphos-methyl caused severe signs of neurotoxicity (the abnormal exposure of the head-foot region and the loss of adherence) after 7 and 14 days of exposure. Cholinesterases were more sensitive than carboxylesterases. Inhibition of 89% of cholinesterase activity was observed after 7 days of exposure, while the decrease of carboxylesterase activity occurred after 14 days. Additionally, azinphos-methyl decreased protein content in C. gibbosa. On the other hand, the subchronic exposure to carbaryl only produced an inhibition of carboxylesterase activity measured with p-nitrophenyl butyrate as substrate.
We conclude that insecticides applied in Argentina can cause toxic effects in the native gastropod C. gibbosa and that azinphos-methyl is more toxic than carbaryl. The presence of these insecticides in water bodies could put this species at risk, negatively disturbing the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Chilina gibbosa (Sowerby 1841) is a freshwater gastropod from the Chilinidae (pulmonate) family which is endemic to South America, especially abundant in southern Chile and Argentina. In Argentina, it is found in lakes and rivers of Río Negro and Neuquén provinces, North Patagonia (Bosnia et al. 1990; Rumi et al. 2008; Gutiérrez Gregoric 2010; Valdovinos Zarges 2006). It has an important role in the ecosystem as it is a food source for birds and fishes, some of which have commercial value such as the native silverside Odontesthes hatcheri and the rainbow trout Oncorhynchus mykiss (Bosnia et al. 1990). C. gibbosa has several points of interest as model organism for ecotoxicology studies: (1) it is easy to collect and handle, as individuals can usually be found in shallow waters, in an aggregated dispersion pattern (Bosnia et al. 1990), and (2) their limited mobility and ability to excrete pollutants may result in several negative effects at low environmental concentrations of toxicants (Oehlmann and Schulte-Oehlmann 2003), which ensures its effective exposure to any pollutant present in the environment.
The use of native species in ecotoxicological studies has been suggested by US EPA (1976). They have been recommended by several authors as advantageous because the organisms are already acclimated to environmental conditions and are possibly more susceptible to contaminants than invasive species, and results could be considered more ecologically relevant (Baird et al. 2007; Buikema et al. 1982; Krull et al. 2012; Gagneten et al. 2012).
In the Upper Valley of Río Negro and Neuquén (North Patagonia, Argentina), agriculture represents the second most important economic activity (Loewy et al. 2011). Irrigation and pest control are common practices required for agricultural development in this region. Pesticides are applied by ground-based spraying equipment, and a substantial fraction of them reaches surface drainage water and soil (Loewy et al. 1999, 2011). The organophosphate (OP ) azinphos-methyl (AZM ) and the carbamate carbaryl (CAR ) have been two of the main insecticides used in this region. The recommended concentrations in water of AZM and CAR for aquatic life protection in Argentina are ≤0.02 μg L−1 and 0.05 μg L−1, respectively. Nevertheless, Loewy et al. (1999, 2011) detected a maximum concentration of 79.30 μg L−1 of AZM and 45.7 μg L−1 of CAR in surface and subsurface waters of the Upper Valley of Río Negro and Neuquén region. AZM and CAR share the same mechanism of action: they are inhibitors of cholinesterase activity (ChE), which can cause neurotoxicity and eventually death. They can also cause other effects such as the inhibition of carboxylesterase activity (CE) (Sanchez-Hernandez 2007; Timbrell 2000).
Inhibition of ChE and CE by AZM and CAR has been previously reported in other aquatic gastropods (Cacciatore et al. 2013; Kristoff et al. 2006, 2010). In C. gibbosa , our group has studied different toxic effects of the acute (48 h) exposure to AZM. We reported that environmental concentrations of AZM caused neurotoxicity, inhibition of ChE in whole organism soft tissue, and immunotoxicity, causing a decrease in hemocyte viability and phagocytic activity (Bianco et al. 2013; Cossi et al. 2015; Herbert et al. 2018). However, subchronic exposure had not been studied yet. Subchronic and chronic assays provide valuable information because they represent a more realistic picture of the impact of contaminants in the environment (Cossi et al. 2018).
The aim of this study was to assess effects on different parameters (lethality, neurotoxicity, and ChE and CE activity) in C. gibbosa after 7 and 14 days of exposure to environmental concentrations of AZM and CAR. Our hypothesis were that (1) a subchronic exposure to AZM produces more severe effects than an acute one and that (2) the exposure to CAR causes similar effects on C. gibbosa than the ones caused by AZM.
5.2 Methodology
5.2.1 Chemicals
Acetylthiocholine iodide (AcSCh ), p-nitrophenyl acetate (p-NPA), p-nitrophenyl butyrate (p-NPB ), 5,5-dithio-2-bis-nitrobenzoate (DTNB ), azinphos-methyl (AZM ) PESTANAL®, and carbaryl (CAR ) PESTANAL® were purchased from Sigma-Aldrich. All other chemicals used were also of analytical reagent grade.
5.2.2 Organisms
C. gibbosa snails were collected by hand from the vegetated bank of the river Chimehuin (39°54′57.15″S 71°06′23″W; province of Neuquén, Argentina) at a depth of 5–70 cm. The river Chimehuin originates 20 km upstream of the collection site, from the glacial lake Huechulafquen, located within the Lanín National Park, Neuquén. The collection site can be considered free from agrochemical pollution because agricultural exploitation is banned upstream from it. The snails were then transported to the Laboratorio de Ecotoxicología Acuática: Invertebrados Nativos (EAIN), Ciudad Autónoma de Buenos Aires (CABA), Buenos Aires, Argentina, where bioassays were carried out after at least 20 days of acclimatization in aerated glass aquaria (10 L) at 12 ± 2 °C, under a 12:12 h (L:D) artificial photoperiod regime and with ad libitum goldfish flakes (TetraFin) as food. Adult snails of similar size, 1.6 ± 0.4 mm of shell length, and weight, 0.27 ± 0.08 g, were selected for all the bioassays.
5.2.3 Bioassays
All bioassays were carried out at 12 ± 2 °C under a photoperiod of 12:12 h (L:D). Tap water dechlorinated passively during 72 h was used for the bioassays. Insecticide stock solutions were prepared by dissolving the insecticides in acetone due to their low solubility in water. The concentrations of AZM and CAR used for the bioassays were obtained by diluting the stock solutions with dechlorinated tap water. Solutions were renewed every 96 h for AZM and daily for CAR according to previous stability studies (Cacciatore et al. 2013, 2018). The molar concentration of AZM and CAR used in the bioassays was 0.063 nM, which corresponds to 20 μg L−1 AZM and 13 μg L−1CAR. Both of these concentrations are environmental concentrations found in freshwater of the region of the Upper Valley of Río Negro and Neuquén, Argentina (Loewy et al. 1999, 2011).
Two 14-day subchronic bioassays were carried out, one for each insecticide, by exposing 7 snails per glass vessel containing either 0.05% acetone in dechlorinated tap water as solvent control (SC) or the corresponding concentration of insecticide in dechlorinated tap water (20 μg L−1 for AZM and 13 μg L−1 for CAR). Six aerated vessels were used for each treatment. Snails were fed TetraFin goldfish flakes every 96 h after solution renewal. At 7 and 14 days, mortality and neurotoxicity signs (adherence and conspicuous protrusion of the head-foot region) were recorded, and homogenates of one snail per vessel were then carried out for measurement of enzymatic activities and protein content. Whole tissue homogenates were carried according to Cossi et al. (2015) after anesthetizing snails on ice during 6–8 minutes, wiping them clean and dry and gently removing their shells.
5.2.4 Enzymatic Activity Assays
Protein content was determined in order to express enzyme activity results as μmol of substrate hydrolyzed per min per mg of protein by following the method of Lowry et al. (1951), using bovine serum albumin as standard. Protein content was expressed as mgprotein mlhomogenate−1.
ChE activity was measured following the method of Ellman et al. (1961), previously adapted for this species (Bianco et al. 2013), using 100 mM phosphate buffer pH 8, 0.2 mM DTNB, 1.5 mM AcSCh as substrate and 200 μL of the supernatant fraction. Specific activity was calculated using 13.6 mM−1 cm−1 as the molar extinction coefficient.
CE activity was determined by measuring the hydrolysis of p-NPA and p-NPB following the method of Kristoff et al. (2010), adapted for this species by Bianco et al. (2013), using 2.5 mL 100 mM phosphate buffer pH 8.0 containing 5% acetone, 1.5 mM p-NPA or p-NPB, and 150 μL of the supernatant fraction. Specific activity was calculated using 18.6 mM−1 cm−1as the molar extinction coefficient for p-nitrophenol.
5.2.5 Data Analysis
Differences in neurotoxic responses in SC and insecticide-exposed snails for each day were tested using Fisher’s exact test. Differences in enzyme activity in SC and insecticide-exposed snails between days were tested using two-way ANOVA. Assumptions of normality and homogeneity of variances were tested by Shapiro-Wilk’s normality test and Levene’s test, respectively. A log transformation was applied for ChE activity of AZM-exposed snails and CE (p-NPA and p-NPB) activity of CAR-exposed snails in order to meet assumptions. Tukey tests were used to perform post hoc comparisons, and Fisher’s Least Significant Difference (LSD) test was applied when interaction between factors resulted significant. The level of significance used was set at 0.05 for all analyses. Statistical analyses were performed using GraphPad Prism and Statistica 7 software.
5.3 Results
Mortality in both bioassays was not significant for SC snails nor for insecticide-exposed snails. Only one snail died after 14 days of AZM exposure.
Exposure to AZM for 7 days reduced the ability of the snails to adhere to the walls of the vessels to 10% adherence. After 14 days, there was no adherence at all (Table 5.1; Fisher’s exact test, P < 0.0001). AZM also had a strong neurotoxic effect, causing the protrusion of the head-foot region in 100% and 97% of the snails after 7 and 14 days of exposure, respectively (Table 5.1; Fisher’s exact test, P < 0.0001). In the case of CAR, subchronic exposure had no effect on adherence, and head-foot protrusion was not observed (Table 5.1; Fisher’s exact test, P < 0.0001).
In the case of AZM, protein content was negatively affected by AZM exposure both after 7 and 14 days, decreasing 16% and 9%, respectively, compared to SC snails (two-way ANOVA; main effect insecticide: F1,20 = 10.3888, P = 0.0043). Protein content increased between 7 and 14 days in both groups (two-way ANOVA; main effect time: F1,20 = 6.2744, P = 0.0210). In the case of CAR, protein content did not vary between SC and exposed snails (two-way ANOVA; main effect insecticide: F1,20 = 0.0099, P = 0.6578) nor between 7 and 14 days of exposure (two-way ANOVA; main effect time: F1,20 = 0.6317, P = 0.4361).
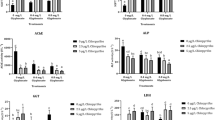
Exposure to AZM caused 87% inhibition of ChE activity after 7 days and 91% after 14 days with respect to SC snails, and overall ChE activity decreased after 14 days with respect to the activity after 7 days (Fig. 5.1; two-way ANOVA; main effect insecticide, F1,20 = 0.6317, P = 0.0001; main effect time, F1,20 = 5.1295, P = 0.0348).
Chilina gibbosa cholinesterase (ChE) activity after 7 and 14 days of a subchronic exposure to 20 μg L−1 azinphos-methyl (AZM ). SC = solvent control (0.05% acetone). Data are expressed as mean ± SD. Different letters indicate significant differences between SC and AZM; different casing (upper or lower case) indicates differences between days (two-way ANOVA; P < 0.05)
CE activity, measured using p-NPA as substrate, was not affected by a 7-day exposure to AZM. Nevertheless, after 14 days, CE activity of snails exposed to AZM decreased 40% with respect to SC snails and 34% compared to snails exposed for 7 days (Fig. 5.2a; two-way ANOVA; interaction insecticide x time, F1,20 = 6.1334, P = 0.0223; Fisher’s LSD test for the interaction insecticide x time, df = 20, 14 days SC vs. 14 days AZM P = 0.0062, 7 days AZM vs. 14 days AZM P = 0.0259). In addition, CE activity measured using p-NPB as substrate was affected by AZM exposure only after 14 days of exposure, causing 30% inhibition (Fig. 5.2b; two-way ANOVA; interaction insecticide x time, F1,20 = 4.8823, P = 0.0390; Fisher’s LSD test for the interaction insecticide x time, df = 20, 14 days SC vs. 14 days AZM P = 0.0142).
Chilina gibbosa carboxylesterase (CE) activity after 7 and 14 days of a subchronic exposure to 20 μg L−1 azinphos-methyl (AZM ). CE activity was determined using (a) p-nitrophenyl acetate (p-NPA) or (b) p-nitrophenyl butyrate (p-NPB ) as substrates. SC = solvent control (0.05% acetone). Data are expressed as mean ± SD. Different letters indicate significant differences between SC and AZM (two-way ANOVA; P < 0.05)
Contrastingly, exposure to CAR did not have a significant effect on ChE activity, even though the activity of this enzyme tended to decrease in CAR-exposed snails (25 and 35% after 7 and 14 days of exposure) (Fig. 5.3; two-way ANOVA; main effect insecticide, F1,20 = 4.2082, P = 0.0536; main effect time, F1,20 = 0.0581, P = 0.8119).
Exposure to CAR did not inhibit CE activity measured using p-NPA. Activity increased between 7 and 14 days, regardless of snails having been exposed to CAR or not. (Fig. 5.4a; two-way ANOVA; main effect insecticide, F1,20 = 1.4541, P = 0.2419; main effect time, F1,20 = 20.4203, P = 0.0002). Overall CE activity measured using p-NPB also increased after 14 days (Fig. 5.4b; two-way ANOVA; main effect time: F1,20 = 20.2461, P = 0.0002). However, in this case, CAR caused 60% inhibition at 7 days and 14 days of exposure with respect to SC group. (Fig. 5.4b; two-way ANOVA; main effect insecticide: F1,20 = 51.3028, P < 0.0001).
Chilina gibbosa carboxylesterase (CE) activity after 7 and 14 days of a subchronic exposure to 13 μg L−1 carbaryl (CAR ). CE activity was determined using (a) p-nitrophenyl acetate (p-NPA) or (b) p-nitrophenyl butyrate (p-NPB ) as substrates. CAR concentration was chosen to be the molar equivalent of 20 μg L−1AZM. SC = solvent control (0.05% acetone). Data are expressed as mean ± SD. Different letters indicate significant differences between SC and AZM; different casing (upper or lower case) indicates differences between days (two-way ANOVA; P < 0.05)
5.4 Discussion
Subchronic exposure to environmental concentrations of AZM and CAR caused sublethal toxic effects in the native gastropod C. gibbosa . However, differential responses on biochemical and behavioral biomarkers between insecticides were observed.
AZM caused severe signs of neurotoxicity and decreased protein content and ChE and CE activity. Our original hypothesis was that both insecticides could inhibit ChE because this enzyme is the main mechanism of action of these insecticides. However, CAR did not significantly decrease ChE activity. CAR only inhibited CE activity measured with p-NPB after 14 days of exposure. Other authors have also reported a higher toxicity of AZM than CAR (Kristoff et al. 2006; Kristoff 2010; Ferrari et al. 2004). However, in these cases, significant inhibition of ChE after the exposure to the carbamate was observed.
In C. gibbosa exposed to AZM, ChE activity was more sensitive than CE activity, while in organisms exposed to CAR, CE was more sensitive than ChE. CE has been frequently reported as more sensitive to OPs and carbamates than ChE in invertebrate species (Kristoff et al. 2010; Bianco et al. 2014; Wheelock et al. 2008; Agrelo et al. 2019). However, CE is not directly involved in the acute toxicity of OP and carbamates insecticides. CE can protect ChE by removing a significant amount of OPs and carbamates by two main mechanisms: by detoxification through the hydrolysis of ester bonds in some of these insecticides and by providing alternative binding sites (Sanchez-Hernandez 2007; Jokanović 2001).
Several authors have associated a greater sensitivity of CE with the absence of neurotoxic signs (Cossi et al. 2018; Anguiano et al. 2014; Otero and Kristoff 2016) and a greater sensitivity of ChE with neurotoxicity (Kristoff et al. 2006). Consistently, in our study, severe signs of neurotoxicity (lack of adherence and presence of abnormal head-foot protrusion) were observed only when organisms were exposed to AZM which produced a strong inhibition of ChE activity.
A 14-day exposure to AZM increased the toxicity previously reported after an acute (48 h) exposure in C. gibbosa (Bianco et al. 2013). At the same concentration of AZM (20 μg L−1), CE activity and protein content were not decreased after an acute exposure, but both parameters were affected after a subchronic one. In concordance with our results, Bianco et al. (2014) observed a decrease in the protein content in the gastropod Biomphalaria straminea after 21 days of exposure to AZM, and Rivadeneira et al. (2013) observed inhibition of CE in the gastropod Planorbarius corneus exposed 14 days to the OP chlorpyrifos, which had not occurred due to acute exposure (48 h). Some responses to toxicants can appear only after several days of exposure to low concentrations of pollutants, showing the relevance of studying subchronic and chronic effects of contaminants in exposed organism.
The family Chilinidae is considered vulnerable due to its restricted geographic distribution, reduction of habitat availability, aquatic contamination, presence of invasive species, and climate change (Valdovinos Zarges 2006). C. gibbosa proved to be a sensitive species when it was exposed in laboratory conditions to insecticides used in Argentina. This could imply that natural populations of this species are threatened in contaminated regions of our country. Since C. gibbosa is an important item in aquatic food chains, these effects could represent a risk at higher trophic levels in the ecosystem.
5.5 Conclusions
Insecticides applied in Argentina can cause toxic effects in the native freshwater gastropod C. gibbosa , with AZM being more toxic than CAR. The presence of these insecticides in water bodies could put this species at risk, negatively disturbing the environment.
Abbreviations
- AcSCh:
-
Acetylthiocholine iodide
- AZM:
-
Azinphos-methyl
- CAR:
-
Carbaryl
- CE:
-
Carboxylesterase
- ChE:
-
Cholinesterase
- DTNB:
-
5,5-dithio-2-bis-nitrobenzoate
- OP:
-
Organophosphate insecticide
- p-NPA:
-
p-nitrophenyl acetate
- p-NPB:
-
p-nitrophenyl butyrate
- SC:
-
Solvent control
References
Agrelo M, Rivadeneira PR, Cossi PF et al (2019) Azinphos-methyl causes in Planorbarius corneus toxic effects on reproduction, offspring survival and B-esterases depending on the exposure time. Comp Biochem Physiol C Toxicol Pharmacol 217:114–121
Anguiano OL, Castro C, Venturino A, Ferrari A (2014) Acute toxicity and biochemical effects of azinphos methyl in the amphipod Hyalella curvispina. Environ Toxicol 29:1043–1053
Baird DJ, Brown SS, Lagadic L et al (2007) In situ-based effects measures: determining the ecological relevance of measured responses. Integr Environ Assess Manag 3:259–267
Bianco K, Yusseppone MS, Otero S et al (2013) Cholinesterases and neurotoxicity as highly sensitive biomarkers for an organophosphate insecticide in a freshwater gastropod (Chilina gibbosa) with low sensitivity carboxylesterases. Aquat Toxicol 144–145:26–35
Bianco K, Otero S, Oliver AB et al (2014) Resistance in cholinesterase activity after an acute and subchronic exposure to azinphos-methyl in the freshwater gastropod Biomphalaria straminea. Ecotoxicol Environ Saf 109:85–92
Bosnia AS, Kaisin FJ, Tablado A (1990) Population dynamics and production of the freshwater snail Chilina gibbosa Sowerby 1841 (Chilinidae, Pulmonata) in a North-Patagonian reservoir. Hydrobiologia 190:97–110
Buikema AL, Niederlehner BR, Cairns J (1982) Biological monitoring part IV-toxicity testing. Water Res 16:239–262
Cacciatore LC, Verrengia Guerrero NR, Cochón AC (2013) Cholinesterase and carboxylesterase inhibition in Planorbarius corneus exposed to binary mixtures of azinphos-methyl and chlorpyrifos. Aquat Toxicol 128–129:124–134
Cacciatore LC, Verrengia Guerrero NR, Cochón AC (2018) Toxicokinetic and toxicodynamic studies of carbaryl alone or in binary mixtures with azinphos methyl in the freshwater gastropod Planorbarius corneus. Aquat Toxicol 199:276–284
Cossi PF, Boburg B, Luquet CM, Kristoff G (2015) Recovery study of cholinesterases and neurotoxic signs in the non-target freshwater invertebrate Chilina gibbosa after an acute exposure to an environmental concentration of azinphos-methyl. Aquat Toxicol 167:248–256
Cossi PF, Herbert LT, Yusseppone MS et al (2018) Environmental concentrations of azinphos-methyl cause different toxic effects without affecting the main target (cholinesterases) in the freshwater gastropod Biomphalaria straminea. Ecotoxicol Environ Saf 162:287–295
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ferrari A, Anguiano OL, Soleño J et al (2004) Different susceptibility of two aquatic vertebrates (Oncorhynchus mykiss and Bufo arenarum) to azinphos methyl and carbaryl. Comp Biochem Physiol C Toxicol Pharmacol 139:239–243
Gagneten AM, Tumini G, Imhof A, Gervasio S (2012) Comparative study of lead accumulation in different organs of the freshwater crab Zilchiopsis oronensis. Water Air Soil Pollut 223:617–624
Gutiérrez Gregoric DE (2010) Redescription of two endemic species of Chilinidae (Gastropoda: Hygrophila) from Del Plata basin (South America). J Conchol 40:321–332
Herbert LT, Castro JM, Bianchi VA et al (2018) Effects of azinphos-methyl on enzymatic activity and cellular immune response in the hemolymph of the freshwater snail Chilina gibbosa. Pestic Biochem Physiol 150:71–77
Jokanović M (2001) Biotransformation of organophosphorus compounds. Toxicology 166:139–160
Kristoff G (2010) Estudio comparativo de biomarcadores en los invertebrados acuáticos Biomphalaria glabrata y Lumbriculus variegatus expuestos a pesticidas de relevancia ambiental. Doctoral Thesis, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires
Kristoff G, Verrengia Guerrero N, de D’Angelo AMP, Cochón AC (2006) Inhibition of cholinesterase activity by azinphos-methyl in two freshwater invertebrates: Biomphalaria glabrata and Lumbriculus variegatus. Toxicology 222:185–194
Kristoff G, Guerrero NRV, Cochón AC (2010) Inhibition of cholinesterases and carboxylesterases of two invertebrate species, Biomphalaria glabrata and Lumbriculus variegatus, by the carbamate pesticide carbaryl. Aquat Toxicol 96:115–123
Krull M, Barros F, Barros K (2012) Key issues in aquatic ecotoxicology in Brazil: a critical review. J Braz Soc Ecotoxicol 7027:57–66
Loewy M, Kirs V, Carvajal G et al (1999) Groundwater contamination by azinphos methyl in the Northern Patagonic Region (Argentina). Sci Total Environ 225:211–218
Loewy RM, Monza LB, Kirs VE, Savini MC (2011) Pesticide distribution in an agricultural environment in Argentina. J Environ Sci Health B 46:662–670
Lowry OH, Rosebrough NJ, Lewis Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Oehlmann J, Schulte-Oehlmann U (2003) Bioindicators & Biomonitors - principles, concepts and applications. Trace Met other Contam Environ 6:577–635
Otero S, Kristoff G (2016) In vitro and in vivo studies of cholinesterases and carboxylesterases in Planorbarius corneus exposed to a phosphorodithioate insecticide: finding the most sensitive combination of enzymes, substrates, tissues and recovery capacity. Aquat Toxicol 180:186–195
Rivadeneira P, Agrelo M, Otero S, Kristoff G (2013) Different effects of subchronic exposure to low concentrations of the organophosphate insecticide chlorpyrifos in a freshwater gastropod. Ecotoxicol Environ Saf 90:82–88
Rumi A, Gutiérrez Gregoric DE, Núñez V, Darrigran GA (2008) Malacología Latinoamericana. Moluscos de agua dulce de Argentina. Rev Biol Trop 56:77–111
Sanchez-Hernandez JC (2007). Ecotoxicological perspectives of b-esterases in the assessment. In: Plattenberg R (ed). Environmental pollution: New research (pp 1–45). Nova Science Publishers, Inc, Nueva York, NY
Timbrell J (2000) Principles of biochemical toxicology. Taylor and Francis, London
US EPA (1976) Quality criteria for water, Washington, DC. (EPA-440/9-76-023)
Valdovinos Zarges C (2006) Estado de conocimiento de los Gastrópodos dulceacuícolas de Chile. Gayana 70:88–95
Wheelock CE, Phillips BM, Anderson BS et al (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). In: Whitacre DM (ed) Reviews of environmental contamination and toxicology (pp 117–178). Springer, New York, NY
Acknowledgments
This research was supported by grants of Universidad de Buenos Aires, PIP-CONICET and AGENCIA PICT. We would like to thank QB (Departamento de Química Biológica, FCEyN, UBA), DBBE (Departamento de Biodiversidad y Biología Experimental, FCEyN, UBA), IQUIBICEN (Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales), and CEAN (Centro de Ecología Aplicada del Neuquén). We are also grateful to L. Culasso Prinos for the support with statistical analysis.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Boburg, B., Herbert, L.T., Cossi, P., Kristoff, G. (2019). Differential Responses of Biochemical and Behavioral Parameters in the Native Gastropod Chilina gibbosa Exposed Subchronically to Environmental Concentrations of Two Insecticides Used in Argentina. In: Gómez-Oliván, L. (eds) Pollution of Water Bodies in Latin America. Springer, Cham. https://doi.org/10.1007/978-3-030-27296-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-27296-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27295-1
Online ISBN: 978-3-030-27296-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)