Abstract
The Coatzacoalcos Region in Veracruz, Mexico houses one of the most important industrial complexes in Mexico and Latin America. Lead is an ubiquitous environmental pollutant which represents a great risk to human health and ecosystems. Amphibian populations have been recognized as biomonitors of changes in environmental conditions. The purpose of this research is to measure exposure to lead and evaluate hematological and biochemical effects in specimens of giant toads (Rhinella marina) taken from three areas surrounding an industrial complex in the Coatzacoalcos River downstream. Lead levels in toads' blood are between 10.8 and 70.6 μg/dL and are significantly higher in industrial sites. We have found a significant decrease in the delta-aminolevulinic acid dehydratase (δ-ALAD) activity in blood from 35.3 to 78 % for the urban–industrial and industrial sites, respectively. In addition, we have identified a strong inverse relationship between the δ-ALAD activity and the blood lead levels (r = −0.84, p < 0.001). Hemoglobin and mean corpuscular hemoglobin levels, as well as the condition factor, are found to be lower at industrial sites compared with the reference sites. Our results suggest that the R. marina can be considered a good biomonitor of the δ-ALAD activity inhibition and hematological alterations at low lead concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
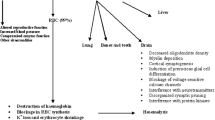
Lead is a heavy metal that due to its distribution and abundance on the earth's crust (15 to 20 mg/Kg of soil) is considered ubiquitous (ATSDR 2007). This metal has been used for a great variety of purposes during the last three centuries. Activities such as mining, refining, and casting of lead have moved great amounts of this metal from its natural deposits to the environment (Gloag 1981). Lead is a toxic element of high risk for the biota and human beings; it is classified as a carcinogen in animals and known as a possible carcinogen (group 2B) in humans (ATSDR 2007). It is not an essential element and it can be absorbed by many means; all of its forms are toxic. In addition, it gradually accumulates in the body affecting the circulatory, nervous, renal, and reproductive system (ATSDR 2007). Nowadays, the magnitude of lead exposure in Mexico and other countries from Latin America is critical due to the industrial growth in the region (Flores and Albert 2004; Trejo-Acevedo et al. 2009).
The Coatzacoalcos industrial area (in the State of Veracruz) was established during 1970s by the estuary margins of the Coatzacoalcos River and houses one of the biggest and most important petrochemical complexes of Mexico and Latin America. Its total production of petrochemical products is approximately 1.6 thousand million tons/year. Coatzacoalcos estuary has experienced a fast industrial and urban growth which combined with other productive activities such as agriculture and cattle farming have triggered a severe impact in the region's ecosystems. At present, the Coatzacoalcos River's downstream is considered one of the most polluted sites in Mexico. Environmental research in the area has shown the presence of hydrocarbon, volatile organic compounds, persistent organic pollutants, and heavy metals (Rosales-Hoz and Carranza-Edwards 1998; Bahena-Manjarrez et al. 2002; Rosales-Hoz et al. 2003; Espinosa-Reyes et al. 2010; Gonzalez-Mille et al. 2010).
The traditional approaches to the evaluation of ecosystem health around the Coatzacoalcos area have emphasized the evaluation of abiotic components (soil, sediments, water, and air), and at a lower degree, the exposure of organisms to pollutants (Ruelas-Inzunza et al. 2007, 2009). These environmental monitoring schemes have shown to be insufficient because of the lack of information about possible effects in the resident biota. From the ecotoxicological point of view, exposure to pollutants does not always result in lethal effects in the biota, but on the other hand, subtle sublethal effects can be produced at different levels of biological organization which eventually can influence the survival of the populations in the long term (Triebskorn et al. 2001). In this sense, a comprehensive approach that includes abiotic and biotic measures can provide a better understanding of possible effects of pollution on natural populations, becoming an environmental diagnostic tool to design strategies of control and prevention of pollution.
Biomonitoring wildlife can be used to detect chemical pollution as well as to evaluate the ecosystems health through using the species as systematic models in the risk evaluation associated to exposure routes and hazard assessment of chemical compounds. Wildlife species residing in polluted sites are exposed to complex mixtures of pollutants by multiple pathways which could hardly be evaluated in lab studies (Bernanke and Köhler 2009). Amphibians have been recently proposed as biomonitors of environmental conditions due to the characteristics associated with their metabolism, life cycle, and ecology (Sparling et al. 2010). During the last decades, amphibian populations have decreased worldwide; it has been argued that because of direct and indirect relations with human activities such as environmental degradation and climate (Collins and Storfer 2003). That is how monitoring amphibian populations has become relevant to evaluate the environmental pollution (Burger 2006; Bernanke and Köhler 2009) even though there has been a little emphasis on the evaluation of sublethal effects (Venturino et al. 2003).
There are relatively few studies concerning toxic effects of compounds, such as lead, in adult amphibians (Linder et al. 2003; Sparling et al. 2010), but some lab studies have shown alterations in the synthesis of the heme group through inhibition of the activity of the enzyme delta-aminolevulinic acid dehydratase (δ-ALAD) affecting the synthesis of protoporphyrin IX and hemoglobin (Perí et al. 1998; Arrieta et al. 2000; 2004). The inhibition of the δ-ALAD activity is one of the most important biomarkers and a well-known indicator of lead toxicity (ATSDR 2007), and it has been described in human beings and other vertebrates. At the same time, in adult anura that have been exposed to lead, other effects have been found such as an increase in erythrocytes' osmotic fragility (Rosenberg et al. 1998), increase in levels of erythrocyte protoporphyrin (Arrieta et al. 2004), alterations in the serum profile of proteins and in the count of blood cells (Perí et al. 1998), oxidative damage (Wang and Jia 2009), micronucleus induction and nuclear abnormalities (Zhang et al. 2007), hypoxia (Rice et al. 1999), growth and development delay (Pérez-Coll et al. 1988), skeletal malformations (Te-Hao et al. 2006), behavioral alterations (Strickler-Shaw and Taylor 1991), and reduction in survival and recruitment of juveniles (Rowe et al. 2001).
The giant toad (Rhinella marina, after Bufo marinus) is a native and geographically widespread species in Mexico and Central America (Zug and Zug 1979). It is an omnivorous and opportunistic species (Zug and Zug 1979), which indicates that toads would reflect different exposure routes due to the ingestion of a wide variety of food items and amphibious living habits. The giant toad is one of the largest amphibians in Mexico (adult body length ranges from 10 to 17 cm), with a life expectancy of 10 to 15 years in the wild. The high lipid somatic index (2 to 10 % compared to less than 0.1 % in most anuran species after the spawning period) and the elevated hepatosomatic index (Feder and Burggren 1992) along with its breeding biology make this species prone to bioaccumulation of organic and inorganic pollutants and their toxicological effects (Linder et al. 2003; Sparling et al. 2010). Recently, the giant toad has been used as an aquatic ecosystem biomonitor in the evaluation of air pollution (Dohm et al. 2008), infectious diseases (Zupanovic et al. 1998), organochlorine pesticides (Linzey et al. 2003), and endocrine disruptors (McCoy et al. 2008).
The purpose of this research is to measure lead exposure and sublethal effects in giant toad specimens (R. marina) taken from the Coatzacoalcos River downstream in order to obtain an indication of adverse health effects present in the region's wildlife. Lead is evaluated as a priority pollutant. δ-ALAD activity, hematologic parameters (hemoglobin, mean corpuscular hemoglobin, and hematocrit), and the condition factor are used as stress biological indicators in these amphibians.
Materials and methods
Study area and sampling sites
The Coatzacoalcos River downstream area is located in the southeast of Veracruz, Mexico (18°08′56″ N; 94°24′41″ W)—an area that is mainly formed by urban, industrial, livestock, and agricultural areas that are immersed in wetlands. The region's wetlands are fed by the Coatzacoalcos River which is 322 km long, starting in the State of Oaxaca and going down to its mouth, draining an area of about 21,120 km2.
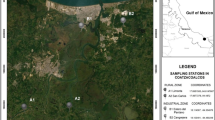
The sampling was done in seven adjacent sites to the Coatzacoalcos River in May 2008 (Fig. 1). In order to make comparisons, the sites were clustered according to the productive activity and degree of urbanization. The sites' characteristics are: (a) industrial sites: They are located near industrial areas and have a limited urban development. 1—Ejido Cangrejera (communal land): This site borders with the Pajaritos and Cangrejera petrochemical complexes where ethylene- and chlorine-derived compounds are produced as well as vinyl chloride monomer (MCV) and volatile organic compounds (COV). 2—Santa Alejandrina: adjacent site to the “General Lázaro Cárdenas del Río” refinery in Minatitlán, where energy products (220,000 barrels/day) and petrochemical products (2,500 barrels/day, PEMEX 1999) are produced. (b) Urban–industrial sites: They are located close to industrial complexes and show a significant urban development. 3—Estero del Pantano: It is located by the banks of the Calzadas River, 15 km east of the Pajaritos and Cangrejera petrochemical complexes; the river receives wastewater from close urban and industrial areas. 4—Mundo Nuevo: It is located 10 km south of the Pajaritos petrochemical complex. At this site, important pollutants such as dioxins and hexachlorobenzene have been found (Stringer et al. 2001). 5—Nanchital: It is located by the edge of the Nanchital stream, 10 km southeast of the Minantitlán's refinery; it is well known for frequent hydrocarbon spills. (c) Rural sites: They lack industrial activities and have a very limited urban development, with ecosystems that seem to be well-preserved. 6—San Carlos: It is located by the Uxpanapa River's banks, one of the Coatzacoalcos River tributaries, and it is considered as one of the main tributaries for the region's wildlife preservation. 7—Las Palomas: It is located upstream of the Coatzacoalcos River, in the municipality of Hidalgotitlán, where there is scarce urbanization and holds well-preserved ecosystems. During this study, rural sites were used as reference sites with the purpose to compare them with sites with industrial influence.
Biological and environmental sampling
Ten sexually mature toads, weighing an average of 183.8 g (±8.6) and an average snout vent length (SVL) of 12.5 cm (±0.1) were collected from each site using nets in nocturnal transects within an area of 10,000 m2 and transported to the laboratory. In the laboratory, 5 mL of blood was collected using cardiac puncture with heparinized syringes and stored in Vacutainer® tubes with sodium heparin. Aliquots of 1.5 mL of whole blood were put in cryogenic vials, flash frozen in liquid nitrogen and stored at −80°C for later analysis of δ-ALAD enzymatic activity and lead levels in blood. All toads were collected with authorization of the Natural Resources and Environment Secretariat of Mexico (SEMARNAT-No. FAUT-0133). Three soil samples were taken within the same toad location areas. Surface soil samples (1–5 cm) were obtained with a stainless steel scoop on approximately 1 m2 surface area and stored in polyethylene bags for further analysis.
Soil lead concentration
Soil samples were oven-dried at 30°C for 1 to 2 days. The <600-μm fraction was separated with a sieve (28 mesh Tyler Series). The samples were acid digested (50 % HNO3) for 60 min at 80 psi using a microwave extraction system (CEM MDS-2000). After digestion, the extracted solution was filtered through a filter paper (Whatman 1). Analyses for lead were carried out in an atomic absorption spectrophotometer Perkin-Elmer 3110 coupled with a graphite furnace (HGA 600, Perkin-Elmer). A primary standard reference material (NIST-SRM 2711-Montana soil) was included in each run as an internal control for quality purposes. Recoveries of standard reference materials were 97 to 98 %. Lead concentrations in soil samples were expressed in milligrams per kilogram dry weight.
Blood lead concentration
Blood lead levels were determined following the protocol by Subramanian (1989). In brief, a matrix modifier (diammonium hydrogen phosphate-Triton X-100 in the presence of 0.2 % of nitric acid) was mixed with samples in a 1:5 proportion. Standard reference material (bovine whole blood—CDC08PB06) was included in each run as an internal quality control, with recoveries ranging from 91 to 100 %. Blood samples were analyzed with an atomic absorption spectrophotometer (Perkin-Elmer 3110) coupled with a graphite furnace (HGA 600, Perkin-Elmer). Lead concentration in blood samples was expressed in micrograms per deciliter. Lead concentrations in soil and toad blood samples were quantified in the Toxicology Laboratory of the Medicine School at the Autonomous University of San Luis Potosí. This laboratory currently participates in the Blood Lead Proficiency Testing Program of Centers for Disease Control and Prevention in Atlanta.
δ-ALAD activity
The activity of enzyme delta-aminolevulinic dehydratase was determined according to the standardized European method (Berlin and Schaller 1974) with minor modifications. A 0.05-mL blood aliquot was mixed with 1.45 mL of deionized water (30:1 water/blood dilution ratio) adding 1 mL of aminolevulinic acid 10 mM in a phosphate buffer adjusted to a 6.4 pH. The samples were incubated in a wet bath at 38°C for 60 min. The reaction was stopped with trichloroacetic acid (10 %); later on, samples were centrifuged for 10 min at 2,000 rpm (Hettich Universal 32). The supernatant was mixed with Erlich's solution (1:1). After 10 min, absorbance was measured at 555 nm in a UV–Visible spectrophotometer (Barnstead/Thermolyne SP8001). The enzyme activity was expressed as units per liter of erythrocytes (RBC) per minute and it was calculated according to the next equation:
where Abs = absorbance of sample, 2 = (δ-ALAD conversion factor to porphobilinogen), DF = dilution factor, 60 = incubation time (minutes), and 0.062 = extinction coefficient (in liters per micromole × centimeter), and Htc = hematocrit (percent).
Hemoglobin, hematocrit, and corpuscular hemoglobin determination
Hematocrit (percent of whole blood volume occupied by packed red cells) was determined by centrifuging blood in heparinized capillary tubes (50 μL) at 7,000 rpm for 5 min in a hematocrit centrifuge (Sol Bat M-08). Measurement was done with a hematocrit standard chart (Critops® tube reader). The hemoglobin content (in grams per deciliter) was measured using a diagnostic kit (HemoCueHb 201 Microcuvettes® and HemoCueHb 201 Analyzer®). The test was performed according to the protocol of the instrument operation manual. The mean corpuscular hemoglobin concentrations (hemoglobin in a given volume of packed red blood cells) were calculated to integrate the levels of hematocrit and hemoglobin according to the formula:
All chemicals used were of analytical grade. All measurements were carried out in duplicate and the mean was reported.
Condition factor (K)
The SVL was measured using a digital caliper (near to 0.01 mm), and the eviscerated weight (EW) with an electronic balance (0.1 g precision). The condition factor (K) was calculated with the following formula:
Statistical analysis
The differences between lead concentration in soil and blood, as well as the hematological parameters and condition index by type of site were evaluated using a one-way analysis of variance (one-way ANOVA) followed by a test of multiple comparisons of Fisher means (Fisher's LSD). The relation among the lead levels in blood (transformed to logarithm to linearize the variable) and the lead concentrations in soil and the activity of δ-ALAD and MCHC in erythrocytes were analyzed through the Pearson association test (r), verifying the significance with the ANOVA. The significance was determined in 5 and 1 %. The analyses were performed with the software STATISTICA 8.0 (StatSoft, Inc. 2007).
Results and discussion
Lead in soil
Lead concentrations in soil at the Coatzacoalcos River research area vary from 4.7 to 23.5 mg/Kg. According to the classification by type of site, the lead concentrations in soil (mean ± SE) of the rural, urban–industrial, and industrial sites were 10.2 ± 0.9, 9.3 ± 1.3, and 13.8 ± 1.9 mg/Kg, respectively. Nonsignificant differences of lead levels in soil were registered (p = 0.09) among the sites, probably explained by the small sample size and high variability of data.
However, an additional explanation is possible. It has been demonstrated that organic matter influenced heavy metal absorption in soils due to the low caption exchange capacity of organic material (Kabata-Pendias 2007). The high soil organic matter content is favorable for heavy metal absorption in soils (Kabata-Pendias 2007). Dominant soil types in the Coatzacoalcos Region are histosols, gleysols, cambisols, and vertisols with high organic material contents; therefore, it is possible that the low lead concentrations in soils studied in our work are due to the rapid turnover of organic material in the area. Lead concentrations in soil were low compared with national guidelines (NOM-147-SEMARNAT/SSA1-2004) and international ones (Canadian Sediment Quality Guidelines for the Protection of Aquatic Life and screening quick reference tables of the National Oceanic and Atmospheric Administration), which would suggest that lead levels in soil represent a low risk for the environment.
Lead in blood
Lead levels in toads' blood are between 10.8 and 70.6 μg/dL; those from urban–industrial and industrial sites were significantly higher (F 2, 67 = 22.711, p < 0.001) than the levels of toads from rural sites (Table 1). The increase of lead concentration in toads' blood from urban–industrial and industrial sites versus the ones from rural sites was from 180 to 468 %, respectively.
The metabolic rate and physiological characteristics of poikilotherms are correlated with a long red cell life span. The mean life span of the giant toad red cells probably lies between 700 and 1,400 days (Altland and Brace 1962). This suggests that lead levels in the toads' blood reflected lead input approximately 46 months ago. Also, lead concentration in blood reflects the redistribution during the course of long-term exposures. Therefore, lead in toads' blood can be considered a biomarker that reflected a chronic exposure by a punctual and constant lead input to the environment.
Even though the lead concentration in blood is the most common used indicator of exposure, there is little information on this biomarker, and as a result, comparison with other studies is difficult. Literature reports some cases which illustrate lead accumulation in wildlife amphibian tissues related to pollution intensity originated from industrial sources or agriculture. Linzey et al. (2003) reported values of 3.07–3.15 μg/Kg in skin and 0.05–0.06 μg/Kg in muscles of giant toads collected in agricultural sites of Bermuda; Lee and Stuebing (1990) showed lead accumulation in toads' liver (Bufo yuxztaper) collected at the proximities of a copper mine. Research conducted by Arrieta et al. (2001) on South American toads (Bufo arenarum) collected near the petrochemical industry at La Plata, Argentina showed blood lead intervals from 1.99 to 4.66 mg/dL; furthermore, the levels did not present relevant variations during the sampling period (3 years). Arrieta et al. (2004, 2000) reported blood lead levels from 0.76 to 8.8 mg/dL in lead exposure experimental studies. Our results show blood lead levels from 70 to 125 times lower than those found in these studies.
Possible lead sources
Studies conducted by Rosales-Hoz et al. (2003, 2005) concluded that the intense industrialization of the Coatzacoalcos River is associated with the increase of metal concentrations in sediment (including lead at concentrations from 0.02 to 78.7 mg/Kg) in comparison with samples taken from the industrialized area upstream. Ruelas-Inzunza et al. (2007) reported lead concentrations from 0.2 to 5.4 μg/g in muscles of four fish species from the Coatzacoalcos Region, where most concentrations were present in the collected species in the sites with most industrial influence. Pelallo-Martínez et al. (2011) found an increase of lead in the blood of turtles (Trachemys scripta) and children associated to the location around the industrial complex. Currently, the Pollutant Transfer and Emission Records (by its acronym in Spanish) published by SEMARNAT (2004–2009) reports an estimate of 1.8 t/year of lead compounds released in the environment (air and water) by chemical and petrochemical industries located in the municipalities of Coatzacoalcos and Minatitlán. It is worth mentioning that before 1997, Tetraetilo de Mexico (TEMSA), the only company in Mexico that produced lead tetraethyl (used as antiknock additive for gasoline), operated and it could have been an important industrial source for the region (Flores and Albert 2004).
Based on the results obtained in this study, we can establish an increase in pattern of lead levels in blood in the industrial sites (Table 1). Our results agree with the findings of other authors who studied in the area both in environmental samples (Rosales-Hoz and Carranza-Edwards 1998; Rosales-Hoz et al. 2003) and biological ones (Ruelas-Inzunza et al. 2007; Pelallo-Martínez et al. 2011). According to these results, we can infer that there are current sources of emission at the industrialized areas and/or probably the existence of residual lead as a result of historical industrial activities performed in the area.
Exposure pathways
Our data show a positive and statistically significant correlation (r = 0.82, p < 0.05, n = 6) between lead concentrations in soil and the lead content in blood (Fig. 2). Various studies on food preferences of the giant toad have identified a great variety of stomach contents, as well as rock fragments and soil particles (Zug and Zug 1979). According to Gans and Gorniak (1982), during the feeding movement of the giant toad (lingual flip), the tongue gets in direct contact with the soil, which explains the presence of soil particles in the stomach contents and also suggests that accidental ingestion of soil can be considered an important pathway to lead exposure. However, the relatively low lead concentrations in soil at the study area suggest that there could be other important exposure pathways which are not evaluated in this study.
In drought seasons, water levels of the Coatzacoalcos River tend to decrease considerably and sediments on the riverbanks can be exposed and this generates beaches where amphibians can feed themselves as well as dry sediment particles can be transported by the wind. In addition, it must be mentioned that these amphibians build galleries on the riverbanks to protect themselves from the sun during the day getting directly in touch with sediment particles.
In preliminary studies in the zone, we have found the presence of lead in sediment (2.8–57.8 mg/Kg, unpublished results) and in dust particles (32.0–549.0 mg/Kg, unpublished results). This is particularly important because organisms can be exposed to metal in sediment from early stages of development and later on in adult stages from breathing particles with lead. Rosales-Hoz and Carranza-Edwards (2005) found that bioavailable fractions of heavy metals in sediment samples from Coatzacoalcos present a high lead bioavailability (26 %) in comparison to the other evaluated metals. On the other hand, the air-breathing rate for giant toad is high compared with other species of anurans (Dohm et al. 2008) and the exposure to airborne lead particles could be important if we consider the dust lead levels mentioned above.
The characteristic of the giant toad is that it is a species with voracious appetite; in eating behavior researches (feeding behavior), a wide prey variety within their stomach content has been reported (including from insects to congeners; Pizzatto and Shine 2008). Their preys' lead contribution and their respective exposure sources (air, soil, sediments, and water) have not been evaluated yet but it may be relevant because of the high rate of prey intake of these organisms (up to 50 preys/toad/day; Zug and Zug 1979).
It is known that age has an influence in biological levels of environmental pollutants. As it was mentioned before, blood lead levels can reflect the redistribution of this metal from deposits such as bone and other tissues (liver and kidney). Taking into account the high life expectation in wildlife (10–15 years) and this species' low mobility range (home range of 160 m2, Zug and Zug 1979), it is possible that differences found between blood lead levels in this study may be explained through differences in exposure during the species' early development and the exposure at the long term at low lead concentrations in the environment (as those reported in several studies in the area) bringing as a result high tissue residues late in life (Hopkins 2006).
δ-ALAD activity
In Table 1, a significant decrease in δ-ALAD activity in toads from urban–industrial and industrial sites is shown, in comparison with toads from rural zones (F 2, 67 = 50.425, p < 0.001). The decrease in the δ-ALAD activity in blood was from 35.3 to 78 % for the urban–industrial and industrial sites, respectively. Our results show a significant strong decreasing relationship (r = −0.84, p < 0.001, n = 70) between the δ-ALAD activity and the concentration of lead in blood (Fig. 3a).
The pattern of enzyme inhibition observed in our results matches what is registered in adult amphibians exposed to lead in an experimental way (Perí et al. 1998; Arrieta et al. 2000). These works have found inhibitions of the δ-ALAD activity from 27 to 90 % in relation to the controls, showing a clear pattern of concentration–effect. Previous studies and our results here show that the inhibition of the δ-ALAD activity can be considered a biomarker of the exposure to lead in adult amphibians which is applicable in lab conditions and in field ones. Lead levels in blood which cause a significant inhibition in the δ-ALAD activity in studies done by Arrieta et al. (2000, 2004) and Perí et al. (1998a) in B. arenarum are higher (7.26–8.8 mg/dL) in comparison with the levels established in our work (IC50 = 7.04 μg/dL). Activity δ-ALAD inhibition at low lead exposure concentrations (background levels) as the one in this study has been reported in several studies with wildlife birds (Martínez-López et al. 2010; Gómez-Ramírez et al. 2011; Martinez-Haro et al. 2011); these have proven inhibitions from 30 to 55 % to concentrations from 4 to 15 μg/dL, which suggest that the giant toad (R. marina) could be a good biomonitor of the enzyme inhibition at low concentrations.
Hematological parameters
Hemoglobin concentrations (F 2, 67 = 3.251, p < 0.05) and the values of mean corpuscular hemoglobin concentration (F 2, 67 = 7.398, p < 0.05) in toads' blood from rural sites were significantly higher than those from industrial and urban–industrial sites (Table 1). The decrease in the hemoglobin concentration in blood and the mean corpuscular hemoglobin concentrations for toads of the urban–industrial and industrial sites was from 11.1 to 17.8 and from 9.8 to 10.5 %, respectively. There were no statistical differences found in the levels of hematocrit in blood (F 2, 67 = 0.431, p > 0.05; Table 1). A decrease in the activity δ-ALAD concentration (excluding hematocrit) derived from lead exposure has been reported in B. arenarum (Rosenberg et al. 1998; Arrieta et al. 2000).
There are no reference values on “normal” intervals of the hematological parameters in the giant toad; however, for the first time, Hall (1966) described the hemoglobin functions in the giant toad and reported some hematological parameters that could be used as reference. According to this information, the percentage of toads from the Coatzacoalcos Region with hemoglobin values below the optimum increases in the order: rural (20 %) < urban–industrial (56.7 %) < industrial (80 %). A percentage from 23 to 25 % of toads with abnormal hematocrit values without an apparent impairment pattern by site type was also found (Table 2).
Although nonsignificant association was found between lead levels in blood (log transformed) and hemoglobin (r = 0.18, p = 0.137, n = 70), we found a statistically significant negative association (r = −0.41, p < 0.001, n = 70) between lead levels in the blood (log transformed) and mean corpuscular hemoglobin concentration (Fig. 3b). Media corpuscular hemoglobin is a relevant auxiliary indicator in microcytic anemia diagnosis that is a characteristic of lead poisoning. Decrease pattern of hemoglobin concentration and media corpuscular hemoglobin by site can be interpreted as a secondary anemia in toads with high lead levels in blood, especially in sites with industrial influence as a direct consequence of the decrement of the synthesis of hemoglobin by the inhibition of the enzyme activity of δ-ALAD.
It is known that exposure to lead in amphibians causes anemia and hypoxic conditions due to the decrease in hemoglobin levels (Rice et al. 1999). Physiological responses that lead to hypoxia in vertebrates are widely documented and are generally centered in two adaptive mechanisms: the increase in the volume of ventilation and the increase of the carrying capacity of oxygen (O2) of blood (Brauner and Wang 1997). During hypoxia, in many vertebrates, including amphibians, the oxygen-carrying capacity increases by increasing the number of red blood corpuscles (a phenomenon known as polycythemia); the polycythemia degree and its duration depend on environmental conditions, while some other cases have been reported where this mechanism has not shown significant changes in the hematocrit (Brauner and Wang 1997). Our results show a positive correspondence (r = 0.31, p < 0.01, n = 70) between lead levels in blood (log transformed) and the hematocrit (Fig. 3c). The increase pattern in the hematocrit and other hematological parameters has been registered in experimental studies on giant toads with anemia and exposed to different degrees of hypoxia (Wood 1990; Pörtner et al. 1991; Wood and Malvin 1991; Andersen et al. 2003). We suggested that the increase of hematocrit as a function of lead in blood can be interpreted as a mechanism of compensation to the anemic hypoxia.
As previously mentioned, in the downstream region of the Coatzacoalcos River, the presence of pollutant mixes has been proven (Rosales-Hoz and Carranza-Edwards 1998; Bahena-Manjarrez et al. 2002; Rosales-Hoz et al. 2003; Espinosa-Reyes et al. 2010; Gonzalez-Mille et al. 2010); this is particularly important because in addition to lead, these pollutants may be contributing with the observed hematological and biochemical effects. Barni et al. (2007) found several hematological alterations and nuclear abnormalities in green frogs (Rana esculenta) derived from the exposure to persistent organic pollutants, heavy metals, fertilizers, and organic matter. Similar results to the ones from this study were found in neotropical fish (Corydoras paleatus) by Cazenave et al. (2005) where the increase of hematocrit, hemoglobin, and media corpuscular hemoglobin was related with the organic enrichment and hypoxia conditions in a polluted river. Folmar (1993) compiled several studies on fish that are related to changes in hematological parameters due to the exposure of pollutant mixes (PCBs, HAPs, heavy metals, and pesticides).
Because of ethical and preservation reasons, the development and use of nondestructive biomarkers in wildlife organisms have been promoted (Fossi 1994; Fossi and Marsili 1997). Hematological and biochemical parameters evaluated in this study may be very useful in field conditions because a greater number of samples can be obtained with the minimum of stress for the animal and its population (it is not necessary to kill the animals); another advantage is that it is possible to follow the organisms in time and space, providing priceless information over the efficiency of remedy program applications as well as other intervention measures in polluted sites. It must be mentioned that the biomarkers evaluated in this study may be useful for the establishment of threshold levels of lead exposure for hematological effects in amphibians and their later application in risk evaluation (Buekers et al. 2009).
Condition factor
We found a decrease of 14.4 % in the condition factor in toads from industrial sites in relation to those from rural sites (F 2, 67 = 3.381, p < 0.05, Table 1). The decrease in the condition factor is generally interpreted as a decrease in the energy of reserve stored in fat and liver with possible negative implications for the growth, survival, and reproduction of organisms (Stevenson and Woods 2006). Diverse studies in aquatic invertebrates, fish, and birds show a significant decrease in the condition factor in organisms exposed to environmental pollutants. The decrease in conditions rates in toads from industrial sites can be due to the fact that some pollutants create alterations in the food intake rate and the metabolic rate, as well as in the absorption of nutrients in the gastrointestinal tract (Rice et al. 1999).
There are a few studies concerning the lead effects in amphibian nutrition and metabolism; however, it has been reported that this metal can cause changes in the digestive tract, and as a result, can alter the absorption of nutrients, as well as increase the demand of oxygen due to energetic costs to repair the damages (Rice et al. 1999). During hypoxic conditions, ectothermic organisms response to stress with a decrease in preferred temperature (P T), followed by a reduction in body temperature—T b, considered by some authors as hypothermia (Wood 1991; Wood and Malvin 1991; Feder and Burggren 1992; Wood and Gonzales 1996)—causing the reduction of consumption of O2. Hypothermia as a behavioral response to hypoxia and anemia has been widely documented in giant toads (Pörtner et al. 1991; Wood 1991; Wood and Malvin 1991; Wood and Gonzales 1996). Wood (1990) found a direct relation between the levels of hematocrit and the selection of P T, proving that anemia can lead to hypothermia in these organisms. The most significant effect of hypothermia is the decrease in the metabolic rate, which could have direct effects in the diet and the condition factor because of the reduction of the intake rate, an increase in the physiological stress (production of catecholamine and lactate), and alterations in the locomotor behavior (escape and search of food) finally putting at risk the survival of the organism (Feder and Burggren 1992; Wood and Gonzales 1996; Chapman and McKenzie 2009). It must be said that the low availability of food can also be a decisive factor influencing sites with high anthropogenic impact; however, this scenario has not been researched.
Conclusion
We conclude that lead concentrations in blood, the enzyme δ-ALAD activity, and the hematological parameters in the giant toad (R. marina) can be used as sensitive biomarkers of lead exposure in field conditions. The physiological, ecological, and ecotoxicological characteristics of the giant toad make them good candidates for pollution biomonitors of lead in environmental monitoring programs for the Coatzacoalcos Region and other tropical regions in Mexico and South America. Even when the lead levels in soil and sediment do not go over the established limits by the Mexican normative and international guides, the concentrations in the environment found in this study represent a high exposure for the giant toad populations living in the region. This study offers a baseline for the status of lead pollution in the Coatzacoalcos industrial region and the sublethal effects of that metal in the wild populations of the giant toad. We consider that more studies are required in the amphibian populations and other wild organisms in Coatzacoalcos, and in particular, about the establishment of the toxic levels of reference (TRVs) based on the lead levels in blood, the identification of possible exposure pathways to that metal and to other pollutants in the aquatic and land systems, and the evaluation of various effects of ecological relevance (example: genetic, neurologic, and endocrine), with the purpose to identify and quantify the associated risks in the populations of amphibians in the region, as well as their implications in human health and ecosystems.
References
Altland, P. D., & Brace, K. C. (1962). Red cell life span in the turtle and toad. The American Journal of Physiology, 203, 1188–1190.
Andersen, J. B., Hedrick, M. S., & Wang, T. (2003). Cardiovascular responses to hypoxia and anaemia in the toad Bufo marinus. The Journal of Experimental Biology, 206, 857–865.
Arrieta, M. A., Peri, S. I., Apartin, C., Rosenberg, C. E., Fink, N. E., & Salibian, A. (2000). Blood lead concentration and delta-aminolevulinic acid dehydratase activity in adult Bufo arenarum. Archives of Physiology and Biochemistry, 108, 275–280.
Arrieta, M. A., Apartin, C., Rosenberg, C. E., Fink, N. E., & Salibian, A. (2001). Blood lead content in peri-urban population of the South American toad Bufo arenarum. The Science of the Total Environment, 271, 99–105.
Arrieta, M. A., Bruzzone, L., Apartín, C., Rosenberg, C. E., Fink, N. E., & Salibián, A. (2004). Biosensors of inorganic lead exposure and effect in an adult amphibian. Archives of Environmental Contamination and Toxicology, 46, 224–230.
ATSDR (2007). Toxicological profile for lead. Agency for Toxic Substances and Disease Registry.
Bahena-Manjarrez, J. L., Rosales-Hoz, L., & Carranza-Edwards, A. (2002). Spatial and temporal variation of heavy metals in a tropical estuary. Environmental Geology, 42, 575–582.
Barni, S., Boncompagni, E., Grosso, A., Bertone, V., Freitas, I., Fasola, M., et al. (2007). Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquatic Toxicology, 81, 45–54.
Berlin, A., & Schaller, K. H. (1974). European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Zeitschrift für Klinische Chemie und Klinische Biochemie, 12, 389–390.
Bernanke, J., & Köhler, H. R. (2009). The impact of environmental chemicals on wildlife vertebrates. Reviews of Environmental Contamination and Toxicology, 198, 1–47.
Brauner, C. J., & Wang, T. (1997). The optimal oxygen equilibrium curve: a comparison between environmental hypoxia and anemia. American Zoologist, 37, 101–108.
Buekers, J., Redeker, E. S., & Smolders, E. (2009). Lead toxicity to wildlife: derivation of a critical blood concentration for wildlife monitoring based on literature data. The Science of the Total Environment, 407, 3431–3438.
Burger, J. (2006). Bioindicators: types, development, and use in ecological assessment and research. Environmental Bioindicators, 1, 22–39.
Cazenave, J., Wunderlin, D. A., Hued, A. C., & Bistoni, M. A. (2005). Haematological parameters in a neotropical fish, Corydoras paleatus (Jenyns,1842) (Pisces, Callichthyidae), captured from pristine and polluted water. Hydrobiologia, 537, 25–33.
Chapman, L. J., & McKenzie, D. J. (2009). Behavioral responses and ecological consequences. In J. G. Richards, A. P. Farrell, & C. J. Brauner (Eds.), Fish physiology: hypoxia (pp. 25–77). New York: Academic.
Collins, J. P., & Storfer, A. (2003). Global amphibian declines: sorting the hypotheses. Diversity and Distributions, 9, 89–98.
Dohm, M. R., Mautz, W. J., Doratt, R. E., & Stevens, J. R. (2008). Ozone exposure affects feeding and locomotor behavior of adult Bufo marinus. Environmental Toxicology and Chemistry, 27, 1209–1216.
Espinosa-Reyes, G., Ilizaliturri, C. A., González-Mille, D. J., Costilla, R., Díaz-Barriga, F., Cuevas, M. C., et al. (2010). DNA damage in earthworms (Eisenia spp.) as an indicator of environmental stress in the industrial zone of Coatzacoalcos, Veracruz, Mexico. Journal of Environmental Science and Health Part A, 45, 49–55.
Feder, M. E., & Burggren, W. W. (1992). Environmental physiology of the amphibians. Chicago: Chicago Press. University of Chicago.
Flores, J., & Albert, L. A. (2004). Environmental lead in Mexico, 1990–2002. Reviews of Environmental Contamination and Toxicology, 181, 37–109.
Folmar, L. C. (1993). Effects of chemical contaminants on blood chemistry of teleost fish: a bibliography and synopsis of selected effects. Environmental Toxicology and Chemistry, 12, 337–375.
Fossi, M. C. (1994). Nondestructive biomarkers in ecotoxicology. Environmental Health Perspectives, 102, 49–54.
Fossi, M. C., & Marsili, L. (1997). The use of non-destructive biomarkers in the study of marine mammals. Biomarkers, 2, 205–216.
Gans, C., & Gorniak, G. C. (1982). Functional morphology of lingual protrusion in marine toads (Bufo marinus). The American Journal of Anatomy, 163, 195–222.
Gloag, D. (1981). Sources of lead pollution. British Medical Journal (Clinical Research Ed), 282, 41–44.
Gómez-Ramírez, P., Martínez-López, E., María-Mojica, P., Leó-Ortega, M., & García-Fernández, A. J. (2011). Blood lead levels and δ-ALAD inhibition in nestlings of Eurasian eagle owl (Bubo bubo) to assess lead exposure associated to an abandoned mining area. Ecotoxicology, 20, 131–138.
Gonzalez-Mille, D. J., Ilizaliturri-Hernández, C. A., Espinosa-Reyes, G., Costilla-Salazar, R., Díaz-Barriga, F., Ize-Lema, I., et al. (2010). Exposure to persistent organic pollutants (POPs) and DNA damage as an indicator of environmental stress in fish of different feeding habits of Coatzacoalcos, Veracruz, Mexico. Ecotoxicology, 19, 1238–1248.
Hall, F. G. (1966). Hemoglobin functions in the blood of Bufo marinus. Journal of Cellular Physiology, 68, 69–73.
Hopkins, W. (2006). Use of tissue residues in reptile ecotoxicology: a call for integration and experimentalism. In S. Gardner & E. Oberdorster (Eds.), Toxicology of reptiles (pp. 35–62). Boca Raton: CRC Press. Taylor and Francis Group.
Kabata-Pendias, A. (2007). Trace elements in soils and plants (3rd ed.). Boca Raton: CRC Press.
Lee, Y. H., & Stuebing, R. B. (1990). Heavy metal contamination in the river toad, Bufo juxtasper (Inger), near a copper mine in East Malaysia. Bulletin of Environmental Contamination and Toxicology, 45, 272–279.
Linder, G. L., Krest, S. K., & Sparling, D. W. (2003). Amphibian decline: an integrated analysis of multiple stressor effects. Pensacola: Society of Environmental Toxicology and Chemistry (SETAC).
Linzey, D., Burroughs, J., Hudson, L., Marini, M., Robertson, J., Bacon, J., et al. (2003). Role of environmental pollutants on immune functions, parasitic infections and limb malformations in marine toads and whistling frogs from Bermuda. International Journal of Environmental Health Research, 13, 125–148.
Martinez-Haro, M., Green, A. J., & Mateo, R. (2011). Effects of lead exposure on oxidative stress biomarkers and plasma biochemistry in waterbirds in the field. Environmental Research, 111, 530–538.
Martínez-López, E., Sousa, A. R., María-Mojica, P., Gómez-Ramírez, P., Guilhermino, L., & García-Fernández, A. J. (2010). Blood δ-ALAD, lead and cadmium concentrations in spur-thighed tortoises (Testudo graeca) from Southeastern Spain and Northern Africa. Ecotoxicology, 19, 670–677.
McCoy, K. A., Bortnick, L. J., Campbell, C. M., Hamlin, H. J., Guillette, L. J., & St Mary, C. M. (2008). Agriculture alters gonadal form and function in the toad Bufo marinus. Environmental Health Perspectives, 116, 1526–1532.
Pelallo-Martínez, N. A., Ilizaliturri-Hernández, C. A., Espinosa-Reyes, G., Carrizales-Yáñez, L., & González-Mille, D. J. (2011). Assessment of exposure to lead in humans and turtles living in an industrial site in Coatzacoalcos Veracruz, Mexico. Bulletin of Environmental Contamination and Toxicology, 86, 642–645.
PEMEX. (1999). La primera refinería de Latinoamérica Refinería Gral. Lázaro Cárdenas. Revista octanaje. Petróleos Mexicanos (PEMEX). Available from http://www.ref.pemex.com/octanaje/23laza.htm. Accessed 3 June 2012.
Pérez-Coll, C. S., Herkovits, J., & Salibián, A. (1988). Embryotoxicity of lead on Bufo arenarum. Bulletin of Environmental Contamination and Toxicology, 41, 247–252.
Perí, S. I., Arrieta, M. A., Fink, N. E., & Salibián, A. (1998). Delta-aminolevulinic acid dehydratase (ALAD) activity in blood of Bufo arenarum (Anura). Biological Research, 31, 339–342.
Perí, S. I., Fink, N. E., & Salibián, A. (1998). Hematological parameters in Bufo arenarum injected with sublethal dose of lead acetate. Biomedical and Environmental Sciences, 11, 70–74.
Pizzatto, L., & Shine, R. (2008). The behavioral ecology of cannibalism in cane toads (Bufo marinus). Behavioral Ecology and Sociobiology, 63, 123–133.
Pörtner, H. O., MacLatchy, L. M., & Toews, D. P. (1991). Metabolic responses of the toad Bufo marinus to environmental hypoxia: an analysis of the critical PO2. Physiological Zoology, 64, 836–849.
Rice, T. M., Blackstone, B. J., Nixdorf, W. L., & Taylor, D. H. (1999). Exposure to lead induces hypoxia-like responses in bullfrog larvae (Rana catesbeiana). Environmental Toxicology and Chemistry, 18, 2283–2288.
Rosales-Hoz, L., & Carranza-Edwards, A. (1998). Heavy metals in sediments from Coatzacoalcos River, Mexico. Bulletin of Environmental Contamination and Toxicology, 60, 553–561.
Rosales-Hoz, L., & Carranza-Edwards, A. (2005). Estudio geoquímico de metales en el estuario del Río Coatzacoalcos. In: V. A. Botello, J. Rendón-Von Osten, G. Gold-Bouchot, C. Agraz-Hernández (Eds.) Golfo de México contaminación e impacto ambiental: diagnostico y tendencias (pp. 389–406). Universidad Autónoma de Campeche, Universidad Autónoma de México, Instituto de Ecología.
Rosales-Hoz, L., Cundy, A. B., & Bahena-Manjarrez, J. L. (2003). Heavy metals in sediment cores from a tropical estuary affected by anthropogenic discharges: Coatzacoalcos estuary, Mexico. Estuarine, Coastal and Shelf Science, 58, 117–126.
Rosenberg, C. E., Perí, S. I., Arrieta, M. A., Fink, N. E., & Salibián, A. (1998). Red blood cell osmotic fragility in Bufo arenarum exposed to lead. Archives of Physiology and Biochemistry, 106, 19–24.
Rowe, C. L., Hopkins, W. A., & Coffman, V. R. (2001). Failed recruitment of southern toads (Bufo terrestris) in a trace element-contaminated breeding habitat: direct and indirect effects that may lead to a local population sink. Archives of Environmental Contamination and Toxicology, 40, 399–405.
Ruelas-Inzunza, J., Gárate-Viera, Y., & Páez-Osuna, F. (2007). Lead in clams and fish of dietary importance from Coatzacoalcos estuary (Gulf of Mexico), an industrialized tropical region. Bulletin of Environmental Contamination and Toxicology, 79, 508–513.
Ruelas-Inzunza, J., Páez-Osuna, F., Zamora-Arellano, N., Amezcua-Martínez, F., & Bojórquez-Leyva, H. (2009). Mercury in biota and surficial sediments from Coatzacoalcos estuary, Gulf of Mexico: distribution and seasonal variation. Water, Air, and Soil Pollution, 197, 165–174.
Sparling, D. W., Linder, G., & Bishop, C. A. (2010). Ecotoxicology of amphibians and reptiles (2nd ed.). Pensacola: Society of Environmental Toxicology and Chemistry (SETAC).
StatSoft. (2007). STATISTICA (data analysis software system), version 8.0. Tulsa, OK: Statsoft.
Stevenson, R. D., & Woods, W. A. (2006). Condition indices for conservation: new uses for evolving tools. Integrative and Comparative Biology, 46, 1169–1190.
Strickler-Shaw, S., & Taylor, D. H. (1991). Lead inhibits acquisition and retention learning in bullfrog tadpoles. Neurotoxicology and Teratology, 13, 167–173.
Stringer, R., Labunska, I., Brigden, K. (2001). Organochlorine and heavy metal contaminants in the environment around the complejo petroquimicos Paharitos, Coatzacoalcos, Mexico (Technique Note). United Kingdom: University of Exeter, Greenpeace Research Laboratories.
Subramanian, K. S. (1989). Determination of lead in blood by graphite furnace atomic absorption spectrometry—a critique. The Science of the Total Environment, 89, 237–250.
Te-Hao, C., Gross, J. A., & Karasov, W. H. (2006). Sublethal effects of lead on northern leopard frog (Rana pipiens) tadpoles. Environmental Toxicology and Chemistry, 25, 1383–1389.
Trejo-Acevedo, A., Díaz-Barriga, F., Carrizales, L., Domínguez, G., Costilla, R., Ize-Lema, I., et al. (2009). Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere, 74, 974–980.
Triebskorn, R., Böhmer, J., Braunbeck, T., Honnen, W., Köhler, H. R., Lehmann, R., et al. (2001). The project VALIMAR (VALIdation of bioMARkers for the assessment of small stream pollution): objectives, experimental design, summary of results, and recommendations for the application of biomarkers in risk assessment. Journal of Aquatic Ecosystem Stress and Recovery, 8, 161–178 (formerly Journal of Aquatic Ecosystem Health).
Venturino, A., Rosenbaum, E., De Castro, A. C., Anguiano, O. L., Gauna, L., De Schroeder, T. F., et al. (2003). Biomarkers of effect in toads and frogs. Biomarkers, 8, 167–186.
Wang, M. Z., & Jia, X. Y. (2009). Low levels of lead exposure induce oxidative damage and DNA damage in the testes of the frog Rana nigromaculata. Ecotoxicology, 18, 94–99.
Wood, S. C. (1990). Effect of hematocrit on behavioral thermoregulation of the toad Bufo marinus. The American Journal of Physiology, 258, 848–851.
Wood, S. C. (1991). Interactions between hypoxia and hypothermia. Annual Review of Physiology, 53, 71–85.
Wood, S. C., & Gonzales, R. (1996). Hypothermia in hypoxic animals: mechanisms, mediators, and functional significance. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 113, 37–43.
Wood, S., & Malvin, G. (1991). Physiological significance of behavioral hypothermia in hypoxic toads (Bufo marinus). The Journal of Experimental Biology, 159, 203–215.
Zhang, Y., Huang, D., Zhao, D., Long, J., Song, G., & An’na, L. (2007). Long-term toxicity effects of cadmium and lead on Bufo raddei tadpoles. Bulletin of Environmental Contamination and Toxicology, 79, 178–183.
Zug, G. R., & Zug, P. B. (1979). The marine toad, Bufo marinus: a natural history resumé of native populations. Washington: Smithsonian Institution Press.
Zupanovic, Z., Musso, C., Lopez, G., Louriero, C. L., Hyatt, A. D., Hengstberger, S., et al. (1998). Isolation and characterization of iridoviruses from the giant toad Bufo marinus in Venezuela. Diseases of Aquatic Organisms, 33, 1–9.
Acknowledgments
This work was supported by a grant from the Dirección General de Investigación sobre la Contaminación Urbana y Regional del Instituto Nacional de Ecología [No. de convenio INE/A1-047/2007], SEMARNAT, and El Colegio de la Frontera Sur [No. de Fondo FORDECyT-143303]. Special thanks to Prof. J. Jesus Guerrero Cabrera from language center of San Luis Potosí Autonomous University and Biol. Susan Quackenbush for English language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilizaliturri-Hernández, C.A., González-Mille, D.J., Mejía-Saavedra, J. et al. Blood lead levels, δ-ALAD inhibition, and hemoglobin content in blood of giant toad (Rhinella marina) to asses lead exposure in three areas surrounding an industrial complex in Coatzacoalcos, Veracruz, Mexico. Environ Monit Assess 185, 1685–1698 (2013). https://doi.org/10.1007/s10661-012-2660-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2660-7