Abstract
Mangroves have wide applications in traditional medicines due to their several therapeutic properties. Potentially toxic elements (PTEs), in mangrove habitats, need serious concern because of their toxicity, bioaccumulation capacity and ecotoxicological risks. In the current study, we aimed to examine sediment quality and bioaccumulation of PTEs in a mangrove-dominated habitat of Sundarban, India, and their relation with antimicrobial property of ten mangrove species of the region. Antimicrobial activity of different solvent fractions of mangrove leaves was assessed against seven microorganisms. The highest antimicrobial activity was detected in ethyl acetate and acetone-extracted fractions of Avicennia alba. Various sediment quality indices revealed progressively deteriorating nature of surface sediment having moderate contamination, however, low ecotoxicological risk. The accumulation factors (AF) for different PTEs indicate a gradual metal bioaccumulation in leaf tissue. Antimicrobial activities indicated both positive and negative correlations with manganese (Mn), copper (Cu), iron (Fe) and zinc (Zn) concentrations of mangrove species. Concentration of Mn showed a significant correlation with almost all the fractions, whereas Cu had correlation with ethyl acetate, acetone and methanol fractions (P < 0.05). The AF of Mn and Cu exhibited correlation with antimicrobial activities of acetone and methanol fractions, whereas Fe and Zn had correlation with hexane and ethyl acetate fractions. Overall, Mn, Fe, Cu and Zn concentrations of Acanthus ilicifolius and Avicennia alba leaves and in the surface sediments demonstrated the strongest association (P < 0.05) with their antimicrobial activity as also depicted in correlation and cluster analysis studies. Thus, this study will help to establish a link between the PTEs in mangrove ecosystem with their bioactivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are the intertidal salt tolerant plant species which have a wide range of applications in traditional medicines since ages due to their antimicrobial, anti-inflammatory, anticancer and other therapeutic properties. Their ability to survive under stressful conditions has endowed them with some unique morphological features and prima facie unusual physiological processes (Bandaranayake 2002). Despite their ecological, societal and economical functions, these habitats are continuously under stress due to anthropogenic pressure and changing climatic vulnerability.
The global mangrove cover has been estimated to be 156, 220 km2 (Ghosh et al. 2015), of which India shares a part of the largest estuarine mangrove habitat of the world, i.e., ‘Sundarban’—spanning about 350 km in width along Bangladesh (Gopal and Chauhan 2006). Due to huge bioprospecting potential, the naturally occurring estuarine mangroves have recently been explored for their organic content, precisely secondary metabolites, elemental compositions along with their antimicrobial and other medicinal properties (Arivuselvan et al. 2011; Bakshi and Chaudhuri 2014; Bakshi et al. 2015).
Mangrove species play a crucial role in conserving the coastal estuarine habitats in tropics and subtropics worldwide (Bayen 2012). Generally, estuarine sediments are anaerobic and reduced by nature, thus leading to retention of waterborne trace metals (Lacerda and Abrao 1984). Organic component and sulfide-rich mangrove sediments enhance the possibility of formation of insoluble metal sulfides and organometallic complexes causing elevated trace metal sequestration (Zhou et al. 2011; Sałata and Dąbek 2017). Typically, this has resulted to their wide range of trace metal distribution and their consequent bioaccumulation potential in mangroves. Moreover, they act as a natural reservoir of nutrients, especially organic carbon and sink for trace metals, thereby influencing the biogeochemical cycles in this unique habitat (Jennerjahn and Ittekkot 2002).
Several studies have been carried out globally on different aspects of potentially toxic elements (PTEs), including their distribution, toxicity, persistent nature, bioaccumulation capacity, potential ecotoxicological risks to assess the anthropogenic influence in various estuarine ecosystems (Zhang et al. 2010; Antoniadis et al. 2017a, b; Sarkar et al. 2017). Environmental stress encountered by the habitat can be monitored by studying the concentration of the PTEs in the sediments, their degree of contamination, consequent biomarker response and associated ecological risks (Qi et al. 2015; Menghan et al. 2015; Goldyn et al. 2015; Rinklebe et al. 2017). PTEs accumulated in soils, sediments and plants can be estimated by calculating several mathematical indices (Farsad et al. 2011; Ravisankar et al. 2015; Ferati et al. 2015; Zhao et al. 2015).
The presence of different bioactive metabolites, organic compounds and trace elements is the characteristics of the plants having several pharmacological attributes (Han et al. 2001; Xie et al. 2004). Some of them have direct effects on human health (Tolonen 1990; Chen et al. 1993; Obiajunwa et al. 2002) and required to be monitored in terms of their regulatory limit, allowable concentration, toxicity level and their potential ecotoxicological risks (Bodin et al. 2013; Altundag et al. 2015; Cheng and Yap 2015). The trace elements such as Mn, Fe, Cu and Zn are involved in different metabolic and cellular functions, like primary and secondary metabolism, gene regulation, cell protection, signal transduction and hormone perception (Hänsch and Mendel 2009; Altundag et al. 2015). Many trace elements play a significant role in the formation of active constituents of plants which are responsible for their medicinal, healing properties and nutritive value supplementation (Pytlakowska et al. 2012; Zeiner et al. 2015). On the other hand, synthesis of bioactive secondary metabolites may get compromised in plants growing in polluted habitats contaminated by toxic metals (Lajayer et al. 2017). Hence, quantitative data on major and trace element contents in medicinal or edible plants are of considerable significance in order to understand their pharmacological activity (Chuang et al. 2000; Gowrishankar et al. 2010; Swain et al. 2012), as well as to analyze their potential risk upon consumption (Tyokumbur and Okorie 2011; Moore et al. 2015).
In contrast to the non-medicinal plants, some medicinal plants contain higher concentration of trace elements possibly due to their higher capacity for uptake from soil (Rajukar and Damame 1998). Characterization of the major and trace elements of common medicinal plants is reasonably available in the research databases. Minerals and trace elements present in two tropical medicinal plant Syzygium caryophyllatum and Syzygium densiflorum having several pharmacological attributes including antibacterial and antifungal activities have been investigated by Subramanian et al. (2012). In a recent work by Giordani et al. (2017), the possible trace element contamination of traditional herbal preparations of lichen Cetraria islandica having antioxidant, antimicrobial and anti-inflammatory activities was studied.
But unfortunately, plants like mangroves which have wide traditional application as biomedicine are undervalued and the presence of microelements in these plants has never been correlated with their bioactivity. The study of bioaccumulation of PTEs in various parts of mangrove and their consequent biotic response could provide an insight to the plant–sediment interactions (Nath et al. 2013; Chaudhuri et al. 2014; Nath et al. 2014a, b).
Mangroves have been reported to possess a number of phytoalexins and phytoanticipin compounds which have an active role in plant defense mechanism and have potential for their commercial exploitation (Sutton et al. 1985; Liebezeit and Rau 2006; Zhu et al. 2009; Eldeen and Effendy 2013). Different mangroves have been used widely for their pharmaceutical values, especially against asthma, diabetes, diuretic, dyspepsia, hepatitis, leprosy, neuralgia, paralysis, ringworms, rheumatism, skin diseases, snake bites, tiger bites, stomach pains, etc. (Bandaranayake 1998). Several scientific studies have been carried out to validate the antimicrobial, antiviral, anticancer properties of mangroves (Bobbarala et al. 2009; Arivuselvan et al. 2011; Prabhu and Guruvayoorappan 2013). Several mangroves and back mangroves of Indian Sundarban, namely Heritiera fomes, Ceriops decandra, Ceriops tagal, Excoecaria agallocha, Rhizophora apiculata, Xylocarpus granatum, Sonneratia apetala, Nypa fruticans, Bruguiera gymnorhiza, Avicennia sp., Phoenix paludosa, Hibiscus tiliaceous, Suaeda maritima, Clerodendrum inerme, Sarcolobus carinatus have been used for different therapeutic applications. Researchers have documented various applications of these plants against ailments like fever, malaria, cold and cough, bronchitis, asthma, skin diseases, ulcers, leprosy, small pox, diarrhea, dysentery, diabetes, antifertility (Naskar et al. 2002; Mondal and Mondal 2011; Datta et al. 2011; Bakshi and Chaudhuri 2014). Antimicrobial activity of mangroves like Acanthus ilicifolius, Aegiceras corniculatum, Aegialitis rotundifolia, Avicennia alba, Avicennia marina, Avicennia officinalis, Bruguiera gymnorrhiza, Cerbera odollam, Ceriops decandra, Cynometra iripa, Heritiera fomes, Nypa fruticans, Phoenix paludosa, Rhizophora mangle, Sonneratia alba, Xylocarpus granatum and others have been studied against several animal and plant pathogens (Chaudhuri and Guha 2010; Khajure and Rathod 2010; Bakshi and Chaudhuri 2014).
On the other hand, this ecologically sensitive region has been reported to have deposition of several PTEs generated from natural weathering processes and anthropogenic contamination (Ghosh et al. 2016; Bakshi et al. 2017; Chowdhury et al. 2017; Ghosh et al. 2018). Eventually, the phytoavailable PTEs result in their uptake, translocation and bioaccumulation in the dominant mangrove species of the region (Antoniadis et al. 2017c). Accumulation factors of PTEs in mangrove leaves have been used by the researchers to estimate the potential of metal bioaccumulation of the plant and the plant–sediment interaction (Bakshi et al. 2017). A recent study on elemental accumulation and distribution pattern was carried out in 13 mangroves of Indian Sundarban, showing higher enrichment of the essential microelements like Mn, Fe, Zn, Cu, Co, Ni in different plant parts. Among the studied plants, Sonneratia apetala and Avicennia officinalis were found to be the most potent accumulator of trace elements. To the best of our knowledge, no previous study has been conducted incorporating these accumulated PTEs in the mangroves which are playing significant role in formation of active constituents of plants responsible for their pharmacological properties. This study was aimed to assess the bioaccumulation of PTEs in mangroves from their habitat sediment and their relation with the antimicrobial property of mangroves.
Materials and methods
Study area
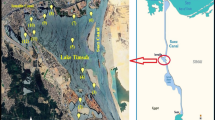
The Indian Sundarban, a part of the world’s largest mangrove habitat, is situated at the dynamic estuarine region of Bengal and sustained by complex Hooghly Matla estuarine system which includes numerous tributaries, distributaries, confluences and tidal creeks.
Present study was conducted at an island of Indian Sundarban which is known as Jharkhali island (22°00′N–22°12′N and 88°38′E–88°47′E) (Fig. 1). The island has diverse mangrove vegetation, including Avicennia sp., Aegiceras sp., Aegialites sp., Bruguiera sp., Excoecaria sp. and Sonneratia sp. along with various back mangroves (Thespesia sp., Derris sp.). The island, previously a part of the Namkhana Reserve Forest, experiences regular tidal influx and currently inhabited by a strong population of around 1,58,092. This ecologically sensitive region has been able to confront the climatic and anthropogenic vulnerabilities including coastal erosion up to a certain extent. The island has also received appreciable acknowledgment for remarkable in situ mangrove conservation. There has been a significant increase in mangrove cover area by 265 ha during 2008–2010 by plantation in and around the island (Manna et al. 2013).
Sampling of mangrove leaves and sediment
Surface sediment (at 5–10 cm depth) samples were collected in triplicate from 4 different locations (S1, S2, S3 and S4) of intertidal mud flats of Jharkhali island (Fig. 1). Mature and healthy leaves of similar height and health conditions were collected from at least 10 plants of each species, namely Avicennia marina, Avicennia alba, Avicennia officinalis, Sonneratia apetala, Sonneratia caseolaris, Excoecaria agallocha, Aegiceras corniculatum, Acanthus ilicifolius, Ceriops decandra and Nypa fruticans; and pooled into one sample for each site. The genus and species of the plants have been confirmed following the manual of Banerjee et al. (1989). Collected sediment samples were dried in the laboratory and ground to fine powder (< 63 µm) with a mortar and pestle prior to determine physicochemical properties and elemental concentrations. Mangrove leaves were dried and powdered (< 63 µm) to extract bioactive metabolites responsible for their antimicrobial properties. The leaf powder (50 g) was extracted in Soxhlet apparatus using hexane, ethyl acetate, acetone and methanol according to polarity gradient and processed further for analyzing their antimicrobial activity (Bakshi and Chaudhuri 2014).
Physicochemical parameters of sediments
Organic carbon content of sediment was measured by standard Walkey and Black titrimetric method (Walkley and Black 1934). The sediment pH and electrical conductivity (EC) were measured using a Wenser WPH 10 pH meter and Labman LMCM 20 conductivity meter, respectively (Ghosh et al. 2016; Bakshi et al. 2017).
Elemental analysis
The elemental concentrations of intertidal sediments and mangrove leaves were estimated using Xenemetrix (Ex-3600)) energy-dispersive X-ray fluorescence (ED-XRF) spectrometer consisting of an oil-cooled Rh anode X-ray tube (maximum voltage 50 kV, current 1 mA). The ED-XRF technique is multi-elemental, rapid, nondestructive and sensitive (ppm level) which has been successfully applied to analyze sediment and plant materials worldwide (Yagi et al. 2013; Schneider et al. 2016; Ravisankar et al. 2015). Measurements were taken for 400 s in vacuum using filters for optimum detection of elements and quantitatively analyzed by nExt software. The X-rays were detected using a liquid-nitrogen-cooled 12.5 mm2 Si(Li) semiconductor detector (resolution 150 eV at 5.9 keV).
Sediment samples
The concentration of aluminum (Al), calcium (Ca), chromium (Cr), copper (Cu), iron (Fe), lead (Pb), manganese (Mn), nickel (Ni), potassium (K), silicon (Si), titanium (Ti), vanadium (V) and zinc (Zn) in sediment samples was estimated. 150 mg of each dried sediment sample, sieved through 63-µm acid-washed nylon sieve, was pelletized using tabletop pelletizer (pressure 110–130 kg/cm2 for 2 min) and measured in triplicates for quantitative elemental analysis in ED-XRF.
Leaf samples
Dried and ground mangrove leaf samples were subjected to analyze for the estimation of elements (Ca), chlorine (Cl), copper (Cu), iron (Fe), potassium (K), manganese (Mn), phosphorus (P), sulfur (S) and zinc (Zn). 120 mg of each dried and sieved leaf sample was pelletized using tabletop pelletizer (pressure 100–110 kg/cm2 for 1 min) and measured in triplicates for quantitative elemental analysis.
Leaf fractions
The hexane, ethyl acetate, acetone and methanol fractions of the leaf samples were also undergone qualitative spectra analysis for four microelements, i.e., Mn, Fe, Cu and Zn. 5 µL of each of the fractions were poured on membrane filters, air-dried for 1 h and used for qualitative elemental analysis. Blank membrane filters and solvent-soaked membrane filters have been also run as negative and positive control.
Antimicrobial activities of the mangrove leaves
Microorganisms used in this study were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India. Four bacterial strains (Escherichia coli (MTCC 3221), Agrobacterium tumefaciens (MTCC 609), Streptococcus mutans (MTCC 497), Staphylococcus aureus (MTCC 7405)) and three fungal strains (Aspergillus flavus (MTCC 1884), Tricophyton rubrum (MTCC 296), Fusarium oxysporum (MTCC 284)) were used to estimate antimicrobial activity of mangrove leaf fractions, using disk assay method. In brief, leaf fractions were concentrated using rotary evaporator and paper disks of 5 mm diameter soaked with the concentrated (1 mg/mL) leaf fractions have been placed on agar plates inoculated with the organisms (Bakshi and Chaudhuri 2014). The zone of inhibition (ZoI) has been measured using Hi Media Antibiotic Zone Scale-C (PW297) and compared with the positive controls ampicillin (for bacteria) and fluconazole (for fungi) and negative control DMSO.
Bioaccumulation of potentially toxic elements (PTEs) in mangrove species
Mangrove species play a crucial role in maintaining elemental concentration in an estuarine ecosystem (He et al. 2014). The bioaccumulation of PTEs in mangrove leaves was calculated to estimate degree of PTE accumulation in mangrove leaf tissues from ambient sedimentary environments (MacFarlane et al. 2007). Accumulation factor (AF) in mangrove leaves was calculated using Eq. (1) where CBiota and Csediment are total metal concentrations in plant and sediment, respectively, in mg/kg.
Sediment quality indices
Sediment quality indices are important tools to quantify magnitude of the degree of elemental contamination in sediment system in contrast to geochemical background values which indicate concentration of a certain element in pristine sediment without any anthropogenic input. Calculation of different sediment indices largely depend on correctly chosen background values and a standard classification structure (Birch 2016). Different researchers have used Wedepohl’s shale values (Wedepohl 1995) and/or upper continental crust (UCC) values of Taylor and McLennan (1985) as geochemical background in Indian subcontinental estuarine system which also covers the area investigated in the current study (Banerjee et al. 2012; Ghosh et al. 2016, 2018; Sarkar et al. 2017). Hence, the UCC (Taylor and McLennan 1985) values have been considered as geochemical background due to the absence of specific geochemical background value in the Sundarban estuarine systems.
Enrichment factor (EF)
Enrichment factor (EF) was estimated to quantify magnitude of human-induced changes in this complex estuarine region based on standardizing the tested element against one reference element with low occurrence variability such as Mn, Ti, Al and Fe (Schiff and Weisberg 1999; Reimann and de Carital 2000; Sutherland 2000). The EF has been calculated according to Eq. (2), where Ex and Fex are the sediment sample concentrations of the element and Fe, while Eb and Feb are their concentrations in the geochemical background (Salomons and Förstner 1984; Abrahim and Parker 2008; Islam et al. 2015).
Geo-accumulation Index (I geo)
The magnitude of contamination in sediments was quantified and classified according to seven enrichment classes of geo-accumulation Index (Igeo) (Muller 1969; Antoniadis et al. 2017b, c). It is calculated using Eq. (3), where Cn and Bn are the concentration of the trace elements in the sediment and geochemical background of that element.
Contamination factor (C f)
The degree of contamination by each element in the sediment can be assessed from the contamination factor (Cf) (Hakanson 1980; Rinklebe and Shaheen 2017). It has been calculated using Eq. (4), where Ex and Eb are the concentrations of the trace elements in the sediment and geochemical background value of the element, respectively (Islam et al. 2015; Shaheen et al. 2017).
Pollution load index (PLI)
The pollution load index (PLI) is a significant key to estimate degree of contamination in coastal and estuarine sediments (Tomlinson et al. 1980; Antoniadis et al. 2017c). It has been calculated using Eq. (5) where Cf is the contamination factor.
Potential ecological risk index (PERI)
The ecological risk of the elements has been evaluated as the quantitative expression of the risk degree based on some common PTEs of ecological importance (Hakanson 1980; Gong et al. 2008; Sany et al. 2012; Guo et al. 2015) followed by Eqs. (6) and (7).
Here, Er is the potential risk of individual element; Cf is the contamination factor; Tr is the toxic response factor (TRF) for a given metal (TRF of Zn = 1, Cr = 2, Cu = 5 and Pb = 5) (Hakanson 1980); PERI is the sum of potential risk of individual metal (potential ecological risk index); and m is the number of individual metals. The standard gradation system of ecological risk has been modified in our study as we have considered only Zn, Cr, Cu and Pb unlike the classic PERI method which considers eight pollutants, including PCBs, Hg, Cd, As, Pb, Cu, Cr and Zn (Jiang et al. 2014) (Table S1).
Another important parameter to assess the ecotoxicological risk is the effect range low (ERL)/effect range median (ERM) and the threshold effect level (TEL)/probable effect level (PEL) values of the respective elements as per the sediment quality guideline (SQG) (MacDonald et al. 1996; Long and MacDonald 1998) (Table S1, Supporting Material). Values with low-range elemental concentrations (within TEL or ERL) suggest less adverse effects and low ecological risk, whereas high range values (more than PEL and ERM) represent high ecological risk (Long and MacDonald 1998). The level of toxicity has been monitored by comparing the elemental concentration of sample with these limit values (Long et al. 1995).
Statistical analysis and quality assurance
The quality assurance in PTEs analysis using ED-XRF was maintained using standard reference material (SRM) [Estuarine sediment- SRM 1646 and Apple leaf- SRM1515] from National Institute of Standards and Technology (NIST). Analytical accuracy, reported as recovery, was 94–107%, and relative standard deviation was < 5% for all measured elements. All the antimicrobial activities and elemental analysis have been carried out in triplicates, and the mean value was taken with the standard deviation. Differences between means were estimated using Duncan’s multiple range test at P < 0.05. Further Pearson’s correlation analysis was used to assess the association between the elements detected in different leaf and sediment samples. The antimicrobial activity of the leaf extracts was correlated with the elemental concentration of the crude leaf dust and the accumulation factors (AFs) to find out whether they are having any positive or negative effect on their bioactivity. The correlation statistics study was also performed between each pair of elements in both sediment and leaf and among the plants as well. Cluster analysis was carried out to determine the linkage between the mangrove plants according to the elemental concentration.
Results and discussion
Physicochemical parameters of sediments
Throughout the sampling locations, the range of pH, conductivity and organic carbon of the samples has been varied between 7.4–7.81, 1512–1701 µS/cm and 0.71–0.87%, respectively. Within the same island, this variation among the physicochemical characteristics found may be because of different land use pattern, variation in sources of organic matter, and their deposition and the distance of the sampling location from the riverside (Ghosh et al. 2016). The source of organic carbon in the studied region is largely because of agricultural and aquaculture runoff along with domestic sewage, erosion and deposition (Banerjee et al. 2012).
Elemental analysis of sediment samples
The elemental concentration of Al, Ca, Cr, Cu, Fe, K, Mn, Ni, Pb, Si, Ti, V and Zn in the sediment samples is represented in Table 1. The four locations showed variable range of elemental concentration mostly due to the influence of different environmental and tidal parameters, several sources of contamination and diverse land use pattern. Among the macro-elements, maximum concentration of Al was found to be present in site S2 (10,724 mg/kg) along with Si (227,140 mg/kg), K (26,353 mg/kg), Ca (20,153 mg/kg), Ti (3525 mg/kg) and Mn (1101 mg/kg).
Comparing to all other sites, S1 showed lower range of average elemental concentration except that of Cr, i.e., 169.65 mg/kg. Most trace elements were present in maximum concentration in site S3 including Fe (75,037 mg/kg), V (147.29 mg/kg), Ni(68.42 mg/kg), Cu (67.39 mg/kg), Zn (129.81 mg/kg) and Pb (15.48 mg/kg).
Our study showed an increase in most of the studied elements (V, Cr, Mn, Fe, Ni, Cu and Zn) than the earlier works carried out by several researchers in this region (Table 2). In last two decades, Jharkhali island situated in the downstream of Hooghly Matla estuarine system is continuously experiencing steady growth in population which has reached up to 1,58,092 in 2011 from 92,276 in 1991 (Samanta and Hazra 2012; Manna et al. 2013). The increasing population pressure has also led to loss of ~ 16 km2 mangrove cover in the island (Manna et al. 2013). The surrounding estuarine region of the island has come across immense industrial growth in sectors like textiles, paper, pesticides, pharmaceuticals, jute, plastic, leather (Mitra et al. 2012). Moreover, the estuary receives huge sewage input from the drainage system of the adjacent urban ecosystem (Pramanick et al. 2015). The contaminated sewages, industrial effluents, surface and agricultural runoffs of various origin like vehicular emission, construction work, open dumping of solid waste, boating and fishing activities, burning of waste, domestic cooking, etc. (Bakshi et al. 2017; Chowdhury et al. 2017) have also led to gradual deposition and accumulation of PTEs. These factors chiefly contribute to increased accumulation of elements in sediments of Jharkhali, which in turn results into their greater bioaccumulation in plants and surrounding habitat. Interestingly, Pb is the only element in our study which showed notably lower concentration than the earlier decades mostly because of the use of unleaded petrol since 2000 (Singh and Singh 2006).
Elemental analysis of mangrove leaves
This study depicts elemental distribution of Ca, Cl, Cu, Fe, K, Mn, P, S and Zn in leaves of ten mangrove plants (Table 3). Among all the studied elements, maximum concentration of P (1820 mg/kg) and K (11,514 mg/kg) has been observed in the leaves of S. caseolaris, whereas the highest Cl (19,953 mg/kg) was recorded in A. marina. E. agallocha showed maximum amount of Ca (15,623 mg/kg), and C. decandra leaves had highest concentration of S (3568 mg/kg), respectively.
For microelements, A. ilicifolius showed the maximum amount of Fe (846 mg/kg and Zn (69 mg/kg); highest concentration of Mn (1154 mg/kg) has been recorded in N. fruticans leaves, and the highest Cu (16.96 mg/kg) concentration has been estimated in the leaves of A. officinalis. Our study observed some contrasting results in terms of elemental concentration in different mangrove plants in India. In most of the cases, the concentration of trace elements, namely Cu, Cr, Fe, Zn, showed twofold–20-fold increase than the earlier reports of other researchers (Agoramoorthy et al. 2008; Akhand et al. 2012; Chakraborty et al. 2013; Badarudeen et al. 2014). The increased deposition of contaminants may have resulted in elevated elemental bioaccumulation in mangroves and hence resulted in this changing scenario (Chaudhuri et al. 2014; Bakshi et al. 2017).
Elemental transfer or extraction rate in plant or biological preparations is extremely element specific (Giordani et al. 2017). In our study, the qualitative spectra of ED-XRF were analyzed for four PTEs (Mn, Fe, Cu and Zn) showing signature peaks at their corresponding energy (5.9, 6.4, 8.1 and 8.6 kV). The presence of characteristics peaks in the qualitative spectra suggests that most of the microelements which are present in the crude leaf dusts are also extractable in the solvent fractions. The figures of solvent specific (i.e., hexane, ethyl acetate, acetone and methanol) microelement spectrum of ten mangroves are given in supporting material (Fig. S1, Supporting Material). One channel number in the figures corresponds to 10 kV energy; hence, the peaks found at around 590, 640, 804 and 863 represent the qualitative peak of Mn, Fe, Cu and Zn, respectively. The crude leaf powder of N. fruticans, A. ilicifolius and A. officinalis showed the highest concentrations of microelements (Mn, Cu, Fe, Zn) and in case of the solvent fractions the strong intensity of four micronutrients were recorded in case of A. corniculatum, C. decandra, A. ilicifolius, S. apetela and N. fruticans. This is a primary confirmation of the fact that the higher PTE concentration in crude leaf powder of N. fruticans, A. ilicifolius and A. officinalis results better extractability of those elements in the above-mentioned plant fractions. This is worth-mentioning that the strongest PTE intensities were found mostly in the plants, showing good antimicrobial activities. Overall, the work revealed the extractable nature of essential microelements which were found in the leaf fractions of mangroves and can play a crucial role in their antimicrobial activity (Xie et al. 2004; Pytlakowska et al. 2012; Zeiner et al. 2015).
Antimicrobial activities of the mangrove leaves
Results depicted zones of inhibition (ZoI) found in disk assay study with leaf extract fractions of ten mangrove species against four bacterial and three fungi species. Figure 2 shows antibacterial activity of four solvent fractions, i.e., hexane, ethyl acetate, acetone and methanol, respectively. Among different solvents, the maximum activity was found in ethyl acetate and acetone fractions. A. marina, A. alba, A. ilicifolius, C. decandra and E. agallocha showed better antimicrobial potential than the others. In our study, the strongest antimicrobial potential was exhibited by the A. marina extracts with 21.9 mm and 17.7 zone of inhibition against S. aureus and S. mutans, respectively.
Hexane fractions of nearly all ten plants showed the poorest results in the disk assay method where the highest activity found against Staphylococcus aureus against A. ilicifolius and E. agallocha. The ethyl acetate fractions were found to be the most active in our experiment. These fractions of almost all the plants showed strong inhibitory effects against all four bacteria. A. marina showed the maximum activity against E. coli and S. aureus (15.8 and 21.9 mm ZoI, respectively).
Acetone fractions also exhibited good result against most of the organisms specially E. coli, A. tumefaciens and S. aureus. Methanol fractions also demonstrated moderate activity against the pathogenic bacteria. Previous reports showed that among these plants A. ilicifolius and A. marina extracts are most effective even in very low concentrations (Bakshi and Chaudhuri 2014). The antifungal activity of the chosen mangrove plants of Indian Sundarban was not satisfactory (Fig. 3).
Although antifungal activity of different mangrove plants has been determined partially (Choudhury et al. 2005; Khajure and Rathod 2010; Chaudhuri and Guha 2010; Bobbarala et al. 2009), in our study only A. alba, A. marina and S. caseolaris showed potential of antifungal activity against A. flavus and T. rubrum. The ethyl acetate and acetone extract of A. marina exhibited growth inhibitory effect against A. flavus with 6 and 6.5 mm zone of inhibition. In case of A. alba, the only active extract was acetone with 6.9 mm zone of inhibition. Antifungal action against T. rubrum was found only in case of ethyl acetate and acetone extracts of S. caseolaris and A. alba leaves.
Bioaccumulation of PTEs in mangrove species
Accumulation factors (AFs) in leaves can significantly portray the magnitude of elemental bioaccumulation in a plant from the habitat sediment and also offers an insight to changes in plant–sediment interaction from the earlier period (Mahdavian et al. 2017). In our study, the maximum AF for K has been found in A. marina (0.424), for Ca it was E. agallocha (1.442), AF for Mn was highest in N. fruticans (1.048), for Fe and Zn A. ilicifolius showed the highest AF (0.011 and 0.536), for Cu it was A. officinalis (0.252) (Table 4).
The AF of Fe in Sundarban mangroves leaves found in the previous work was in between 0.015 and 0.021 which is almost similar to our work (Akhand et al. 2012). The range of AF in the case of Cu and Zn was from 0.05–0.31 and 0.13–0.34 by the similar author, whereas the same was observed to be 0.46 and 0.69 in another work by Chakraborty et al. 2013. The AF values found in our study correspond to the highest elemental concentrations of the plants and indicate the gradual accumulation of trace elements in the leaf tissues.
The abundance of PTEs in sediment system leading to their bioaccumulation in plants indicates the phytoremediation potential of mangroves, which can essentially reduce metal leaching into the environment. Aerial parts of mangrove can be very efficient for remediating PTEs from sediment system by phytoextraction or/and phytomining (Rizwan et al. 2016; Naeem et al. 2016). In our study, A. officinalis, A. ilicifolius and N. fruticans were found to have the better potential for phytoremediation of Cu, Fe, Zn and Mn from contaminated sediments.
Sediment quality indices
The sediment quality was assessed by calculating various indices followed by categorization based on the standard classification system. The classification of the sediments based on the EF values (Liu et al. 2005), Igeo values (Muller 1969), Cf values (Hakanson 1980) and PLI values is shown in Table S1.
In sediment samples, Cu and Cr showed the highest EF values which varied from 1.36 to 1.47 for Cu and from 1.08 to 1.23 for Cr followed by V, Mn, Ni and Zn with mean EFs between 0.5 and 1 (Table 5). These results indicate that according to the level of contamination of EF (Table S1) there is no significant enrichment by the studied elements in all sites. Elements including Al, Si, K, Ca, Ti and V revealed negative geo-accumulation index (Igeo) values reflecting their uncontaminated status in the four locations (Table 5). Positive Igeo values for Cr (0.33–0.50), Cu (0.65–0.85) and Zn (0.11–0.29) were observed in all the locations, suggesting uncontaminated to moderately contaminated state for these elements (Müller 1969). Sampling locations, S1 and S2, showed positive Igeo values for Mn (0.043–0.069), whereas Ni has only positive value at location S3 (0.053). The result of Igeo values matches with the result of EF values where the elements showing higher EFs also showed positive Igeo number (Cr, Mn, Ni, Cu, Zn). In terms of contamination factor (Cf), all locations were observed to possess moderate contamination where most of the Cf values for Cu, Cr, Fe, Zn, Mn, V and Ni was between 1 and 3 (Hakanson 1980). The K, Ca and Pb showed low contamination status with Cf < 1. The highest Cf values were observed for Cu in all 4 locations (2.34–2.69). Similarly, Cr, Mn, Fe and Ni also exhibited higher Cf values (1.02–2.11) (Table 5). The calculated pollution load index for S1–S4 was found to be between 1.08 and 1.30, suggesting the progressively deteriorating quality of the habitat sediment in the study area. The increasing order of PLI is S3 > S2 > S1 > S4.
Overall, studied sediment quality indices suggested complementary results revealing the low to moderate contamination (in terms of Cf) and progressive deterioration (in terms of PLI) in the sediment quality of the island. In all 4 locations of the island, the higher concentrations of most of the elements especially Cu, Cr, Mn, Ni, V and Zn from the geochemical background value suggest increased upstream weathering along with rapid urbanization and industrial growth in this extremely sensitive region due to release of industrial waste and domestic sewage, brick kilns, boating and shipping and fishing activity, vehicular emission, etc. (Sarkar et al. 2004; Gopal and Chauhan 2006; Chatterjee et al. 2007; Banerjee et al. 2012). In location S3, the EF value of Zn is 0.99 and the same spot also has the highest Igeo factor for Zn, i.e., 0.286. The highest Zn intensity in the leaf fractions was observed in two plants collected from the similar spot (S3), i.e., A. marina and A. officinalis, which in turn exhibit considerable antimicrobial property. Copper also followed comparable trend, where plants A. alba and S. apetela at spot S1 (EF 1.412 and Igeo 0.043) showed adequate antimicrobial activity. The PLI in both these spots (S3 and S1) was higher, i.e., 1.30 and 1.20, respectively (Table 6).
Potential ecological risk index
The potential risk of individual element (Er) and comprehensive potential ecological risk index (PERI) was calculated to quantify the degree of risk associated with some common PTEs of ecological importance (Table 6). The Er values of Cr, Cu, Zn and Pb ranged from 3.77–4.28, 11.73–13.48, 1.62–1.83 and 2.39–4.55, respectively. The highest Er values were found mostly in spot S3 (Cu, Zn and Pb) and S1 (Cr). The potential ecological risk index (PERI) of mangrove sediments in four spots lies from 20.04 to 24.01. The risk values suggest degree of pollution and risk level to be within ‘low ecological risk’ category (A) despite moderate and progressive contamination in sediment (Table S1).
Ecological risk assessment of the elements was also evaluated by comparing their concentrations with TELs and PELs (Long and MacDonald 1998) and ERL and ERM (Long et al. 1995). The Cr concentration in all 4 sites was < PEL and ERL values. Cu values are higher than TEL and ERL, but much lower than PEL and ERM values. The Ni concentrations in all spots are higher than PEL and ERL values, and in two spots (S1 and S3) these values are even more than ERM. All of the spots had Pb amount much lower than TEL and ERL. The Zn level crossed TEL limit in one spot (S3) and in the rest of spots remained under TEL limit. Locations with low-range elemental concentrations (within TEL or ERL) suggest less adverse effects and low ecological risk, whereas high ecological risk prevails in the spots with high range values (more than PEL and ERM) (Long and MacDonald 1998).
Relationships between elements and plant antimicrobial activity
Result confirmed a wide variation in elemental concentrations in mangroves, even in the case of leaves collected from the same site. This is mostly due to difference in uptake and accumulation potential of macro- and microelements in different plant species which is largely controlled by their genotype (Shtangeeva 1994; Rai et al. 1995; Willey and Fawcett 2006). Pearson’s correlation analysis showed a significant association among some of the leaves and sediment elemental concentration (Table 7). No precise pattern of correlation between the elemental concentration with respect to species and solvent specific antimicrobial activity of mangroves was observed. However, in most of the cases elemental concentrations found to have either positive or negative regulation on antimicrobial activities except ethyl acetate fractions against A. tumefaciens. In the case of microelements, Mn showed a range of correlation (r = − 0.59–0.5, n = 30) with almost all the fractions, whereas Cu had correlation with ethyl acetate, acetone and methanol fractions.
Zn and Fe showed correlation with hexane fractions only; Mn had negative correlation with the antimicrobial activities against A. tumefaciens and S. aureus except for the acetone fractions. Fe showed positive correlation with only the hexane fractions against E. coli, S. aureus and S. mutans. The relation of bioactivities of mangrove leaves with accumulated elements is shown by means of their appreciable correlation with the accumulation factors (AF). The AF of Mn and Cu mostly shows correlation with the antimicrobial activities of acetone and methanol fractions of mangroves, whereas Fe and Zn exhibit correlation with the hexane and ethyl acetate fractions (Table 8).
The trace elements can play a vital role in propagation of various body functions, metabolic processes, enzymatic activities and immunologic reactions, eventually exhibiting both positive and negative impact on several diseases (Chaturvedi et al. 2004; Selvaraju et al. 2009; Emsley 2011; Gowrishankar et al. 2010). In a study by Mulaudzi et al. (2017), commonly used herbal mixtures of South Africa has been characterized by broad-spectrum of antimicrobial activities against Escherichia coli and Neisseria gonorrhoeae and high amount of Cr, Cu, Cd, Mn and Pb (Mulaudzi et al. 2017). In our study, mangrove leaves from the study site contained optimum amount of elements like Fe, Mn, Cu and Zn which are required by body for their role in combating pathogenic infection (Gowrishankar et al. 2010; Rajan et al. 2014). Use of these plants by the local inhabitants of Sundarban and other mangrove habitats as indigenous medicines for the treatment of wound, inflammation, skin irritation, diarrhea, etc. can thus be explained in terms of the role of these elements in boosting immune system and metabolism of the end users (Bandaranayake 2002; Valentino 2006; Borkow et al. 2009; Ayodele and Bayero 2010; Cobanoglu et al. 2010).
The positive association and linkage between the plants and surface sediments on the basis of elemental concentration are represented in Table S2 in supplementary material. Close association between mangrove plant A. marina with A. alba, A. officinalis, S. apetela, S. caseolaris, A. ilicifolius and N. fruticans was observed. Elemental accumulation in specific tissues and cellular organelles in plants are also facilitated by complex mechanisms involving multiple hormonal, genomic and metabolic events. In our study, plant-sediment association is not clearly evident and no significant correlation was found between element concentrations in plant and sediment. This supports the findings of Shtangeeva et al. (2009) and Reimann et al. (2015), who suggested good correlations between element concentrations as measured in plant–soil system are seldom observed irrespective of the fact that most plants accumulate their nutrients from the soil horizons beneath.
The cluster analysis, deriving dendograms constructed with the concentration of elements in different mangrove leaves from all sites, showed A. marina and A. ilicifolius are linked closely. Close association and similarity were also observed among C. decandra, A. corniculatum and E. agallocha (Figure S2 in supplementary material). This result justifies the Pearson correlation analysis where similar kind of positive association was established.
The entire study considering three components can be summed up in the three-dimensional relationship among the microelemental concentrations of the sediment, plant species and average plant bioactivity where the strong association was found in A. ilicifolius and A. alba (Fig. 4). The relationship was strong with almost all four microelements (Mn, Fe, Cu and Zn) as also suggested in correlation and cluster analysis studies. Moreover, our results indicate that the nature of sediment was moderately contaminated and suffering from progressive deterioration in terms of sediment quality indices.
Mangrove plant species showed significant antimicrobial properties, which was closely associated with plant microelements accumulated from the sediments. The deterioration in sediment quality might affect elemental accumulation process of plants which can in turn influence their antimicrobial properties. This study helps to identify the relationship between elemental composition of mangroves and their bioactivity and as such highlight the effect of sediment quality on this association.
Conclusions
This study examined the influence of sediment quality and elemental bioaccumulation on bioactivity of mangrove leaves of Indian Sundarban. Among the ten mangrove plant species, the most active plants (having maximum antimicrobial potential) are Avicennia marina, Avicennia alba, Acanthus ilicifolius, Ceriops decandra and Excoecaria agallocha. The elemental profiling of mangrove leaves, surrounding sediment quality and their close association with their bioactivity suggested the role of available PTEs toward antimicrobial activity. Mangrove leaves contained optimum amount of elements like Fe, Mn, Cu and Zn, which play some crucial roles in different physiological, metabolic and immunologic functions. Overall, our research revealed the presence of essential microelements in leaf fractions of mangroves having significant antimicrobial activity. The concentrations of various PTEs and respective AF values showed distinctly different results than the studies of previous researchers which indicate the changing deposition and accumulation pattern of elements in the study area. Various indices (EF, Igeo, Cf, PLI) indicated that the surface sediment is facing moderate contamination and low ecological risk, where only Cr and Ni were found to cross the limit of ERL and PEL. The progressively deteriorating sediment contaminated from various point and nonpoint sources can certainly influence and affect the elemental profiling of the surrounding biotic community by bioaccumulation and biomagnification. Mangrove plants having unique morphology and unusual physiological processes show various significant bioactivities like antimicrobial properties, which is closely associated with the studied PTEs specially in A. ilicifolius and A. alba. Monitoring of the habitat sediment and the influence of the changing bioaccumulation pattern on the bioactivities of mangrove can be a useful tool to establish the pharmacological potential of the bioaccumulated PTEs in the studied mangrove species.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136, 227–238.
Agoramoorthy, G., Chen, F. A., & Hsu, M. J. (2008). Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environmental Pollution, 155(2), 320–326.
Akhand, A., Chanda, A., Dutta, S., Hazra, S., & Sanyal, P. (2012). Comparative study of heavy metals in selected mangroves of Sundarban ecosystem, India. Journal of Environmental Biology, 33, 1045–1049.
Altundag, H., Albayrak, S., Dundar, M. S., Tuzen, M., & Soylak, M. (2015). Investigation of the influence of selected soil and plant properties from sakarya, turkey, on the bioavailability of trace elements by applying an in vitro digestion model. Biological Trace Element Research, 168(1), 276–285.
Antizar-Ladislao, B., Mondal, P., Mitra, S., & Sarkar, S. K. (2015). Assessment of trace metal contamination level and toxicity in sediments from coastal regions of West Bengal, eastern part of India. Marine Pollution Bulletin, 101(2), 886–894.
Antoniadis, V., Golia, E. E., Shaheen, S. M., & Rinklebe, J. (2017a). Bioavailability and health risk assessment of potentially toxic elements in Thriasio Plain, near Athens, Greece. Environmental Geochemistry and Health, 39(2), 319–330.
Antoniadis, V., Levizou, E., Shaheen, S. M., Ok, Y. S., Sebastian, A., Baum, C., et al. (2017b). Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Science Reviews, 171, 621–645.
Antoniadis, V., Shaheen, S. M., Boersch, J., Frohne, T., Du Laing, G., & Rinklebe, J. (2017c). Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. Journal of Environmental Management, 186, 192–200.
Arivuselvan, N., Silambarasan, D., Govindan, T., & Kathiresan, K. (2011). Antibacterial activity of mangrove leaf and bark extracts against human pathogens. Advances in Biological Research, 5(5), 251–254.
Ayodele, J. T., & Bayero, A. S. (2010). Manganese concentrations in hair and fingernail of some Kano inhabitants. Journal of Applied Sciences and Environmental Management, 14(1), 17–21.
Badarudeen, A., Sajan, K., Srinivas, R., Maya, K., & Padmalal, D. (2014). Environmental significance of heavy metals in leaves and stems of Kerala mangroves, SW Coast of India.
Bakshi, M., & Chaudhuri, P. (2014). Antimicrobial potential of leaf extracts of ten mangrove species from Indian Sundarban. Internationl Journal of Pharma and Bio Science, 5(1), 294–304.
Bakshi, M., Ghosh, S., & Chaudhuri, P. (2015). Green synthesis, characterization and antimicrobial potential of sliver nanoparticles using three mangrove plants from Indian Sundarban. BioNanoScience, 5(3), 162–170.
Bakshi, M., Ram, S. S., Ghosh, S., Chakraborty, A., Sudarshan, M., & Chaudhuri, P. (2017). Micro-spatial variation of elemental distribution in estuarine sediment and their accumulation in mangroves of Indian Sundarban. Environmental Monitoring and Assessment, 189(5), 221.
Bandaranayake, W. M. (1998). Traditional and medicinal uses of mangroves. Mangroves and Salt Marshes, 2(3), 133–148.
Bandaranayake, W. M. (2002). Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management, 10(6), 421–452.
Banerjee, L. K., Sastry, A. R. K., & Nayar, M. P. (1989). Mangroves in India: identification manual. Calcutta: Botanical Survey of India.
Banerjee, K., Senthilkumar, B., Purvaja, R., & Ramesh, R. (2012). Sedimentation and trace metal distribution in selected locations of Sundarbans mangroves and Hooghly estuary, northeast coast of India. Environmental Geochemistry and Health, 34(1), 27–42.
Bayen, S. (2012). Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environment International, 48, 84–101.
Birch, G. F. (2016). Determination of sediment metal background concentrations and enrichment in marine environments—a critical review. The Science of the Total Environment, 580, 13–831.
Bobbarala, V., Vadlapudi, V. R., & Naidu, C. K. (2009). Antimicrobial potentialities of mangrove plant Avicennia marina. Journal of Pharmacy Research, 2(6), 1019–1021.
Bodin, N., N’Gom-Kâ, R., Kâ, S., Thiaw, O. T., De Morais, L. T., Le Loc’h, F., et al. (2013). Assessment of trace metal contamination in mangrove ecosystems from Senegal, West Africa. Chemosphere, 90(2), 150–157.
Borkow, G., Zatcoff, R. C., & Gabbay, J. (2009). Reducing the risk of skin pathologies in diabetics by using copper impregnated socks. Medical Hypotheses, 73(6), 883–886.
Chakraborty, D., Bhar, S., Majumdar, J., & Santra, S. C. (2013). Heavy metal pollution and phytoremediation potential of Avicennia officinalis L. in the southern coast of the Hoogly estuarine system. International Journal of Environmental Science, 3(6), 2291–2303.
Chatterjee, M., Massolo, S., Sarkar, S. K., Bhattacharya, A. K., Bhattacharya, B. D., Satpathy, K. K., et al. (2009). An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environmental Monitoring and Assessment, 150(1), 307–322.
Chatterjee, M. V. S. F. E., Silva Filho, E. V., Sarkar, S. K., Sella, S. M., Bhattacharya, A., Satpathy, K. K., et al. (2007). Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environment International, 33(3), 346–356.
Chaturvedi, U. C., Shrivastava, R., & Upreti, R. K. (2004). Viral infections and trace elements: A complex interaction. Current Science, 87(11), 1536–1554.
Chaudhuri, P., & Guha, S. (2010). Potentiality of mangrove plant extracts for biocontrol of a pathogenic fungi, Fusarium oxysporum. Science and Culture, 76(7–8), 271–274.
Chaudhuri, P., Nath, B., & Birch, G. (2014). Accumulation of trace metals in grey mangrove Avicennia marina fine nutritive roots: the role of rhizosphere processes. Marine Pollution Bulletin, 79(1), 284–292.
Chen, K. S., Tseng, C. L., & Lin, T. H. (1993). Trace elements in natural drugs determined by INAA. Journal of Radioanalytical and Nuclear Chemistry, 170(1), 265–280.
Cheng, W. H., & Yap, C. K. (2015). Potential human health risks from toxic metals via mangrove snail consumption and their ecological risk assessments in the habitat sediment from Peninsular Malaysia. Chemosphere, 135, 156–165.
Choudhury, S., Sree, A., Mukherjee, S. C., Pattnaik, P., & Bapuji, M. (2005). In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fisheries Science, 18(3/4), 285–294.
Chowdhury, R., Favas, P. J., Jonathan, M. P., Venkatachalam, P., Raja, P., & Sarkar, S. K. (2017). Bioremoval of trace metals from rhizosediment by mangrove plants in Indian Sundarban Wetland. Marine Pollution Bulletin, 124(2), 1078–1088.
Chuang, I. C., Chen, K. S., Huang, Y. L., Lee, P. N., & Lin, T. H. (2000). Determination of trace elements in some natural drugs by atomic absorption spectrometry. Biological Trace Element Research, 76(3), 235–244.
Cobanoglu, U., Demir, H., Sayir, F., Duran, M., & Mergan, D. (2010). Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pacific Journal of Cancer Prevention: APJCP, 11(5), 1383–1388.
Datta, D., Chattopadhyay, R. N., & Deb, S. (2011). Prospective livelihood opportunities from the mangroves of the Sunderbans, India. Research Journal of Environmental Sciences, 5(6), 536.
Eldeen, I. M., & Effendy, M. A. (2013). Antimicrobial agents from mangrove plants and their endophytes. In A. Méndez-Vilas (Ed.), Microbial pathogens and strategies for combating them: Science, technology and education (pp. 872–882). Spain: Formatex Research Centre.
Emsley, J. (2011). Nature’s building blocks: an AZ guide to the elements. Oxford: Oxford University Press.
Farsad, F., Karbassi, A., Monavari, S. M., Mortazavi, M. S., & Farshchi, P. (2011). Development of a new pollution index for heavy metals in sediments. Biological Trace Element Research, 143(3), 1828–1842.
Ferati, F., Kerolli-Mustafa, M., & Kraja-Ylli, A. (2015). Assessment of heavy metal contamination in water and sediments of Trepça and Sitnica rivers, Kosovo, using pollution indicators and multivariate cluster analysis. Environmental Monitoring and Assessment, 187(6), 338.
Ghosh, S., Bakshi, M., Bhattacharyya, S., Nath, B., & Chaudhuri, P. (2015). A review of threats and vulnerabilities to mangrove habitats: with special emphasis on east coast of India. Journal of Earth Science and Climate Change, 6(4), 270.
Ghosh, S., Bakshi, M., Kumar, A., Ramanathan, A. L., Biswas, J. K., Bhattacharyya, S., et al. (2018). Assessing the potential ecological risk of Co, Cr, Cu, Fe and Zn in the sediments of Hooghly–Matla estuarine system, India. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0119-7.
Ghosh, S., Ram, S. S., Bakshi, M., Chakraborty, A., Sudarshan, M., & Chaudhuri, P. (2016). Vertical and horizontal variation of elemental contamination in sediments of Hooghly Estuary, India. Marine Pollution Bulletin, 109(1), 539–549.
Giordani, P., Minganti, V., Brignole, D., Malaspina, P., Cornara, L., & Drava, G. (2017). Is there a risk of trace element contamination in herbal preparations? A test study on the lichen Cetraria islandica. Chemosphere, 181, 778–785.
Gołdyn, B., Chudzińska, M., Barałkiewicz, D., & Celewicz-Gołdyn, S. (2015). Heavy metal contents in the sediments of astatic ponds: Influence of geomorphology, hydroperiod, water chemistry and vegetation. Ecotoxicology and Environmental Safety, 118, 103–111.
Gong, Q. J., Deng, J., Xiang, Y. C., Wang, Q. F., & Yang, L. Q. (2008). Calculating pollution indices by heavy metals in ecological geochemistry assessment and a case study in parks of Beijing. Journal of China University of Geosciences, 19, 230–241.
Gopal, B., & Chauhan, M. (2006). Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquatic Sciences-Research Across Boundaries, 68(3), 338–354.
Gowrishankar, R., Kumar, M., Menon, V., Divi, S. M., Saravanan, M., Magudapathy, P., et al. (2010). Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biological Trace Element Research, 133(3), 357–363.
Guo, W., Huo, S., Xi, B., Zhang, J., & Wu, F. (2015). Heavy metal contamination in sediments from typical lakes in the five geographic regions of China: distribution, bioavailability, and risk. Ecological Engineering, 81, 243–255.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001.
Han, Y., Nishibe, S., Noguchi, Y., & Jin, Z. (2001). Flavonol glycosides from the stems of Trigonella foenum-graecum. Phytochemistry, 58(4), 577–580.
Hänsch, R., & Mendel, R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Current Opinion in Plant Biology, 12(3), 259–266.
He, B., Li, R., Chai, M., & Qiu, G. (2014). Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environmental Geochemistry and Health, 36(3), 467–476.
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M., & Hoque, M. F. (2015). Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environmental Earth Sciences, 73(4), 1837–1848.
Jennerjahn, T. C., & Ittekkot, V. (2002). Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften, 89(1), 23–30.
Jiang, X., Lu, W. X., Zhao, H. Q., Yang, Q. C., & Yang, Z. P. (2014). Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Natural Hazards and Earth System Sciences, 14(6), 1599–1610.
Khajure, P. V., & Rathod, J. L. (2010). Antimicrobial activity of extracts of Acanthus ilicifolius extracted from the mangroves of Karwar coast Karnataka. Recent Research in Science and Technology, 2(6), 98–99.
Lacerda, L. D., & Abrao, J. J. (1984). Heavy metal accumulation by mangrove and saltmarsh intertidal sediments. Revista Brasileira de Botanica, 7, 49–52.
Lajayer, B. A., Ghorbanpour, M., & Nikabadi, S. (2017). Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicology and Environmental Safety, 145, 377–390.
Liebezeit, G., & Rau, M. T. (2006). New Guinean mangroves—Traditional usage and chemistry of natural products. Senckenbergiana maritima, 36(1), 1–10.
Liu, W. H., Zhao, J. Z., Ouyang, Z. Y., Söderlund, L., & Liu, G. H. (2005). Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environment International, 31(6), 805–812.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19(1), 81–97.
Long, E. R., & MacDonald, D. D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Human and Ecological Risk Assessment, 4(5), 1019–1039.
Macdonald, D. D., Carr, R. S., Calder, F. D., Long, E. R., & Ingersoll, C. G. (1996). Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology, 5(4), 253–278.
MacFarlane, G. R., Koller, C. E., & Blomberg, S. P. (2007). Accumulation and partitioning of heavy metals in mangroves: a synthesis of field-based studies. Chemosphere, 69(9), 1454–1464.
Mahdavian, K., Ghaderian, S. M., & Torkzadeh-Mahani, M. (2017). Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. Journal of Soils and Sediments, 17(5), 1310–1320.
Manna, S., Mondal, P. P., Mukhopadhyay, A., Akhand, A., Hazra, S., & Mitra, D. (2013). Vegetation cover change analysis from multitemporal satellite data in Jharkhali Island, Sundarbans, India. Indian Journal of Geo-Marine Sciences, 42(3), 331–342.
Massolo, S., Bignasca, A., Sarkar, S. K., Chatterjee, M., Bhattacharya, B. D., & Alam, A. (2012). Geochemical fractionation of trace elements in sediments of Hugli River (Ganges) and Sundarban wetland (West Bengal, India). Environmental Monitoring and Assessment, 184(12), 7561–7577.
Menghan, W., Stefano, A., Annamaria, L., Claudia, C., Antonio, C., Wanjun, L., et al. (2015). Compositional analysis and pollution impact assessment: A case study in the Gulfs of Naples and Salerno. Estuarine, Coastal and Shelf Science, 160, 22–32.
Mitra, A., Barua, P., Zaman, S., & Banerjee, K. (2012). Analysis of trace metals in commercially important crustaceans collected from UNESCO protected world heritage site of Indian Sundarbans. Turkish Journal of Fisheries and Aquatic Sciences, 12(1), 53–66.
Mondal, C. K., &, Mondal. B. (2011). Ethno-traditional medicinal uses of mangrove plants of Sundarbans-a study. In Proceedings of the international symposium on minor fruits and medicinal plants for health and ecological security (ISMF & MP), West Bengal, India, (pp. 91–93). Bidhan Chandra Krishi Viswandyalaya.
Moore, F., Akhbarizadeh, R., Keshavarzi, B., & Tavakoli, F. (2015). Potential health risk of herbal distillates and decoctions consumption in Shiraz, Iran. Biological Trace Element Research, 167(2), 326–337.
Mulaudzi, R. B., Tshikalange, T. E., Olowoyo, J. O., Amoo, S. O., & Du Plooy, C. P. (2017). Antimicrobial activity, cytotoxicity evaluation and heavy metal content of five commonly used South African herbal mixtures. South African Journal of Botany, 112, 314–318.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Naeem, A., Rehman, M. Z. U. S., Akhtar, T., Ok, Y. S., & Rengel, Z. (2016). Genetic variation in cadmium accumulation and tolerance among wheat cultivars at the seedling stage. Communications in Soil Science and Plant Analysis, 47(5), 554–562.
Naskar, K., Mandal, R., & Ghosh, A. (2002). Important medicinal plants from Indian Sunderbans. In Proceedings of national seminar and workshop on Indian systems of medicine and homeopathy, Ramakrishna Mission Ashrama, Narendrapur, West Bengal, India.
Nath, B., Birch, G., & Chaudhuri, P. (2013). Trace metal biogeochemistry in mangrove ecosystems: a comparative assessment of acidified (by acid sulfate soils) and non-acidified sites. Science of the Total Environment, 463, 667–674.
Nath, B., Birch, G., & Chaudhuri, P. (2014a). Assessment of sediment quality in Avicennia marina-dominated embayments of Sydney Estuary: the potential use of pneumatophores (aerial roots) as a bio-indicator of trace metal contamination. Science of the Total Environment, 472, 1010–1022.
Nath, B., Chaudhuri, P., & Birch, G. (2014b). Assessment of biotic response to heavy metal contamination in Avicennia marina mangrove ecosystems in Sydney Estuary, Australia. Ecotoxicology and Environmental Safety, 107, 284–290.
Obiajunwa, E., Adebajo, A., & Omobuwajo, O. (2002). Essential and trace element contents of some Nigerian medicinal plants. Journal of Radioanalytical and Nuclear Chemistry, 252(3), 473–476.
Prabhu, V. V., & Guruvayoorappan, C. (2013). Inhibition of metastatic lung cancer in C57BL/6 mice by marine mangrove Rhizophora apiculata. Asian Pacific Journal of Cancer Prevention, 14(3), 1833–1840.
Pramanick, P., Zaman, S., Rudra, T., Guha, A., & Mitra, A. (2015). Heavy metals in a dominant seaweed species from the Islands of Indian Sundarbans. International Journal of Life Science and Pharma Research, 5(2), 64–71.
Pytlakowska, K., Kita, A., Janoska, P., Połowniak, M., & Kozik, V. (2012). Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chemistry, 135(2), 494–501.
Qi, H. X., Li, H. Z., Ma, P., & You, J. (2015). Integrated sediment quality assessment through biomarker responses and bioavailability measurements: Application in Tai Lake, China. Ecotoxicology and Environmental Safety, 119, 148–154.
Rai, U. N., Sinha, S., Tripathi, R. D., & Chandra, P. (1995). Wastewater treatability potential of some aquatic macrophytes: removal of heavy metals. Ecological Engineering, 5(1), 5–12.
Rajan, J. P., Singh, K. B., Kumar, S., & Mishra, R. K. (2014). Trace elements content in the selected medicinal plants traditionally used for curing skin diseases by the natives of Mizoram, India. Asian Pacific Journal of Tropical Medicine, 7, S410–S414.
Rajurkar, N. S., & Damame, M. M. (1998). Mineral content of medicinal plants used in the treatment of diseases resulting from urinary tract disorders. Applied Radiation and Isotopes, 49(7), 773–776.
Ramesh, R., Ramanathan, A. L., James, R. A., Subramanian, V., Jacobsen, S. B., & Holland, H. D. (1999). Rare earth elements and heavy metal distribution in estuarine sediments of east coast of India. Hydrobiologia, 397, 89–99.
Ravisankar, R., Sivakumar, S., Chandrasekaran, A., Kanagasabapathy, K. V., Prasad, M. V. R., & Satapathy, K. K. (2015). Statistical assessment of heavy metal pollution in sediments of east coast of Tamilnadu using energy dispersive X-ray fluorescence spectroscopy (EDXRF). Applied Radiation and Isotopes, 102, 42–47.
Reimann, C., & de Caritat, P. D. (2000). Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environmental Science and Technology, 34(24), 5084–5091.
Reimann, C., Englmaier, P., Fabian, K., Gough, L., Lamothe, P., & Smith, D. (2015). Biogeochemical plant–soil interaction: variable element composition in leaves of four plant species collected along a south–north transect at the southern tip of Norway. Science of the Total Environment, 506, 480–495.
Rinklebe, J., Knox, A. S., & Paller, M. (2017). Trace elements in waterlogged soils and sediments: CRC Press. New York: Taylor & Francis Group.
Rinklebe, J., & Shaheen, S. M. (2017). Geochemical distribution of Co, Cu, Ni, and Zn in soil profiles of Fluvisols, Luvisols, Gleysols, and Calcisols originating from Germany and Egypt. Geoderma, 307, 122–138.
Rizwan, M., Ali, S., Adrees, M., Rizvi, H., Zia-ur-Rehman, M., Hannan, F., et al. (2016). Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environmental Science and Pollution Research, 23(18), 17859–17879.
Sałata, A., & Dąbek, L. (2017). Sediments from stormwater drainage system as sorbents of organic and inorganic pollutants. In E3S Web of Conferences (Vol. 22, p. 00152). EDP Sciences.
Salomons, W., & Förstner, U. (1984). Sediments and the transport of metals. In Metals in the hydrocycle (pp. 63–98). Berlin: Springer.
Samanta, K., & Hazra, S. (2012). Landuse/landcover change study of Jharkhali Island Sundarbans, West Bengal using remote sensing and GIS. International Journal of Geomatics and Geosciences, 3(2), 299.
Sany, S. B. T., Salleh, A., Sulaiman, A. H., Sasekumar, A., Tehrani, G. H. A. Z. A. L. E. H. M. O. N. A. Z. A. M. I., & Rezayi, M. A. J. I. D. (2012). Distribution characteristics and ecological risk of heavy metals in surface sediments of West Port, Malaysia. Environment Protection Engineering, 38(4), 139–155.
Sarkar, S. K., Frančišković-Bilinski, S., Bhattacharya, A., Saha, M., & Bilinski, H. (2004). Levels of elements in the surficial estuarine sediments of the Hugli River, northeast India and their environmental implications. Environment International, 30(8), 1089–1098.
Sarkar, S. K., Mondal, P., Biswas, J. K., Kwon, E. E., Ok, Y. S., & Rinklebe, J. (2017). Trace elements in surface sediments of the Hooghly (Ganges) estuary: Distribution and contamination risk assessment. Environmental Geochemistry and Health, 39(6), 1245–1258.
Schiff, K. C., & Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in southern California coastal shelf sediments. Marine Environmental Research, 48(2), 161–176.
Schneider, A. R., Cancès, B., Breton, C., Ponthieu, M., Morvan, X., Conreux, A., et al. (2016). Comparison of field portable XRF and aqua regia/ICPAES soil analysis and evaluation of soil moisture influence on FPXRF results. Journal of Soils and Sediments, 16(2), 438–448.
Selvaraju, R., Raman, R. G., Narayanaswamy, R., Valliappan, R., & Baskaran, R. (2009). Trace element analysis in hepatitis B affected human blood serum by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Romanian Journal Biophys, 19(1), 35–42.
Shaheen, S. M., Shams, M. S., Khalifa, M. R., Mohamed, A., & Rinklebe, J. (2017). Various soil amendments and environmental wastes affect the (im) mobilization and phytoavailability of potentially toxic elements in a sewage effluent irrigated sandy soil. Ecotoxicology and Environmental Safety, 142, 375–387.
Shtangeeva, I. V. (1994). Variation of the elemental composition of plants and soils. Journal of Radioanalytical and Nuclear Chemistry, 177(2), 381–391.
Shtangeeva, I., Alber, D., Bukalis, G., Stanik, B., & Zepezauer, F. (2009). Multivariate statistical analysis of nutrients and trace elements in plants and soil from northwestern Russia. Plant and Soil, 322(1–2), 219–228.
Singh, A. K., & Singh, M. (2006). Lead decline in the Indian environment resulting from the petrol-lead phase out programme. Science of the Total Environment, 368(2–3), 686–694.
Subramanian, V. (1993). Phosphorus, silicon, and some trace contaminants in the Ganges Estuary. Estuaries and Coasts, 16(3), 453–458.
Subramanian, V., Jha, P. K., & Van Grieken, R. (1988). Heavy metals in the Ganges estuary. Marine Pollution Bulletin, 19(6), 290–293.
Subramanian, R., Subbramaniyan, P., & Raj, V. (2012). Determination of some minerals and trace elements in two tropical medicinal plants. Asian Pacific Journal of Tropical Biomedicine, 2(2), S555–S558.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental geology, 39(6), 611–627.
Sutton, D. C., Gillan, F. T., & Susic, M. (1985). Naphthofuranonephytoalexins from the grey mangrove, Avicennia marina. Phytochemistry, 24(12), 2877–2879.
Swain, S. S., Ray, D. K., & Chand, P. K. (2012). ED-XRF spectrometry-based trace element composition of genetically engineered rhizoclones vis-à-vis natural roots of a multi-medicinal plant, butterfly pea (Clitoria ternatea L.). Journal of Radioanalytical and Nuclear Chemistry, 293(2), 443–453.
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: its composition and evolution. Oxford: Blackwell.
Tolonen, M. (1990). Vitamins and minerals in health and nutrition. New York: Elsevier.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen, 33(1), 566–575.
Tyokumbur, E. T., & Okorie, T. (2011). Bioconcentration of trace metals in the tissues of two leafy vegetables widely consumed in South West Nigeria. Biological Trace Element Research, 140(2), 215–224.
Valentino, L. A. (2006). Heavy metal FIX for Christmas wounds. Blood, 108(9), 2888.
Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38.
Watts, M. J., Mitra, S., Marriott, A. L., & Sarkar, S. K. (2017). Source, distribution and ecotoxicological assessment of multielements in superficial sediments of a tropical turbid estuarine environment: A multivariate approach. Marine Pollution Bulletin, 115(1), 130–140.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica etCosmochimica Acta, 59(7), 1217–1232.
Willey, N. J., & Fawcett, K. (2006). Inter-taxa differences in root uptake of 103/106 Ru by plants. Journal of Environmental Radioactivity, 86(2), 227–240.
Xie, J. T., Mehendale, S. R., Wang, A., Han, A. H., Wu, J. A., Osinski, J., et al. (2004). American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacological Research, 49(2), 113–117.
Yagi, S., AbdRahman, A. E., ELhassan, G. O., & Mohammed, M. A. (2013). Elemental analysis of ten sudanese medicinal plants using X-ray fluorescence. Journal of Applied and Industrial Sciences, 1(1), 49–53.
Zeiner, M., Cindrić, I. J., Požgaj, M., Pirkl, R., Šilić, T., & Stingeder, G. (2015). Influence of soil composition on the major, minor and trace metal content of Velebit biomedical plants. Journal of Pharmaceutical and Biomedical Analysis, 106, 153–158.
Zhang, J., & Liu, C. L. (2002). Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science, 54, 1051–1070.
Zhang, J. E., Liu, J. L., Ouyang, Y., Liao, B. W., & Zhao, B. L. (2010). Removal of nutrients and heavy metals from wastewater with mangrove Sonneratia apetala Buch-Ham. Ecological Engineering, 36(6), 807–812.
Zhao, D., Wan, S., Yu, Z., & Huang, J. (2015). Distribution, enrichment and sources of heavy metals in surface sediments of Hainan Island rivers, China. Environmental Earth Sciences, 74(6), 5097–5110.
Zhou, Y. W., Peng, Y. S., Li, X. L., & Chen, G. Z. (2011). Accumulation and partitioning of heavy metals in mangrove rhizosphere sediments. Environmental Earth Sciences, 64(3), 799–807.
Zhu, F., Chen, X., Yuan, Y., Huang, M., Sun, H., & Xiang, W. (2009). The chemical investigations of the mangrove plant Avicennia marina and its endophytes. The Open Natural Products Journal, 2(1), 24–32.
Acknowledgements
MB and PC are thankful to UGC, University of Calcutta and Government of West Bengal, India [Grant No. UGC/971/Fellow (Univ)]; SG and PC acknowledge DST, Government of India [Grant number SR/FT/LS-155/2011]; MB, SG and PC acknowledge UGC-DAE, Kolkata Centre for providing financial and infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest among them.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakshi, M., Ghosh, S., Ram, S.S. et al. Sediment quality, elemental bioaccumulation and antimicrobial properties of mangroves of Indian Sundarban. Environ Geochem Health 41, 275–296 (2019). https://doi.org/10.1007/s10653-018-0145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0145-5