Abstract
This study aims to investigate the pollution rate of heavy metals on the western seaboard of Bandarabbas in southeast Iran using a new pollution index. The bulk of the analysis was conducted on sediments, followed by selection of a few samples to perform experiments on chemical partitioning studies as well as biological accessibility. On this basis, the proportions of natural and anthropogenic elements were calculated. Finally, with regard to chemical separation results, the pollution rate was calculated based on Muller's geo-chemical index, enrichment factor, pollution index, and a new "Risk" index. Chemical separation showed the anthropogenic origin of elements are as follows: Ni(27.5%) > Zn(6.5%) > Pb(2%) > Al (0.2%). The newly developed pollution index is indicative of "low environmental pollution "for Ni.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most intensely studied contaminants in marine environments are heavy metals, a group of elements with atomic weights ranging from 63.546 to 200.590 [1]. Given their toxicity to marine organisms above threshold availability and their persistence in the environment, heavy metals pose a potentially hazardous condition [2, 3].
The coastal supply of heavy metals results mainly from atmospheric and riverine delivery systems. Aside from natural sources , a wide range of human activities lead to accumulation of toxic metals in the coastal sediments, which serve as a repository for these elements [4–7] and also as a source of contaminants [8, 9].
Only a minor fraction of metals escape to coastal waters from this system [10].
Bottom sediments contain from three to five times the concentration of heavy metals found in overlying waters, and so bioavailability of even a small fraction of the total sediment burden assumes considerable importance [11].
Enrichment of heavy metals due to industrialization and urbanization was recorded in sediments of coastal seas all over the world [12–22].
The monitoring of metal contents from anthropogenic activities is particularly important for the assessment of environmental quality and protection [23–25].
Many scientists have concentrated on salt marshes and estuaries and coastal areas as they are sensitive, fertile, and often directly and seriously affected by pollutants [26, 27].
Bays comprise a complex system involving interplay among various physical, chemical, and biological factors [28–32]. Heats as well as suspended and dissolved substances are factors likely to affect the dispersion of trace elements in the waters, and the accumulation or mobilization of trace elements in the sediments of bays [20, 33].
Förstner and Wittman [34] used a sediment pollution index to assess heavy metals pollution of rivers in Germany. Geo-chemical studies of sediments for the assessment of pollution are useful [35]. Most of rare elements are more susceptible to enrichment in organic sediments rather than in non-organic sediments [34]. The chemical bonds of metals in sediments and suspended solid particles are important factors in detecting pollution in a specific phase of the aquatic environment. Many sequential digestion and chemical partitioning methods have been developed and used to detect heavy metals bonding and pollution detection in particular phases [36]. Metals that are associated with organics, sulfide and carbonates fractions of sediment can be released into the overlying water under severe anoxic conditions, thus imposing higher risk to the aquatic life [37, 38].
The aim of this study is to determine the risk of accumulation of heavy metals in the near-shore sediments of Bandarabbas, their origin and association with different sedimentary phases. The rate of pollution is described by a new index referred to as "I Risk" and can be compared with Muller's geo-chemical index (I geo), enrichment factor (EF), and pollution index. Geo-chemical indices can shed more light on sediments pollution. The study area is in the geographical location: 55o 58′–56o 31′E longitude and 27o 09′–27o 00′ N latitude, northeast of the Persian Gulf. Bandarabbas has a hot and dry climate. Average annual rain fall in the ten most recent years was about 180 mm. Annual mean temperature has varied in the range of 24–27°C. The soil of the province is mainly salty and alkali. Many industries such as oil refining, aluminum smelting, steel, zinc and lead production facilities, shipbuilding yards, cement factories, and important commercial quays and ports are located in the west of Bandarabbas. Furthermore, most traditional and commercial fishing is carried out in this district and is close to the Hara Protected Area (Mangrove Forests). For the aforementioned reasons, this research was conducted in the west of Bandarabbas.
Materials and Methods
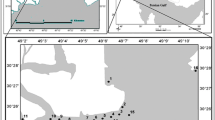
About 26 surficial sediments (top 15 cm) were collected by Peterson grab in the summer of 2009 (Fig. 1).
Grain sizes smaller than 63 μ were separated and dried at a temperature of 50°C for 24 h. Grain size smaller than 63 μ contains more sorbed metals due to its larger surface area [42, 43]. Sampling method was based on the MOOPAM method [39]. Accordingly, a Peterson grab was used to collect the surficial sediment. The samples were powdered by agate mortar and pestle. For bulk digestion, sediment samples were digested by the US Environmental Protection Agency (USEPA), 3050B method. For the digestion of samples, 1 g (dry weight) sample is digested with repeated additions of nitric acid and hydrogen peroxide (H2O2). Then, sample was refluxed by HCl and solution is made up to 100 mL volume.
Then, analysis of samples was conducted using an atomic absorption device (Thermo, model MS). A standard sample IIAEA-433-CRM was used to assess device rate of sensitivity, data accuracy, and reduction of errors. Eleven samples showing minimum, maximum, and medium contents of each metal under study were selected for chemical separation and biological accessibility tests. A three-step sequential extraction including (a) 25% v/v HOAc, (b) 25% v/v HOAc–0.1 M hydroxylamine hydrochloride, and finally (c) 30% w/v H2O2 was used to achieve separation of loosely bonded ions., sulfide and organic bonds [40]. Tests were performed in accordance with the USEPA method to obtain bioaccessibility values. For this reason a solution of pH = 5 was prepared using NaOH and HOAc (USEPA, 1311 method). Cluster analysis and multi-variation method were carried out in this research using MVSP software to establish the statistical origin of elements [41].
In this study, the pollution rate is calculated using Muller’s geo-accumulation index as follows:
- Cn:
-
= measured concentration of the element in the politic sediment fraction (<2 μm)
- Bn:
-
= concentration of element in fossil argillaceous sediment (average shale) [45]
- 1.5:
-
= possible variation of the background data due to lithogenic affects
Also I POLL [42] was used for further comparsion. I POLL can be presented as:
- Bn:
-
= Lithogenous portion of element obtained through chemical partitioning studies
There is a ruler for each of the two aforementioned formulas to find the rate of pollution. The I geo as well as I POLL consists of seven grades (06) where the highest grade [6] reflects 100-fold enrichment above the background level.
These two indices were also compared with Australian scientists' enrichment factor
- CnE :
-
= concentration of metal in sampling station
- AlE :
-
= concentration of aluminum in sampling station
- CnC :
-
= concentration of metal in earth crust
- ALC :
-
= concentration of aluminum earth crust
- EF:
-
= enrichment factor
1 < EF → sure, there is pollution in environment under study.
EF = 1→ the environment under study is perfectly natural and lacks pollution.
EF < 1→ environment under study lacks pollution, but the physical and chemical specifications have changed in a way that have released heavy element from sediment. Results of pollution indices appear in Tables 1, 2, and 3.
Results
The results of elemental contents in coastal sediments of the study area are shown in Table 4.
The average concentration of nickel obtained in this study is about 138 mg/Kg with lowest and highest value is 73 and 211 mg/kg, respectively. A comparison of average nickel concentration in the area of study with its concentration in the earth’s crust (80 mg/Kg) and world sediment average (52 mg/kg) shows that Ni is higher than (2.6 times) the world sediments average and (1.6 times) Persian Gulf values. In order to distinguish between lithogenous and non-lithogenous (anthropogenic) fractions of metals in sediments, the amount of concerned metal obtained from chemical partitioning studies were summed up. Subsequently, 10% of total metal content that is obtained from bulk digestion was deducted and added up to the lithogenous fraction [42]. It is believed that 10% of total elemental contents can naturally appear in chemical partitioning extractions [42]. Results of chemical separation show that 72.5% of nickel in the area originated from land, while only 27.5% has human origin (Table 5).
The results of bulk digestion analysis of Zn show higher concentrations (140 mg/kg) in comparison with the Earth’s crust (75 mg/kg) and world sediments and the Persian Gulf standard (69 mg/kg). The highest Zn content is found near ore mineral loading/unloading sites as well as near-ship painting centers (Table 6).
Average Pb in the area under study is 18.20 mg/Kg. It is higher than Earth’s crust average (14 mg/Kg) and slightly lower than world sediments average (19 mg/Kg). Approximately 98% of total Pb content in the area originates from lithogenous sources and only 2% is derived from human activity (Table 7).
Average values of Sn in sediments is (0.90 mg/Kg) less than Earth’s crust average (2.2 mg/kg).Traces of Sn were not found in chemical partitioning measurements. Aluminum with an average of 8.5% was slightly higher than the world sediment average (7.2%) and Earth’s crust average (8.2%). Results of chemical separation showed that aluminum has a lithogenous source (Table 8).
Aluminum can be considered as source of alumino-silicate parent rocks or rock-bearing aluminum such as bauxite. The basin of area under investigation possesses Al-rich bauxite. It should be pointed out that due to presence of Al in the basin of area of study; an Al smelter company (Almahdi Co.) with production capacity of 25,000 t/year is active. This clearly brings out the importance of geology of area of study in governing the elemental contents in sediments.
Figure 2 shows a dendogram of cluster analysis. The dendogram consists of two dominant clusters (A and B). In cluster A, there are two elements (Al and Pb). These two elements join each other at about insignificant positive similarity coefficient (<0.45). Al is known as an indicator of lithogenous sources. Therefore, in spite of the presence of Pb in the cluster (A), it cannot be inferred that Pb is originates from aluminum–silicate rocks. In cluster B, Ni, Zn, and Sn are joined to each other at positive, and significant similarity coefficients (>0.45). Nickel is considered as an indicator of oil pollution [43]; therefore, its higher similarity coefficient with Zn may be indicative of the similar source for zinc. However, justification of this statement needs more investigation.
A very weak connection between Sn and the other two elements in cluster (B) needs further investigation. Two clusters (A and B) combine under a negative but insignificant similarity coefficient. Therefore, the origin of elements in clusters "A" and "B" differ from each other.
Subsequently, various indices were used to show the intensity of pollution in the area of study. However, it should be pointed that while these indices have their own applications and merits but they lack on defining the "Risk" within an environment. Therefore, we tried to develop a new index named I Risk in the present investigation that account for "Risk". Presence of an specific amount of metals in an environment may not necessarily be indicative of its interaction with surrounding area. Therefore, we prefered to use "Bio-accessiblity" fraction in deveolping the new pollution/risk index. The new index can be defined as:
where
- BA:
-
Bioaccessibility
- I POLL :
-
Eq. 2.
I geo, I POLL, and I Risk indices show an unpolluted environment for Pb. However, some of the sampling stations show low to medium pollution for Ni. The two indices (I geo and I POLL) indicate an unpolluted environment for Zn while EF and I Risk are indicative of low to medium pollution for Zn in some of the sampling locations.
Conclusions
In the recent year, many researchers have attempted to evaluate the pollution intensity in aquatic environment [46–48]. These studies have repeatedly used the already developed indices. Therefore, in the present investigation, it is tried to bring out an index that can show better results. For this reason, bulk digestions along with chemical partitioning extractions were carried out to distinguish between the anthropogenic and non-anthropogenic contents of metals in aquatic sediment. Subsequently I POLL index was modified using bio-accessibility of metals.
The average metal concentrations obtained in the present investigation were also compared with those published NOAA Marine Sediment Quality Guideline Values (Table 9) as well as the Canadian Interim Marine Sediment Quality Guideline. Table 9 also presents the probable effects level, effects range low (ERL), and effects range medium (ERM) of metals in sediments. It can be noticed that Ni stands at higher concentrations in comparison with ERL and ERM while contents of Zn and Pb are lower than ERL and ERM.
The pattern of anthropogenic portion of metals in the area of study is Ni (27.5%) > Zn(6.5%) > Pb(2%) > Al(0.2%). Comparison with results obtained in this study at Bandarabbas coastal sediments and other standards indicates higher values of Ni and Zn and even Pb in comparison with local standards. Pollution rate indices also show low to medium pollution for Zn and Ni at some locations. Results of the I Risk index show the pollution risk of studied metals as Ni(1.45) > Zn(0.47) > Pb(0.08). It suggests that environmental pollution intensity for Nickel is low, while the profiles for Pb and Zn imply virtually no pollution. The values obtained in the present investigation are approximate due to the low number of samples and hence further studies are recommended.
References
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. Rev Aquat 1(2):295–317
Kennish MJ (1997) Estuarine and marine pollution: heavy metals. CRC Press, Boca Raton, p 253
Clarck RB (1992) Marine pollution. Oxford, Oxford University Press, pp 172–184
Luoma SN (1990) Processes affecting metal concentrations in estuarine and coastal marine sediments. In: Furrness RW, Rainbow PS (eds) Heavy metals in the marine environment. CRC Press Inc, Boca Raton, pp 51–66
Heyvaert AC, Reuter JE, Sloton DG, Goldman CR (2000) Paleo-limnological reconstruction of historical atmospheric lead and Hg deposition at Lake Tahoe, California–Nevada. J Environ Sci Tech 34:3588–3597
Yang H, Rose NL (2003) Distribution of Hg in the lake sediments across the UK. Sci Total Environ 304:391–404
Caccia VG, Millero FJ, Palanques A (2003) The distribution of trace metals in Florida Bay sediments. Mar Pollut Bull 46:1420–1433
Adams WJ, Kimerle RA, Barnett RA Jr (1992) Sediment quality and aquatic life assessment. Environ Sci Tech 26:1865–1875
Burton GA Jr, Scott KJ (1992) Sediment toxicity evaluation, their niche in ecological assessment. Environ Sci Tech 26:2068–2075
Sinex SA, Wright DA (1988) Distribution of trace metals in the sediments and biota of Chesapeake Bay. Mar Pollut Bull 19:425–431
Bryan GW, Langstone WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76:89–131
Allen JRL, Rae JE (1986) Time sequence of metal pollution, Severn Estuary, southwestern UK. Mar Pollut Bull 17:427–431
Barcellos C, Rezende CE, Pfeiffer WC (1991) Zn and Cd production and pollution in a Brazilian coastal region. Mar Pollut Bull 22(11):558–561
Farmer JG (1991) The perturbation of historical pollution records in aquatic systems. Environ Geochem Health 13(2):76–83
Coker WB, Kettles IM, Shilts WW (1995) Comparison of mercury concentrations in modern lake sediments and glacial drift in the Canadian Shield in the region of Ottawa/Kingston to Georgian Bay, Ontario, Canada. Water, Air and Soil Poll 80:1025–1029
Birch GF, Evenden D, Teutsch ME (1996) Dominance of point source in heavy metal distributions in sediments of a major Sydney estuary (Australia). Environ Geo 28(4):169–174
Lottermoser BG (1998) Heavy metal pollution of coastal river sediments, north-eastern New South Wales, Australia: lead isotope and chemical evidence. Environ Geol 36(1–2):118–126
Gallasso JL, Siegel FR, Kravitz JH (2000) Heavy metals in eight 1965 cores from the Novaya Zemlya Trough, Kara Sea, Russian Arctic. Mar Pollut Bull 40(10):839–852
Manta DS, Angelone M, Bellanca A (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300(1–3):229–243
Al-Masri MS, Aba A, Khalil H, Al-Hares Z (2002) Sedimentation rates and pollution history of a dried lake: Al-Oteibeh Lake. Sci Total Environ 293(1–3):177–189
Feng H, Han X, Zhang W, Yu L (2004) A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar Pollut Bull 49(11-12):910–915
Pekey H (2006) The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Mar Pollu Bull 52(10):1197–1208
Owen RB, Sandhu N (2000) Heavy metal accumulation and anthropogenic impacts on Tolo Harbour, Hongkong. Mar Poll Bull 40(2):174–180
Spencer KL (2002) Spatial variability of metals in the inter-tidal sediments of the Medway Estuary, Kent, UK. Mar Pollut Bull 44:933–944
De Carlo EH, Anthony SA (2002) Spatial and temporal variability of trace element concentrations in an urban subtropical watershed, Honolulu, Hawaii. Appl Geochem 17:475–492
Arellano JM, Ortiz JB, Capeta da Silva D, Gonzalez de Cannales MI, Sarasquete C, Blasco J (1999) Levels of copper, zinc, manganese and iron in two fish species from salt marshes of Cadis Bay(southwest Iberian Peninsuala). Boletin del Instituto Espanol de Oceanografia 15(1–4):458–488
Cohen T, Hee S, Ambrose R (2001) Trace metals in fish and invertebrates of three California coastal wetlands. Mar Pollut Bull 42(3):232–242
Hutchinson GE (1957) A treatise on limnology. V.1, Geography, physics and chemistry. Wiley, New York, p 1015
Ruttner F (1963) Fundamentals of limnology. University of Toronto Press, Toronto, p 295
Mortimer CH (1971) The exchange of dissolved substances between mud and water in lakes. Part III and IV. J Ecol 30:147–207
Sain KS, Neufeld RD (1942) A dynamic model of biogeochemical cycle of heavy and trace metals in natural aquatic systems. Paper presented at the Second International Symposium on Environmental Biogeochemistry. Burlington, Ontario, Canada, 20
Coker WB, Shilts WW (1979) Lacustrine geochemistry around the north shore of Lake Superior: implications for the evaluation of the effects of acid precipitation. In Current Research, Part C, Geological Survey of Canada, Paper 79-1°C: 1–15
Butman B, Bothner MH (1998) Predicting the long-term fate of sediments and contaminants in Massachusetts Bay. US Geological Survey Fact Sheet, 172–97
Förstner U, Wittman G (1981) Metal pollution in the aquatic environment, 2nd edn. Springer, NY, pp 110–270
Holm NG (1988) Arsenic regeneration from estuarine sediments of the Bothnian Bay, Sweden. J Chem Geol 68:89–98
Chester R, Hughes RM (1967) A chemical technique for the separation of ferromanganese minerals, carbonate minerals and adsorbed trace elements from pelagic sediments. Chem Geol 2:249–262
Lee SV, Cundy AB (2001) Heavy metal contamination and mixing processes in sediments from the Humber Estuary, Eastern England. Estuar Coast Shelf Sci 53(5):619–636
Karbassi AR, Amirnezhad R (2004) Geochemistry of heavy metals and sedimentation rate in a bay adjacent to the Caspian Sea. Int J Environ Sci Tech 1(3):199–206
MOOPAM–ROPME (1999) Manual of oceanographic observations and pollutant analysis methods. MOOPAM, Kuwait
Chen Z (2004) Low concentration of heavy metals in the Yangtze estuarine sediments, China: a diluting setting. Estuarine Coast Shel Sci 60(1):91–100
Davis JC (1973) Statistics and data analysis in geology. Wiley International, New York
Karbassi AR, Monavari SM, Nabi Bidhendi Gh R, Nouri J (2008) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147:107–116
Karbassi AR (1997) Geochemistry of Ni, Zn, Cu, Pb, Co, Cd, V, Mn, Fe, Al & Ca in sediments of North Western part of the Persian Gulf. Intl J Env Studies 54:205–212
Bowen HJM (1979) Environmental chemistry of the elements. Academic press, London, p 333
Wedepohl KH (1971) Environmental influences on the chemical composition of shales and clays. In: Ahrens LH, Press F, Runcorn SK, Urey HC (eds) Physics and chemistry of the Earth, 8. Oxford, Pergamon, pp 307–331
Priju CP, Narayana AC (2007) Heavy and trace metals in Vembanad Lake sediments. Int J Environ Res 1(4):280–289
Praveena SM, Ahmed A, Radojevic M, Abdullah MH, Aris AZ (2008) Heavy metals in mangrove surface sediment of Mengkabong lagoon, Sabah: multivariate and geo-accumulation index approaches. Int J Environ Res 2(2):139–148
Ratheesh Kumar CS, Joseph MM, Gireesh Kumar TR, Renjith KR, Manju MN, Chandramohanakumar N (2010) Spatial variability and contamination of heavy metals in the inter-tidal system of a tropical environment. Int J Environ Res 4(4):691–700
Acknowledgment
Thanks to all who assisted in this research and especially to Mr. Nasser Aghajeri and Mr. Akbarzadeh in the Persian Gulf and Oman Sea Ecological Research Institute for performing the tests and sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farsad, F., Karbassi, A., Monavari, S.M. et al. Development of a New Pollution Index for Heavy Metals in Sediments. Biol Trace Elem Res 143, 1828–1842 (2011). https://doi.org/10.1007/s12011-011-8983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-8983-3