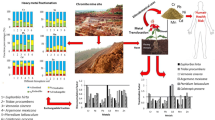
Abstract
The aim of this study was to assess probabilistic human health risk due to ethnobotanical usage of Avicennia officinalis, Porteresia coarctata and Acanthus ilicifolius. The study was conducted at the tannery outfall near Sundarban (Ramsar wetland, India) mangrove ecosystem affected by potentially toxic elements (Cd, Cr, Cu, Hg, Mn, Ni, Pb, and Zn). Total metal concentrations (mg kg-1) were considerably higher in the polluted rhizosphere namely, Cd (1.05–1.97), Cu (36.3–38.6), Cr (144–184), Hg (0.04–0.19), Mn (163–184), Ni (37.7–46.4), Pb (20–36.6), and Zn (97–104). Ecological risk index indicated low to moderate ecological risk in this site, whereas the ecological risk factor showed high potential ecological risk due to Cd pollution. BCR Sequential extraction of metals showed more exchangeable fraction of Cd (47–55%), Cr (9–13%), Hg (11–13%), and Pb (11–15%), at the polluted site. Mercury, though present in trace amount in sediment, showed the highest bioaccumulation in all the three plants. Among the toxic trio, Hg showed the highest bioaccumulation in A. officinalis, Cd in P. coarctata but Pb has the lowest bioaccumulation potential in all the three species. Occasional fruit consumption of A. officinalis and dermal application of leaf, bark of A. officinalis (antimicrobial), A. ilicifolius (anti-inflammatory, pain reliever when applied on wounds) indicated negligible human health risk. However, long-term consumption of P. coarctata (wild rice variety) seeds posed health risk (THQ>1) both in adults and children age groups. This study concludes that nature of ethnobotanical use and metal contamination levels of the mangrove rhizosphere can impact human health. The transfer process of potentially toxic elements from rhizosphere to plants to human body should be considered while planing pollution mitigation measures.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are one of the most resilient ecosystem, playing a vital role in conservation of tropical/sub-tropical region, acting as a buffer between land and sea, as well as protecting the dense population residing along these coasts from natural disasters (Marchand et al. 2006; Maiti and Chowdhury 2013). Despite their role in maintaining the coastal ecosystem, mangroves are threatened and are disappearing worldwide at the rate of 1 to 2% per year, as a result of over-exploitation, pollution, and land conversion (Marchand et al. 2016).
Recent studies have focused on the pollution issues of potentially toxic elements (PTEs) in mangrove habitats spanning across the globe like in China, Japan, Papua New Guinea, Bangladesh, Indonesia Australia, Brazil, USA, and India (Tam and Wong 2000; Akhand et al. 2012; Souza et al. 2015; Rodríguez-Iruretagoiena et al. 2016; Marchand et al. 2016; Anbuselvan and Sridharan 2018). Exposure of PTEs can cause deleterious health impacts on human and environment (Marchand et al. 2016; Naz et al. 2021). Sediment being the “source and sink” of PTEs has the potential to transport PTEs to plants, growing on the contaminated sediment along the riverbanks. Recent studies have identified halophytes with high potential of metal accumulation in their roots and shoots growing in saline/hypersaline coastal soil or estuaries (Agoramoorthy et al. 2008; Akhand et al. 2012; Nath et al. 2014; Sarkar et al. 2014;Akhand et al. 2016; Chowdhury and Maiti 2016a, 2016b). Hence, using these plants with bioaccumulated PTEs in a polluted estuarine environment can cause deleterious human health hazards. Researchers tried to predict the probabilistic health risk associated with consumption/ingestion of water or food products and dermal exposure to toxic metal contaminated dust/soil at polluted regions (Zheng et al. 2007; Chotpantarat et al. 2014; Chowdhury et al. 2015; Naz et al. 2016a, 2016b; Naz et al. 2018). But, other exposure routes such as usage of plants with ethnobotanical properties are generally not investigated by environmentalists while assessing human health hazard. India and most of the Asian countries believe on the ethnobotanical usage of the natural plant and mangroves have widely been used in traditional medicine or as food adjuncts (Bandaranayake 1998; Sheng-Ji 2001; Muthu et al. 2006; Dahdouh-Guebas et al. 2006; Qasim et al. 2014). Recent study by Naz et al. 2020 has identified health risks due to the ethnobotanical usage of plants growing at a Chromite mine area of India contaminated by potentially toxic elements. But till date, there no reported research on metal contamination impact due to the ethnobotanical usage of mangroves on human health.
Mangroves are widely used for ethnomedicinal properties in Asiatic nations (Wu et al. 2008). Research by Nabeelah Bibi et al. 2019indicates that India and Bangladesh accounts for about 51% of global use of mangrove as ethnopharmacological supplements. Avicennia officinalis, Porteresia coarctata, and Acanthus ilicifolius have wide ethnobotanical usage in the region. Scientific evidences prove that leaf of A. officinalis has alkaloids - terpenoids, phenolics, pentacyclic triterpenoids, and flavonoids (Shanmugapriya et al. 2012; Hossain 2016). Leaves of this plant are widely used as powder or paste for ameliorating joint pain as well as healing of wounds by the local community. A.ilicifolius leaf contains terpenoid, phenolic compounds and is widely used across India, Bangladesh as aphrodisiac, to alleviate rheumatism pain, relief for asthma, diabetes, diuretic, dyspepsia, leprosy, hepatitis, blood purifier, cold cure, gangrenous wounds, skin allergies, and snake bites (Bandaranayake et al. 1998; Babu et al. 2001;Hossan et al. 2010; Firdaus et al. 2013; Nabeelah Bibi et al. 2019). P. coarctata is a wild rice variety with a mean carbohydrate content of 39% of dry weight and is sometime taken as a good grain by local communities in times of acute food crisis during cyclone or storm surges. (Sengupta and Majumder 2010; Ghosh and Mitra 2015).
Literature review of available academic studies revealed limited information on metal accumulation in different plant parts of the three mangroves (Avicennia officinalis, Porteresia coarctata, and Acanthus ilicifolius), growing naturally in a contaminated habitat (Chakraborty et al. 2013; Shackira and Puthur 2013; Wu et al. 2015; Chakraborty et al. 2018). Hence, understanding the bioaccumulation processes of PTEs can act as a proxy to comprehend the metal uptake potential and eventual human health risk posed by use of these plant parts in traditional medicine around the mangrove habitats.
In this study, we have considered the PTE transport processes, their chemical fractions in sediment and their transfer to different mangrove plant parts and eventual health hazard due to the traditional use. This study was conducted at tannery, municipal out-fall site near the metropolitan city of Kolkata and its suburbs having an approximate population of 14.3 million as per 2011 census, which is India’s third populous metropolitan area. This outfall site is at the doorway of the world’s largest contiguous mangrove forest of Sundarbans, shared by India (40%) and Bangladesh (60%) and recently declared as a Ramsar Wetland site on February 2019 (site number: 2370). Past researches have shed light into the alarming PTE pollution (Cu, Cd, Cr, Hg, Ni, Mn, Pb, Zn) at Indian Sundarbans due to anthropogenic interventions (Saha et al. 2001;Banerjee et al. 2012; Rodríguez-Iruretagoiena et al. 2016; Akhand et al. 2016; Chowdhury and Maiti 2016a, 2016b).
Thus, the aim of this study was to address the research question, whether PTE accumulation in the rhizosphere and its subsequent bioaccumulation in plant species can be a human health concern due to the ethnobotanical use of the plant parts? The primary objective of this study was, (a) determining the concentration of PTEs (Cd, Cr, Cu, Hg, Ni, Pb and Zn) in the rhizospheric sediment of three halophytes (Avicennia, officinalis, Acanthus ilicifolius and Porteresia coarctata) growing at Kolkata municipality outfall, (b) sequential extraction of PTEs in the rhizospheric sediments of the targeted halophytes to understand the fractions of metal present in exchangeable (bioavailable), oxidisable, reducible and residual form (c) bioaccumulation potential of the concerned PTEs in different plant parts, and (d) Probabilistic human health risk assessment by the traditional usage of target plant parts growing at the study area.
Material and methods
Study site description
Mangrove and halophytes at the Ghushighata outfall (22.52373° N, 88.69492° E), exposed to the municipal sewage and tannery effluent, wasselected as the study area to understand the effect of PTEs contamination on three species, Avicennia officinalis, Acanthus ilicifolius, and Porteresia coarctata. Control site was selected amidst the undisturbed, conserved, pristine mangrove forest of Jharkhali (22.00889°N, 88.7134°E) downstream of the same river (Fig. 1).
The study area and the sampling site, (a) study area is marked out from the map of India, (b) the area of Sundarban Ramsar Wetland site, with the location of the metropolitan city of Kolkata and the un-polluted control patch along with polluted sites, (c) the polluted site is zoomed out showing the municipal and tannery effluent canal (CMC canal) and the outfall, (d) the Kulti sluice gate where the salt water from the estuary mixes with the CMC canal
Sediment sample collection and laboratory treatment
The sediment samples were collected during the monsoon to post monsoon period between August to September of 2015. The time period was selected because of this coincides with fruiting/ seed dispersal time of the three targeted mangroves (Chowdhury et al. 2016b). Considering the spatial distribution and mangrove species composition, the superficial sediment (0–30 cm) was sampled from base of target plant species from sites, near the tannery outfall as well as control site. For each species, seven individual plants were randomly selected from both polluted as well as control site and samples collected from their rhizospheric sediment. All samples were collected during low tide. At each plant rhizosphere, four sub-samples were retrieved manually using acid washed PVC pipes (30 cm length) with help of stainless steel scoop (to aid in removing the PVC pipes from mangrove mud) and mixed into a composite sample. The plant materials/parts were also collected from same plants.
The samples were preserved in ice box till their transport to laboratory. A composite sample was prepared for that particular species from seven replicated sediment samples, sieved (< 62 micron), stored in sterilized zip lock bag and kept at 4 °C in refrigerator for further analysis (APHA 1985; Maiti 2003; Anbuselvan and Sridharan 2018). The pH and electrical conductivity (EC) of the sediment samples were measured in a soil–water suspension (1:1.5; soil: deionised water) using a pH meter (Cyberscan 510) and conductivity meter (EI 601) respectively (Maiti 2013). For soil texture analysis, samples were wet sieved through a 62-μm mesh for 15 min in a mechanical sieve shaker. The sample that retained on the sieve was weighed and expressed as sand. The samples which passed through the sieve were expressed as mud. The finer fraction, mud, i.e., silts and clays (> 0.063 mm) were determined by pipette method. The soil texture characteristics of sediments are given in Table 1.
The collected sediment samples were air-dried, crushed in a porcelain mortar, sieved through a nylon sieve (Pore size, 0.45 mm), and kept in polypropylene air tight zip bags for further analysis. Organic carbon (OC) of sediment samples was determined by the rapid dichromate oxidation method (Walkley and Black 1934; Anderson and Ingram 1998). While preparing reagents for analysis of OC, 0.1 g Ag2SO4 was added before the use of H2SO4 to minimize the interference of Cl- in the saline mangrove soil as the excess Cl- get precipitated in the form of AgCl (Saha et al. 2001; Schumacher 2002; Wang et al. 2013; Chowdhury et al. 2018). K2Cr2O7 solution was used to oxidize the organic carbon in acid medium. The amount of oxidized carbon in the sample was proportional to the amount of chromic ions produced during oxidation. Colorimetric method was employed to determine the chromic ion concentration and the absorbance was recorded by using UV–visible spectrophotometer (Helios Aquamate, Thermo scientific 9423 AQA 151505, made in England) (Schumacher 2002; Perera et al. 2018).Total Kjeldhal Nitrogen (TKN) was determined by alkaline permanganate method by taking 20g of oven dried sediment sample in an 800 mL Kjeldahl flask with 20 mL of water and 100 mL 0.32% KMnO4 and 2.5% NaOH solution and boiled in presence of glass beads. The content was distillated in a Kjeldahl assembly at a steady rate. Ammonia gas released in this process was collected in a 250-mL conical flask containing 20 mL of boric acid indicator solution (pinkish color turns green) and the distillate was titrated against 0.02N H2SO4 ( Jackson 1973; APHA 1985; Maiti 2003).
Sequential extraction of metal in sediment
The optimized three-stage extraction procedure was used to determine the distribution of PTEs in different chemical fractions using the method Community Bureau of Reference (BCR) metal extraction process first introduced by Ure et al. 1993 and later applied by many researchers (Mossop and Davidson 2003; Sahuquillo et al. 2003; Naz et al. 2020). This method also been used for the sequential extraction of Hg by Sahuquillo et al. 2003 and Ge et al. 2020. The steps of the extraction process have been elaborated below:
Exchangeable fraction: A total of 40 mL of 0.11 M acetic acid was added to 1 g air-dried sediment and shaken overnight. The mixture was centrifuged to separate the extract from the residue.
Reducible fraction: A total of 40 mL of 0.5 M hydroxylammonium chloride, adjusted with nitric acid to the pH of 1.5, was added to the residue from Step 1 and the extraction performed as above.
Oxidisable fraction: The residue from Step 2 (Reducible fraction extraction) was treated twice with 8.8 M hydrogen peroxide, evaporated to near dryness, then 50 mL of 1.0 M ammonium acetate, adjusted to pH 2 with nitric acid, was added and the extraction performed as above.
Residual fraction: The material remaining at the end of the step 3 (Oxidisable fraction extraction) was digested in 20 mL aqua regia, with microwave assistance (Model: ETHOS One, Italy) at 100% power with pressure set at 120 psi for 25 min (two cycles).
The aliquots were filtered through Hg-free filter paper (pore size 2.5 μm), and analyzed for Cd, Cr, Cu, Hg, Ni, Mn, Pb, and Zn using a flame atomic absorption spectrophotometer (FAAS-GBC Avanta, Australia) at the most sensitive resonance wavelength of each element. Hg was measured in cold vapour-atomic absorption spectrophotometer (CV-AAS, GBC Avanta, Australia) (Marrugo-Negrete et al. 2015; Raj et al. 2020). The assessed PTEs concentrations were equated to the weight of dried material used for digestion.
Pollution indices
Contamination factor (\( {C}_f^i \))
Contamination factor was initially suggested by Hakanson (1980). Contamination factor was used to assess contaminated sediment by referencing the concentration of metals in the surface layer of bottom sediment to background values of that metal. Contamination factor was calculated as:
where \( {C}_m^i \)= concentration of ith element in sediment and \( {B}_m^i \) = average shale value of earth’s crust for the ith element. Based on the value of \( {C}_f^i \), the sediments are classified as low\( \Big({C}_f^i<1 \)), moderate (\( 1\le {C}_f^i\le 3 \)), considerable (\( 3\le {C}_f^i\le 6 \)) and very high pollution (\( {C}_f^i\ge 6 \)) (Hakanson 1980).
This index shed light into the potential of pollution and used to assess the tolerance of different plants for different ecological risk. The Cif is the ratio of heavy metal concentration in the sediment to the average shale value of earth crust (Hakanson 1980; Raj et al. 2017). For background concentration of metals, the average shale values for different metals were used for Pb: 20 mg/kg, Ni: 68 mg/kg, Cu: 45 mg/kg, Cd: 0.3 mg/kg, Cr: 90 mg/kg, Hg: 0.4 mg/kg, Zn: 95 mg/kg, and Mn: 850 mg/kg (Turekian and Wedepohl 1961; Uluturhan et al. 2011).
Ecological risk index (ERI)
Hakanson (1980) proposed a method to assess the potential ecological risk for areas under special conservation attention. According to this method, the potential ecological risk factor (Eir) of a single element can be assessed with formulae incorporating the toxic response of each metal as given below,
ERI is the summation of the ecological risk factors as stated below,
Eir is categorized into five divisions. Eir< 40= Low ecological risk, 40 <Eir< 80= moderate potential ecological risk, 80 <Eir < 160= considerable potential ecological risk, 160< Eir< 320= high potential ecological risk, Eir> 320= very high ecological risk. The cumulative \( {\mathrm{E}}_{\mathrm{r}}^{\mathrm{i}} \) values for all the elements indicate the level of ecological stress to different study area due to metal pollution.
Ecological risk factor (Eir) is the \( {C}_f^i \) multiplied by toxic response factor (Ti), which differs between different metals, as all metals do not possess similar negative foot print in a particular ecosystem. The toxic response factor (Ti) for Cd = 30; Cr =2; Cu = 5; Hg = 40; Mn = 1; Ni = 5; Pb = 5; and Zn = 1, were considered for the ecological risk factor analysis (Hakanson 1980; Raj et al. 2017).
Ecological risk index (ERI) is the summation of the Eir for all the individual metals. Whereas, 150< ERI= low ecological risk, 150< ERI< 300= moderate ecological risk, 300 < ERI< 600= considerable ecological risk, ERI> 600= very high ecological risk.
Plant sample collection and metal extraction
To determine PTEs in selected halophytes, different parts of the plant were collected (Supplementary materials: Table S1). Avicennia officinalis is a tree of Avicenniaceae family. Roots, barks, leaves and fruits of A.officinalis were collected separately from seven trees. In case of fruits, only one mature tree growing in the polluted site was selected to collect the fruits as other plants were not in the fruiting age. Root samples were collected in the upper 30 cm of rhizospheric sediment at low tide (n = 3 per each type of tree). Bark was collected at breast height from same plants. Porteresia coarctata, a Poaceae herb species, was uprooted with seeds. Seven such plants were collected and separated into root, shoot and spikelet. Acanthus ilicifolius is a thorny shrub of Acanthaceae family. The leaves, fruit, shoot, and roots were collected from the plants for analysis. As the leaves were thorny, extreme care was taken while separating the leaves and fruits from shoot with help of an acid washed stainless steel scissors. All the plant parts were dried, digested, and analyzed three samples for each plant parts (n= 3). That data was used to estimate health risk due to accumulated PTEs. Similar method of sampling was employed by Thành-Nho et al. 2019.
All the collected plant samples were washed several times with distilled water in an ultrasonicator before processing for metal analysis. After that, samples were oven dried at 40°C until a constant weight was achieved and fine particle were made by using mortar and pestle. The fine sized plant samples, (0.5 g) of each parts with three replicates (roots, shoots, fruits, and seeds were digested using microwave-assisted digestion system (Ethos One, Milestone Srl, Italy) HNO3 (9–71% Nitric acid, 5 mL; and 30% Hydrogen peroxide, 2 mL) at 90°C and made up the volume up to 100-mL with 1% HNO3 (Chowdhury et al. 2016a; Marrugo-Negrete et al. 2015; Raj et al. 2017; Raj et al. 2020). Blank acid mixtures were digested in the same way. The digested plant samples were passed through Whatman no. 42 filter paper (pore size 2.5 μm), diluted with Hg-free Millipore water and measured in cold vapour-atomic absorption spectrophotometer (CV-AAS, GBC Avanta, Australia) for Hg estimation. Other trace metal concentrations in all the samples were analyzed using flame atomic absorption spectrophotometer (FAAS-GBC Avanta, Australia). Inter batch variations were monitored by repeated analysis of selected samples in various analytical batches (less than 10% relative variation).
Metal accumulation indices
Following formulae was used to determine metal accumulation factors, i.e., bio-accumulation factor (BAF). The mean metal concentration in the sediments and mangrove plants was considered for calculating the BAF for all three plant species.
Heath risk assessment
The human health risk assessment was carried out by using USEPA model (Wongsasuluk et al. 2014; Naz et al. 2018; Rahman et al. 2019). The exposure of PTEs via ingestion of mangrove plant parts (fruits of A.officinalis and seed of P. coarctata) and by dermal exposure of leaf and bark of A. officinalis and A. ilicifolius) grown in polluted and control (non-polluted) sites were analyzed for children and adult population. The average exposure, frequencies, and dosage were established by consultation with local people and available literature as per Naz et al. 2020 (Table 2). The plant parts, i.e., fruit, leaves, bark were considered in terms of their average weight of use for calculating the human health risk, as elucidated in Table 2. Applied formulae for human health risk calculation was
where ADDing (mg/kg-d) is average daily dose by ingestion, ADDder (mg/kg-d) is average daily dose by dermal contact, C (mg/kg) is average PTE concentration, EF (Events/day) is exposure frequency, ED (years) is exposure duration, Bw (kg) is average body weight, AT (days) is average time, SL(mg/cm2/h) is skin adherence factor, ABS is absorbance. RfD (mg/kg-d) is the reference dose. HQ is hazard quotient and THQ is total hazard quotient. THQ is the cumulative sum of all the individual metal’s HQs (Liu et al. 2020; Tong et al. 2020). In the present study, the exposure frequency (EF) for fruits of A. officinalis was taken 30 days in year, for P. coarctata 150 days in year for ingestion; however, the EF for dermal exposure for leaf and bark of A. officinalis and A. ilicifolius was taken 100 days/year. The exposure duration (ED) for children and adult was taken 12 and 70 years and an average body weight was considered 27 and 70 kg respectively (Naz et al. 2018). The absorbance of each metal was taken 0.001 for people of both age groups (USEPA 2020; Ihedioha et al. 2017). As leaf and bark applied only specific part of the body the skin adherence factor (SL), was considered only 5% of total surface area for both adult and for children (Naz et al. 2018). SA was body surface area (m2). The average surface area was calculated by using the formulae SA = 0.015924 (H × W)0.5, where H is average height (cm) and W is average body weight in kg (Redlarski et al. 2016; Yu et al. 2003). Traditional usage of mangrove plant parts donot involves a whole body exposure and dermal applications are restricted to limited part of the body surface. Hence, the maximum exposed body surface area was taken to be 1.7% of total surface (Table 2). Reference dose (RfD) is no-observed adverse effect level (NOVEL) of any exposure of target toxic substance over a long time duration (up to a lifetime) on the human population. Hence, this assessment is based on “chronic” toxicity and not limited to acute responses of the contaminants. RfD is calculated by dose response curve, in which X-axis denotes dose (in mg/kg of body weight/d), and Y axis is response (Barnes et al. 1988; USEPA 2002). The RfD factor used for the calculation in this health risk study was adopted from literature and presented in the supplementary material (Supplementary materials: Table S2).
Quality control and assurance (QA/QC)
All the analysis was carried out at “Ecological Restoration laboratory, department of Environmental Science and Engineering” in Indian Institute of Technology (Indian School of Mines), Dhanbad, India. Analytical grade glassware and reagents were used in whole analysis, and the standard solutions were prepared with millipore deionized water. The accuracy and precision of the PTE analysis were checked against Certified Reference Material, MESS-4 of marine sediment from the National Research Council, Canada for sediment (Cuong and Obbard 2006; Raj et al. 2017; Pal and Maiti 2019). The accuracy of the microwave-assisted acid digestion procedure for total metal determinations was checked using the Certified Reference Material, MESS-4 of marine sediment from the National Research Council, Canada. To check the accuracy of measurement, reference sample was re-checked after every 10 samples. The percentage recovery values for different PTEs were listed in supplementary material (Table S3). An internal check on the results of the microwave extraction procedure, the sequential extraction procedure was performed by a mass balance analysis by comparing the sum of the 4 steps (acid-soluble + reducible + oxidizable + residual) from the sequential extraction procedure with the total metal content from the microwave-assisted acid digestion procedure. The recovery of the sequential extraction procedure was calculated as follows:
The analysis result varies between 74 and 124%, depicted in supplementary material Table S4. Analytical results obtained for reference materials differed by ±5%. The FAAS detection limits of metals (mg/L) were as follows: Cd = 0.0004, Cr= 0.003, Cu=0.001, Ni= 0.004, Mn=0.0015, Ni= 0.009, Pb= 0.01, and Zn = 0.0005. The instrumental limit of detection (CV-AAS) for Hg was 0.0005 mg/L.
No experiments had been conducted on humans or any animal subjects and the risk assessment were calculated through empirical model prescribed by USEPA guidelines (USEPA 2005). All experiments have been complied with strict scientific ethics and quality control. The respondents of ethnobotanical survey were all above 18 years in age and have willingly given information about the traditional use of the plants and doses.
Statistical analysis
Statistical analysis was carried out to interpret the result by using Data Analysis Package of MS Excel 2007 and SPSS 16 (SPSS Inc. Chicago, USA). Descriptive statistics like one-way ANOVA was used to access the variance between means of analyzed parameter among the sampling sites and where significant F value was observed. Difference between individual means was tested using DMRT (Duncan’s Multiple Range Test) at 5% level of significance. Kruskal-Wallis test was only used when normality (Shapiro-Wilk’s test) and homogeneity (Leven’s test) were violated which was not the case for this study. For soil texture analysis, the sample size was taken as n=3, while for all other statistical analysis regarding sediment samples, it was n=7. Plant samples and estimation of metals in biological samples were done with a sample size of n=3.
Result and discussion
Chemical characteristic of sediments
Sediment samples were collected from the rhizosphere of the target mangrove species from both polluted and control sites. One-way ANOVA revealed that Cd, Cr, Hg, Ni, Mn, and Pb and associated parameters of pH, electrical conductivity (EC), percentage of organic carbon (OC), and available nitrogen (Av N) showed a significant difference in mean (p < 0.05) between the rhizospheres of each plant species in the polluted sites except Cu and Zn (Table 3). But in the control site of Jharkhali, one-way ANOVA showed insignificant results (p > 0.05) when tested for differences in mean between all these parameters of the rhizosphereic sediment samples. The reason can be that in the polluted sites, the plants tend to modify their rhizospheres to ameliorate metal pollution stress unlike the control site. Study conducted in the same sites showed that metal pollution plays an important role in defining the plant biodiversity and plant community composition (Chowdhury and Maiti 2016a). Study revealed that high metal concentrations in the polluted sites have a selection pressure on the plant species, and only the species that can tolerate metal toxicity can survive in the landscape. Mangrove plant community structure also influences the diversity of soil microbiota as evident from works of Li et al. 2011, Chen et al. 2016 and soil microbiota directly influence the nutrient dynamics, as well as overall edaphic environment (Ikenaga et al. 2010).
The concentration (mg/kg) of Cr (143–184), Cd (1.05–1.97), Hg (0.04–0.19), and Pb (20–36.6) were alarmingly high in the polluted site as is evident from current study and past researches in this area (Rodríguez-Iruretagoiena et al. 2016; Chowdhury et al. 2017; Asha et al. 2020). Cr, Cd, and Pb tend to show toxic responses in biota above the level of 90 mg/kg, 0.3 mg/kg, and 20 mg/kg respectively in the sediment (Hakanson 1980; Raj et al. 2017). The average metal concentration in the sediment at the polluted site (A. officinalis rhizosphere) was in the order, Cr> Mn> Zn> Ni> Cu>Pb> Cd> Hg. Metal concentrations at A. ilicifolius rhizosphere were in the order of Mn> Cr> Zn> Ni> Cu>Pb> Cd> Hg at the polluted site. In the same site, P. coarctata rhizosphere was in the order of Mn> Cr> Zn> Ni> Cu>Pb> Cd> Hg.
The sequential extraction of PTEs in polluted and control sediment has been elucidated in Fig. 2a and b respectively. It is evident that the metal partitioning varies with place to place and with mangrove species (polluted vs. control). Studies around the saline estauarine/coastal habitats also shows similar type of metal speciation (Kabata-Pendias and Pendias 2001; Alloway 2013). Here, in the present study, it is evident that polluted site has more exchangeable form of toxic metals like Cr, Pb, Cd, and Hg in comparison with the unpolluted site, proving an extensive risk in the site. But in control site geogenic metals like Zn, Mn has more mobility (exchangeable form) or in form of organic bound fraction in comparison to the polluted site. Microbial activity may be more in the control site, because of less concentration of toxic metals. And Zn, Mn is utilized by microbes owing to its less toxicity. So, both of metals were found to be in higher exchangeable/organic bound fractions in control. Contrary to the prevailing trends, Cd is present in predominant exchangeable form (47–56%) in the polluted rhizospheres. As per similar studies, it is evident that Cd has similar ionic radius like Ca, which is 0.97°A in former and 0.99°A in later. Hence, Cd can co-precipitate with carbonates and favoring its incorporation to the calcite lattice that result in the formation of CdαCa1-αCO3 (Ma et al. 2016; Mondal et al. 2020). Pb was mostly present in oxidizable form (37–53%) in the polluted rhizospheres. Literatures indicate that in presence of organic matter and decomposing biological materials, Pb can be found in the oxidizable form as it binds on the organic matter surface (Anastopoulos et al. 2019). Sundarban ecosystem has high organic matter content and organic carbon residues (Chowdhury et al. 2018, 2019). Most of the other metals were in the residual fractions indicating their lower mobility in the mangrove rhizospheres. Metal fractions from Hoogly river (lower part of Ganges) sediment profile also records similar trends (Mondal et al. 2020). Less pH and finer fractions of sediment indicate more exchangeable form of metals. In saline/hypersaline soil, Na+ and K+ ions compete with the heavy metals ions and metal tend to stay in immobilized form. But with increase in pH and more organic content in soil results in de-mobilization of these metal ions as evident from similar polluted mangrove sites (Chowdhury and Maiti 2016a;Ma et al. 2016; Marchand et al. 2006, 2016)
a Fraction of the Potentially Toxic Elements (PTE) found in rhizospheric sediments collected from study area (Polluted site) as determined by the BCR sequential extraction method. b Fraction of the potentially toxic elements (PTE) found in rhizospheric sediments collected from study area (control site) as determined by the BCR sequential extraction method. Hg is not indicated in the figure of the control site as that metal has been found to be below detection limit
Pollution indices
Contamination factor (Cf)
To properly understand the pollution status on ecosystem ‘contamination factor’ (\( {C}_f^i\Big) \), are used, that takes into account the relative toxicity of a particular metal in the environment (Table 4). Contamination factor (\( {C}_f^i \)) compares the pollution severity in a particular site by comparing the metal concentration of a reference value from pre-industrial period. As per this study, \( {C}_f^i \) for Cd was in the category of “very high pollution” for rhizosphearic sediment from A. officinalis but it was in the category of “considerable pollution” for A. ilicifolius and P.coarctata. However, rhizosphearic sediment of all the three target species was “moderately polluted” with Cr, Pb, and Zn pollution. Other studied metals (Cu, Hg, Mn, and Ni) were in the category of “low pollution.”
In the control sites, the Cf values of all the metals were in low pollution range except Cd which was showing moderate pollution levels. The Sundarban ecosystem overall has high Cd concentration in sediment phase as reported by previous studies (Akhand et al. 2016; Chowdhury and Maiti 2016b).
Ecological Risk Index (ERI)
Ecological Risk Index shows a better outlook level of metal contamination on backdrop of its effect on ecosystem. In this study, all the metals except Cd showed “low ecological risk,” whereas Cd pollution was under “high potential ecological risk” category for A. ilicifolius and A. officinalis sediment samples, and “considerable potential ecological risk” for rhizosphere of P. coarctata (Table 5). Ecological risk index showed “considerable potential ecological risk” for P. coarctata whereas all other two plant species have “high potential ecological risk.” In the control site, the result showed “moderate ecological risk” for Cd whereas all other metals were having “low ecological risk.” The ERI results showed “low- moderate potential ecological risk,” which indicated minimum pollution levels in the control site and substantiated its choice as control for this study.
Potential toxic elements in mangrove plants
Potential toxic elements in root, shoot/bark, leaves, and fruits/seeds of plants at polluted site are elucidated in Fig. 3. The concentration of PTEs in the plant growing in the control site has been depicted in supplementary material (Figure S1). It is evident that Zn and Mn have the highest concentration in plants root system, which play an important part in several key plant enzyme pathways. The roots of A. officinalis showed the highest concentration of Cr (185 mg/kg) followed by the roots of A. ilicifolius (105 mg/kg) and P. coarctata (86 mg/kg). However, in shoots, Cr was the highest in A. officinalis (72 mg/kg) followed by A. ilicifolius (63 mg/kg) and P .coarctata (24 mg/kg). A. officinalis also showed high concentration of Pb and Cd in its roots (Pb—6.63 mg/kg, Cd—2.07 mg/kg), and bark (Pb—2.2 mg/kg, Cd—2.8 mg/kg) . A. ilicifolius has the highest Pb concentration in its root (10.6 mg/kg) amongst the three plants. Comparatively, higher concentrations of Pb were recorded in leaf (3.2 mg/kg) and fruit (1.2 mg/kg) of this plant . Mercury in roots of selected plant species was analyzed to be 0.12, 0.27 and 0.05 mg/kg in A.officinalis, A.ilicifolius and P. coarctata respectively. Table 6 showed the PTE concentration in different parts of the mangrove. There is limited study on metal accumulation in different plant parts of the targeted mangrove species growing naturally in a polluted soil.
In consideration of the ethnobotanical usage of the mangrove plants, this study also explain the PTE contents in respective usable parts. i.e., the leaf, fruit, and seeds. Results showed the absence of Pb in the fruits and seed of the A. officinalis and P. coarctata, whereas trace of Cd and Hg was present in fruits and seeds of the plants growing in the polluted sites. In A. officinalis and A. ilicifolius, significantly high metal concentration was found in its shoot/ bark and leaves.
Bioaccumulation factor (BAF) shows the potential of each species to accumulate PTEs in their body. A. officinalis (12) showed the highest bioaccumulation of Hg in its areal parts followed by A. ilicifolius (8.3) and P. coarctata (5.7) (Fig. 4). Whereas, P. coarctata has the highest capacity to bioaccumulate Cd (7.9) in its tissues followed by A. officinalis (5.2) and A. ilicifolius (4.4). Cd is readily taken by the plants through apoplastic and symplastic transport via xylem-phloem and have low toxicity to macrophytes than other pollutants but have potentials to be bioaccumulated in higher tissues (Alloway 2013; Clemens et al. 2002). Cr also showed a similar trend with P. coarctata having highest bioaccumulation potential (2.0) followed by A. officinalis (1.6) and A. ilicifolius (1.5). Pb was negligible in the fruits of A. officinalis and seeds of P. coarctata.
As most of the toxic metals (Cd, Hg, Cr) had showed a high concentration and bioaccumulation in plant tissues, it can be concluded that these species can cause health hazard if it is regularly ingested/usage by the human. But for health risk assessment, all the metals were considered for THQ calculation. Because even if other metals were showing relatively lower bioaccumulation in the plant parts cumulatively they may impart health impact. In this study, THQ was used to understand the relative human health impact of each metal and to segregate out the PTE’s having highest probable human health risk. Similar literatures on human health risk assessment due to PTE exposures also considered all the investigated metal concentrations to evaluate cumulative human health hazard (Kumari et al. 2018; Naz et al. 2020). THQ empirical model indicates which metal has the highest propensity to cause health impact in respect to all the studies PTE’s.
Human health risk assessment
Results indicated that plants growing in metal polluted areas are prone to cause health risk in adult and children population via regular consumption of the ethnobotanical usage (Fig. 5). However, dermal exposure of bark/stem and leaves of A. officinalis and A. ilicifolius growing in both polluted and non-polluted sites was not posing any potential health risk in both adult and children population but the consumption of P. coarctata seeds harvested from the polluted sites showed high total hazard quotient (THQ>1) 1.06 and 1.37 in adult and children respectively indicating health risk. Although, the fruits of A. officinalis showed THQ 2.14E-02 (adult) and 2.77E-02 (children) respectively, this was indicating negligible health risk (Fig. 5). Among all metals, Cd was responsible for the maximum contribution in the health risk by ingestion of P. coarctata seeds and fruits of A. officinalis, followed by Ni, Mn, Zn, Cr, and Cu (Supplementary material Table S5 a,b).
Total hazard quotient through dermal exposure by leaf of A. officinalis was 9.10E-04 and 3.31E-03 in adult and children respectively. And health risk via dermal exposure by bark of same plant was 1.17E-03 and 4.24E-03 in adult and children respectively (Fig. 5). The trend of the responsible metals for this health risk via usage of leaf of A. officinalis was Cd>Mn>Cr>Zn>Cu>Hg>Pb>Ni and via usage of bark of same plant was Cd>Cr> Mn>Hg>Pb>Zn >Cu>Ni (Supplementary material Table S5a). The THQ by uses of leaf and bark of A. ilicifolius was 4.33E-04 (Adult), 1.58E-03 (children) and 7.75E-04(Adult), 2.82E-03 (children) respectively (Fig. 5).The maximum and minimum health risk posing metal via both pathways (leaf and bark application) was Cd and Mn respectively. Although the overall posed health risk by external usage (dermal) of leaves and bark of A. officinalis and A. ilicifolius was negligible, but the regular exposure can cause threat to local populace.
In this study, Cd was responsible for high THQ in comparison to other exposed metals. Cadmium has been regarded as highly toxic as it does not play any important role in biological functions. Cd+2 form of cadmium can replace Zn2+ from key enzyme motifs due to their similar electronic configuration (Sigel et al. 2013; Chowdhury and Maiti 2016a, 2016b). This replacement occurs often because both metals are present in a same group in the periodic table having similar d10 electronic configuration and coordination numbers. This can be attributed to be the main reason for the high bioaccumulation of Cd in biological systems and Cd has been found to be biomagnified in higher trophic levels. In animals, Cd can bind with the red blood cells which results in accumulation of cadmium in the kidneys and liver tissues. It has a very long biological half life in any tissues (Alloway et al. 2012). Because of its high toxicity, bioaccumulation potential and almost no biological function, Cd is one of the “Toxic Trio,” the most deleterious metal pollutants (Wu et al. 2011).
The quantitative estimation of human health risk has by high degree of uncertainties, which arises from the input parameters and toxicology data used for the study (De Miguel et al. 2007; Yang et al. 2019). Different input parameters, such as the average exposure age, body weight, height, skin surface area, exposure time, and exposure frequency can vary from person to person according to living style, economic, and environmental conditions. Uncertainty in the inputs is reflected in uncertainty in model results and predictions (Borgonovo 2007). Thus, in case of oral and dermal application of mangrove plant parts can vary from person to person and from time to time, further the doses can differ from one person to another. The maximum values of input parameters provide the maximum risk factor, which delivers the maximum extend of risk caused in certain condition (Sahuquillo et al. 2003). Thus, to draw out the maximum extend of health risk and to reduce the uncertainty a certain level, the maximum values of input parameters have been considered except the PTE concentrations (which is taken as average) to identify the health risk in this study. The obtained results may have uncertainties as evident from similar studies, and these results can vary with the variations of input parameters (Yang et al. 2019; Kohzadi et al. 2019).
Conclusion
This study concludes that polluted site had more exchangeable form of toxic metals like Cr, Pb, Cd, and Hg in comparison to the unpolluted site, proving probable ecological and health risk in the region. Ecological risk assessment suggested considerable pollution pressure in the study site. Cd had the highest exchangeable fraction in the polluted soil resulting in high uptake of this PTE by mangroves. A. officinalis had the potential to uptake Cd, Cr, Zn, and Mn as indicated by bioaccumulation factor. Mercury was present in trace amounts at the polluted rhizosphere. This study also indicates that Hg was highly accumulated in the three mangroves showing highest bioaccumulation factor. But this toxic metal poses minimum health risk as evident from this health risk assessment study. Among all three halophytes (A. officinalis, A. ilicifolius, and P. coarctata), human health risk due to bioconcentration of Cd was observed highest due to the ingestion of seeds of P. coarctata seeds. Low health risk was observed by the oral intake of plants grown in the polluted sites. However, the dermal application of plant parts did not show any significant health risk, which indicated that the plants grown in the metal contaminated sites can be used for external applications. Regular assessment and monitoring studies need to be conducted across coastal mangrove areas impacted by PTE contamination along with accounting bioaccumulation potentials of these toxic elements in the native plants. Ethnobotanical usage of these plants needs to be considered while implementing policy level pollution mitigating interventions.
Data availability
The raw data is available with authors and rest are presented in the manuscript and supplementary materials.
References
Agoramoorthy G, Chen FA, Hsu MS (2008) Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environ Pollut 155:320–326
Akhand A, Chanda A, Sanyal P, Hazra S (2012) Pollution load of four heavy metals in water, sediment and benthic organisms in the Kulti River of Sundarban fed by metropolitan sewage. Nat Environ Pollut Technol 11(1):153–156
Akhand A, Chanda A, Das S, Sanyal P, Hazra S (2016) High cadmium contamination at the gateway to Sundarban ecosystem driven by Kolkata metropolitan sewage in India. Curr Sci 110(3):386
Alloway BJ (2013) Heavy metals in soils. Wiley, New York
Alloway BJ (Ed.) (2012) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability (Vol. 22). Springer
Anastopoulos I, Massas I, Pogka EE, Chatzipavlidis I, Ehaliotis C (2019) Organic materials may greatly enhance Ni and Pb progressive immobilization into the oxidisable soil fraction, acting as providers of sorption sites and microbial substrates. Geoderma 353:482–492
Anbuselvan N, Sridharan M (2018) Heavy metal assessment in surface sediments off Coromandel Coast of India: Implication on marine pollution. Mar Pollut Bull 131:712–726
Anderson JM, & Ingram JSI (Eds.), (1998). Tropical Soil Biology And Fertility, A Handbook of Methods. CAB publishing, UK.
APHA, AWWA, WPCF (1985) Standard Methods for the Examination of Water and Waste Water, 16th edn. American Public Health Association, Washington
Asha AS, Saifullah ASM, Uddin MG, Sheikh MS, Uddin MJ, Diganta MTM (2020) Assessment of trace metal in macroalgae and sediment of the Sundarban mangrove estuary. Appl Water Sci 10(1):51
Babu BH, Shylesh BS, Padikkala J (2001) Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia 72(3):272–277
Bandaranayake WM (1998) Traditional and medicinal uses of mangroves. Mangrove Salt Marshes 2(3):133–148
Banerjee K, Senthilkumar B, Purvaja R, Ramesh R (2012) Sedimentation and trace metal distribution in selected locations of Sundarbans mangroves and Hoogly estuary, Northeast coast of India. Environ Geochem Health 34(1):27–42
Barnes DG, Dourson M, Preuss P, Bellin J, Derosa C, Engler R et al (1988) Reference dose (RfD): description and use in health risk assessments. Regul Toxicol Pharmacol 8(4):471–486
Bhimba BV, Meenupriya J, Joel EL, Naveena DE, Kumar S, Thangaraj M (2010) Antibacterial activity and characterization of secondary metabolites isolated from mangrove plant Avicennia officinalis. AsianPac J Trop Med 3(7):544–546
Borgonovo E (2007) A new uncertainty importance measure. Reliab Eng Syst Saf 92(6):771–784
Chakraborty D, Bhar S, Majumdar J, Santra SC (2013) Heavy metal pollution and phytoremediation potential of Avicennia officinalis L. in the southern coast of the Hoogly estuarine system. Int J Environ Sci 3(6):2291–2303
Chakraborty S, Zaman S, Mitra A (2018) Potentiality of mangrove associate species Porteresia coarctata (salt marsh grass) in acting as an agent of phytoremediation. IOCBS 2018:66
Chen Q, Zhao Q, Li J, Jian S, Ren H (2016) Mangrove succession enriches the sediment microbial community in South China. Sci Rep 6:27468
Chotpantarat S, Wongsasuluk P, Siriwong W, Borjan M, Robson M (2014) Non-carcinogenic hazard maps of heavy metal contamination in shallow groundwater for adult and aging populations at an agricultural area in northeastern Thailand. Hum Ecol Risk Assess 20(3):689–703
Chowdhury A, Maiti SK (2016a) Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: A case study from Indian Sunderban. Environ Earth Sci 75(9):744
Chowdhury A, Maiti SK (2016b) Assessing the ecological health risk in a conserved mangrove ecosystem due to heavy metal pollution: A case study from Sundarbans Biosphere Reserve, India. Hum Ecol Risk Assess 22(7):1519–1541
Chowdhury R, Favas PJ, Pratas J, Jonathan MP, Ganesh PS, Sarkar SK (2015) Accumulation of trace metals by mangrove plants in Indian Sundarban Wetland: prospects for phytoremediation. Int J Phytoremed 17(9):885–894
Chowdhury A, Sanyal P, Maiti SK (2016a) Dynamics of mangrove diversity influenced by climate change and consequent accelerated sea level rise at Indian Sundarbans. Int J Global Warm 9(4):486–506
Chowdhury A, Maiti SK, Bhattacharyya S (2016b) How to communicate climate change ‘impact and solutions’ to vulnerable population of Indian Sundarbans? From theory to practice. SpringerPlus 5:1219. https://doi.org/10.1186/s40064-016-2816-y
Chowdhury A, Naz A, Maiti SK (2017) Health risk assessment of ‘Tiger prawn seed’ collectors exposed to heavy metal pollution in the conserved mangrove forest of Indian Sundarbans: A socio-environmental perspective. Hum Ecol Risk Assess 23(2):203–224
Chowdhury A, Naz A, Bhattacharyya S, Sanyal P (2018) Cost–benefit analysis of ‘Blue Carbon’ sequestration by plantation of few key mangrove species at Sundarban Biosphere Reserve, India. Carbon Manag 9(6):575–586
Chowdhury A, Naz A, Bhattacharyya S (2019) Plantation Methods and Restoration Techniques for Enhanced Blue Carbon Sequestration by Mangroves. In Sustainable Agriculture Reviews, 37th edn. Springer, Cham, pp 127–144
Clemens S, Palamgren MG, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7(7):309–315
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21(8):1335–1346
Dahdouh-Guebas F, Collin S, Seen DL, Rönnbäck P, Depommier D, Ravishankar T, Koedam N (2006) Analysing ethnobotanical and fishery-related importance of mangroves of the East-Godavari Delta (Andhra Pradesh, India) for conservation and management purposes. J Ethnobiol Ethnomed 2(1):24
Das SK, Samantaray D, Mahapatra A, Pal N, Munda R, Thatoi H (2018) Pharmacological activities of leaf and bark extracts of a medicinal mangrove plant Avicennia officinalis L. Clinical Phytosci 4(1):13
De Miguel E, Iribarren I, Chacon E, Ordonez A, Charlesworth S (2007) Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 66(3):505–513
Firdaus M, Prihanto AA, Nurdiani R (2013) Antioxidant and cytotoxic activity of Acanthus ilicifolius flower. Asian Pac J Trop Biomed 3(1):17–21
Ge M, Liu G, Liu H, Liu Y (2020) Distribution, bioavailability and contamination assessment of mercury and arsenic in the surface sediments from the Yellow River Estuary, China. Hum Ecol Risk Assess 27:1–15. https://doi.org/10.1080/10807039.2019.1710460
Ghosh R, Mitra A (2015) Effect of salinity on nutritional value of saltmarsh grass (Porteresia coarctata) from Gangetic delta, northeast coast of India. Indian J-Mar Sci 44(7):1043–1052
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001
Hossain ML (2016) Medicinal activity of Avicennia officinalis: Evaluation of phytochemical and pharmacological properties. Saudi J Med Pharm Sci 2:250–255
Hossan S, Agarwala B, Sarwar S, Karim M, Jahan R, Rahmatullah M (2010) Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot Res Appl 8:061–074
Ihedioha JN, Ukoha PO, Ekere NR (2017) Ecological and human health risk assessment of heavy metal contamination in soil of a municipal solid waste dump in Uyo, Nigeria. Environ Geochem Health 39(3):497–515
Ikenaga M, Guevara R, Dean AL, Pisani C, Boyer JN (2010) Changes in community structure of sediment bacteria along the Florida coastal everglades marsh–mangrove–seagrass salinity gradient. Microb Ecol 59(2):284–295
Jackson ML (1973) Soil chemical analysis. Prentice Hall Pvt. Ltd., New Delhi
Jones PW, Lobo ET, Qiuwen MC, Heebyung Lee D, Soejarto D, Geoffrey A et al (2005) Antitumor activity of 3 Chlorodeoxylapachol a naphthoquinone from Avicennia germinans collected from an experimental plot in southern Florida. J Pharm Pharmacol 7(9):1101–1108
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press, London
Kohzadi S, Shahmoradi B, Ghaderi E, Loqmani H, Maleki A (2019) Concentration, source, and potential human health risk of heavy metals in the commonly consumed medicinal plants. Biol Trace Elem Res 187(1):41–50
Kumari P, Chowdhury A, Maiti SK (2018) Assessment of heavy metal in the water, sediment, and two edible fish species of Jamshedpur Urban Agglomeration, India with special emphasis on human health risk. Hum Ecol Risk Assess 24(6):1477–1500
Li M, Hong YG, Cao HL, Gu JD (2011) Mangrove trees affect the community structure and distribution of anammox bacteria at an anthropogenic-polluted mangrove in the Pearl River Delta reflected by 16S rRNA and hydrazine oxidoreductase (HZO) encoding gene analyses. Ecotoxicology 20(8):1780–1790
Liu J, Zhang J, Lu S, Zhang D, Tong Z, Yan Y, Hu B (2020) Interannual variation, ecological risk and human health risk of heavy metals in oyster-cultured sediments in the Maowei Estuary, China, from 2011 to 2018. Mar Pollut Bull 154:111039
Ma X, Zuo H, Tian M, Zhang L, Meng J, Zhou X, Min N, Chang X, Liu Y (2016) Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144:264–272
Maiti SK (2003) Handbook of methods in environmental studies, 2nd edn. ABD Publishers, Jaipur, pp 110–121
Maiti SK (2013) Ecorestoration of the Coalmine Degraded Lands. Springer, New York
Maiti SK, Chowdhury A (2013) Effects of anthropogenic pollution on Mangrove biodiversity: a review. J Environ Prot 4(12):1428–1434
Marchand C, Lallier-Verges E, Baltzer F et al (2006) Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Mar Chem 98(1):1–17
Marchand C, Fernandez MJ, Moreton B (2016) Trace metal geochemistry in mangrove sediments and their transfer to mangrove plants (New Caledonia). Sci Total Environ 562:216–227
Marrugo-Negrete J, Durango-Hernández J, Pinedo-Hernández J, Olivero-Verbel J, Díez S (2015) Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 127:58–63
Mondal P, Schintu M, Marras B, Bettoschi A, Marrucci A, Sarkar SK, Chowdhury R, Jonathan MP, Biswas JK (2020) Geochemical fractionation and risk assessment of trace elements in sediments from tide-dominated Hooghly (Ganges) River Estuary, India. Chem Geol 532:119373
Mossop KF, Davidson CM (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478:111–118
Muthu C, Ayyanar M, Raja N, Ignacimuthu S (2006) Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J Ethnobiol Ethnomed 2(1):43
Nabeelah Bibi S, Fawzi MM, Gokhan Z, Rajesh J, Nadeem N, Rengasamy Kannan RR, Albuquerque RDDG, Pandian SK (2019) Ethnopharmacology, phytochemistry, and global distribution of mangroves―A comprehensive review. Mar Drugs 17(4):231
Nath B, Chaudhuri P, Birch G (2014) Assessment of biotic response to heavy metal contamination in Avicennia marina mangrove ecosystems in Sydney Estuary, Australia. Ecotox Environ Safe 107:284–290
Naz A, Chowdhury A, Mishra BK, Gupta SK (2016a) Metal pollution in water environment and the associated human health risk from drinking water: A case study of Sukinda chromite mine, India. Hum Ecol Risk Assess 22(7):1433–1455. https://doi.org/10.1080/10807039.2016.1185355
Naz A, Mishra BK, Gupta SK (2016b) Human health risk assessment of chromium in drinking water: A case study of Sukinda chromite mine, Odisha, India. Expo Health 8(2):253–264
Naz A, Chowdhury A, Mishra BK, Karthikeyan K (2018) Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environ Geochem Health 40(5):2155–2175
Naz A, Chowdhury A, Chandra R, Mishra BK (2020) Potential human health hazard due to bioavailable heavy metal exposure via consumption of plants with ethnobotanical usage at the largest chromite mine of India. Environ Geochem Health 42:4213–4231. https://doi.org/10.1007/s10653-020-00603-5
Naz A, Chowdhury A, Mishra BK (2021) An Insight into Microbial Remediation of Hexavalent Chromium from Contaminated Water. In: In Contaminants in Drinking and Wastewater Sources. Springer, Singapore, pp 209–224
Pal D, Maiti SK (2019) Evaluation of potential human health risks from toxic metals via consumption of cultured fish species Labeo rohita: a case study from an urban aquaculture pond. Expo Health 11(1):33–46
Perera KARS, De Silva KHWL, Amarasinghe MD (2018) Potential impact of predicted sea level rise on carbon sink function of mangrove ecosystems with special reference to Negombo estuary, Sri Lanka. Glob Planet Chang 161:162–171
Qasim M, Abideen Z, Adnan MY, Ansari R, Gul B, Khan MA (2014) Traditional ethnobotanical uses of medicinal plants from coastal areas. J Coast Life Med 2(1):22–30
Rahman MA, Kumar S, Mohana AA, Islam R, Hashem MA, Chuanxiu L (2019) Coliform Bacteria and Trace Metals in Drinking Water, Southwest Bangladesh: Multivariate and Human Health Risk Assessment. Int J Environ Res 13(2):395–408
Raj D, Chowdhury A, Maiti SK (2017) Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: A case study from the eastern part of a Jharia coal field, India. Hum Ecol Risk Assess 23(4):767–787
Raj D, Kumar A, Maiti SK (2020) Mercury remediation potential of Brassica juncea (L.) Czern. For clean-up of flyash contaminated sites. Chemosphere 248:125857. https://doi.org/10.1016/j.chemosphere.2020.125857
Redlarski G, Palkowski A, Krawczuk M (2016) Body surface area formulae: an alarming ambiguity. Sci Rep 6:27966
Rodríguez-Iruretagoiena A, Chowdhury R, Gredilla A, deVallejuelo SFO, de Diego A, Sarkar SK, Arana G, Madariaga JM, Venkatachalam P (2016) Uptake and Distribution of Trace Elements in Dominant Mangrove Plants of the Indian Sundarban Wetland. Bull Environ Contam Toxicol 97(5):721–727
Saha SB, Mitra A, Bhattacharyya SB, Choudhury A (2001) Status of sediment with special reference to heavy metal pollution of a brackishwater tidal ecosystem in northern Sundarbans of West Bengal. Trop Ecol 42(1):127–132
Sahuquillo A, Rauret G, Bianchi M, Rehnert A, Muntau H (2003) Mercury determination in solid phases from application of the modified BCR-sequential extraction procedure: a valuable tool for assessing its mobility in sediments. Anal Bioanal Chem 375(4):578–583
Saranya A, Ramanathan T, Kesavanarayanan KS, Adam A (2015) Traditional medicinal uses, chemical constituents and biological activities of a mangrove plant, Acanthus ilicifolius Linn.: a brief review. Am-Eurasian J Agric Environ Sci 15(2):243–250
Sarkar SK, Favas PJ, Rakshit D, Satpathy KK (2014) Geochemical speciation and risk assessment of heavy metals in soils and sediments. In Environmental risk assessment of soil contamination. IntechOpen. https://doi.org/10.5772/57295
Schumacher BA (2002) Methods for Determination of Total Organic Carbon (TOC) in Soils and Sediments, NCEA-C-1282 EMASC-001, Ecological Risk Assessment Support Center, Office of Research and Development US. Environmental Protection Agency.
Sengupta S, Majumder AL (2010) Porteresia coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant Cell Environ 33(4):526–542
Shackira AM, Puthur JT (2013) An assessment of heavy metal contamination in soil sediments, leaves and roots of Acanthus ilicifolius L. In Proceedings of 23rd Swadeshi Science Congress. pp 689-692.
Shanmugapriya R, Ramanathan T, Renugadevi G (2012) Phytochemical characterization and antimicrobial efficiency of mangrove plants Avicennia marina and Avicennia officinalis. Int J Pharm Biol Arch 3(2):348–351
Sheng-Ji P (2001) Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharm Biol 39(sup1):74–79
Sigel A, Sigel H, Sigel RK (eds) (2013) Cadmium: from toxicity to essentiality, vol 11. Springer, New York
Singh D, Aeri V (2013) Phytochemical and pharmacological potential of Acanthus ilicifolius. J Pharm Bioallied Sci 5(1):17
Souza ID, Rocha LD, Morozesk M, Bonomo MM, Arrivabene HP, Durate ID et al (2015) Changes in bioaccumulation and translocation patterns between root and leafs of Avicennia schauerianaas adaptive response to different levels of metals in mangrove system. Mar Pollut Bull 94(1):176–184
Tam NFY, Wong WS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110(2):195–205
Thành-Nho N, Marchand C, Strady E, Huu-Phat N, Nhu-Trang TT (2019) Bioaccumulation of some trace elements in tropical mangrove plants and snails (Can Gio, Vietnam). Environ Pollut 248:635–645
Thirunavukkarasu P, Ramanathan T, Ramkumar L, Shanmugapriya R (2010) Anti ulcer effect of Avicennia officinalis leaves in Albino rats. World Appl Sci J 9(1):55–58
Tong R, Fang Y, Zhang B, Wang Y, Yang X (2020) Monitoring and evaluating the control effect of dust suppressant on heavy metals based on ecological and health risks: a case study of Beijing. Environ Sci Pollut Res 1-14. https://doi.org/10.1007/s11356-020-11648-5.
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth's crust. Geol Soc Am Bull 72(2):175–192
Uluturhan E, Kontas A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62(9):1989–1997
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques 14 M. GE ET AL. undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51(1–4):135–115. https://doi.org/10.1080/03067319308027619
USEPA (2002) A review of the reference dose and reference concentration processes. External Review Draft. https://www.epa.gov/sites/production/files/2014-12/documents/rfd-final.pdf. Accessed July 2020
USEPA (2005) Guidelines for carcinogen risk assessment, EPA/630/P-03/001F. Risk assessment forum, Washington
USEPA (2020) Regional Screening Levels (RSLs) - Generic Tables. Tables as of: May 2020. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables. Accessed July 2020
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wang G, Guan D, Peart MR, Chen Y, Peng Y (2013) Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of South China. For Ecol Manag 310:539–546
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36(1):169–182
Wu J, Xiao Q, Xu J, Li MY, Pan JY, Yang MH (2008) Natural products from true mangrove flora: source, chemistry and bioactivities. Nat Prod Rep 25(5):955–981
Wu FZ, Yang WQ, Zhang J, Zhou LQ (2011) Growth responses and metal accumulation in an ornamental plant (Osmanthus fragrans var. thunbergii) submitted to different Cd levels. Int Sch Res Not 2011:738138. https://www.hindawi.com/journals/isrn/2011/738138/. Accessed July 2020
Wu Z, Geng J, Huang L (2015) Heavy metal contamination in sediments and mangroves from Maowei Gulf, South China. Fresenius Environ Bull 24:1091–1097
Yang D, Liu J, Wang Q, Hong H, Zhao W, Chen S et al (2019) Geochemical and probabilistic human health risk of chromium in mangrove sediments: a case study in Fujian, China. Chemosphere 233:503–511
Yu CY, Lo YH, Chiou WK (2003) The 3D scanner for measuring body surface area: a simplified calculation in the Chinese adult. Applied Ergonomics 34(3):273–278
Zheng N, Wang Q, Zheng D (2007) Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc Plant in China via consumption of vegetables. Sci Total Environ 383:81–89
Acknowledgements
The authors are thankful to Indian Institute of Technology (Indian School of Mines) Dhanbad, Tagore Society for Rural Development (Kolkata, India), and O.P. Jindal Global University for their support, laboratory facilities and other resources provided to conduct this research work. This work was funded by Indian Institute of Technology (Indian School of Mines), Dhanbad and Ministry of Human Resource Development (MHRD) through institutional full-time doctoral research fellowship (2013DR0015) grant to the first author. We thank the anonymous reviewers for their comments and constructive suggestions aimed at improvement of this article.
Funding
The first and second author have received institutional fellowship from Indian Institute of Technology (Indian School of Mines), Dhanbad, India for this study.
Author information
Authors and Affiliations
Contributions
A.C. Responsible for designing of research question, sampling, analysis, interpretation of data, and drafting of the manuscript. A.N. Taken part in sequential extraction of PTEs from sediment samples and using human health risk assessment tools to interpret the results. A.N. has equal contribution as the first author. S.K.M. Is responsible for guidance, providing laboratory facilities, supervision of analysis and interpretation of results. The final manuscript and authorship sequence have been approved by all the authors before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research has been conducted following ethical guidelines. Participants of the survey were adult and have given permission to participate in the interview.
Consent for publication
We give permission to publish the manuscript upon acceptance and declare there is no use of any copy righted materials in this manuscript for which any consent from third party is required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Editorial Responsibility: NV. V.S.S. Sarma
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 380 kb)
Rights and permissions
About this article
Cite this article
Chowdhury, A., Naz, A. & Maiti, S.K. Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ Sci Pollut Res 28, 33042–33059 (2021). https://doi.org/10.1007/s11356-021-12566-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12566-w