Abstract
Four sediment cores were collected from selected locations of Sundarbans mangroves and Hooghly estuary, northeast coast of India to establish 210Pb geochronology and trace metal distribution in sediments. Core sites were chosen to reflect a matrix of variable anthropogenic input and hydrological conditions. The vertical distribution of 210Pbxs (210Pbtotal–226Ra) provided reliable geochronological age to calculate the mass accumulation rates and historic trace element inputs and their variations. The mass accumulation rates ranged from 0.41 g cm−2 year−1 (estuarine region) to 0.66 g cm−2 year−1 (mangrove region). Both in mangroves and estuarine systems, Fe–Mn oxy-hydroxides are observed to be a major controlling factor for trace metal accumulation when compared to organic carbon. Core collected from Hooghly estuary shows less contamination when compared to the mangrove region due to high energy and mostly coarse grained. Fe-normalized enrichment factors (EFs) of trace metals were calculated based on crustal trace element abundances. The EFs are typically >1 for Cd, Pb, Co, and Cu indicating that these metals are highly enriched while other metals such as Zn, Ni, Cr, and Mn show no enrichment or depletion. Both Sundarbans mangroves and Hooghly estuary have been receiving considerable pollution loads from anthropogenic sources such as industrial, domestic, and shipping activities in recent times, indicating high concentration of metals in the top few layers. This study suggests that the variation in trace metals content with depth or between mangrove and estuarine system results largely from metal input due to anthropogenic activities rather than diagenetic processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diffusion of toxic metals in the environment as the result of anthropogenic activities poses an increasing risk for ecosystems (Lichtfouse et al. 2005). Many studies have been carried out in coastal environment on cycling of trace metals, because of their toxicity, bio-accumulation capacity, and persistent nature (Tam and Wong 2000; Ip et al. 2004; Marchand et al. 2006; Janaki-Raman et al. 2007; Liu et al. 2009; Zhang et al. 2010). The atmospheric cycle of heavy metals has been highly dependent upon climate during pre-industrial period. Trace metals are not biologically or chemically degradable, thus they may either accumulate locally or get carried away further through rivers (Marchand et al. 2006). In the natural environment, trace metal distribution depends on various parameters like redox potential of water and sediments, organic content, grain size, Fe, and/or Mn oxy-hydroxides (Guo et al. 1997; Dong et al. 2000; Mounier et al. 2001).
The Ganges–Brahmaputra delta is one of the world’s largest delta (80,000 km2) and densely populated low-lying and highly vulnerable coastal environment, formed from sediments deposited by three major rivers, the Ganges, Brahmaputra, and Meghna (Kuehl et al. 1997; Ramesh et al. 2009; Stanley and Hait 2000). The delta harbors the largest single continuous tract of diverse mangrove forest, Sundarbans mangroves (Hussain and Acharya 1994; Stanley and Hait 2000). Mangrove and estuarine ecosystems may act as a sink for trace metals because of their variable physical and chemical properties (Yuan et al. 2010). The waterlogged mangrove sediments are anoxic and reduced in nature (Defew et al. 2005; Marchand et al. 2006) due to good supply of sulfide ions and decomposing organic matter in the sediments (Janaki-Raman et al. 2007).
The dissolved and particulate loads of rivers represent quantitatively the most important input into the oceans. The global flux of sediment is 14 billion metric tons year−1 (Syvitski et al. 2005). The rivers from southeast Asia alone contribute 25–30% of the total sediment discharge to the oceans and are the largest producer of fluvial sediment (Milliman et al. 1995; Syvitski et al. 2005). Several studies have estimated the suspended sediment loads of the Ganges river to vary between 485 and 1,600 million tons year−1 (Milliman and Meade 1983). Recent studies have shown that the Ganges carries 262 million tons year−1 sediment that is much lower than the published values because of either interannual variability or construction of dam (Farakka barrage built in 1974) as observed by Stephanie 2007. Hooghly is the largest estuary of river Ganges in the northeast coast of India. Many mangroves and coastal ecosystems over the world are close to urban development areas (Tam and Wong 2000; MacFarlane and Burchett 2002; Preda and Cox 2002) and are impacted by urban and industrial runoff, which contains trace metals in the dissolved or particulate form. The Sundarbans mangroves and Hooghly estuary are close to metropolis Kolkata (Calcutta) and under direct anthropogenic influence on trace metal contamination (Sarkar et al. 2004).
In the present study, sedimentation and distribution of trace metals were studied in sediment cores collected from Sundarbans mangroves and Hooghly estuary, northeast coast of India. This study examines the pattern and source of contaminant deposition along with the sediment accumulation rate in this ecologically sensitive area.
Study area
Mangroves of India account for about 5% of the world’s mangrove vegetation and are spread over an area of about 6,740 km2 along the coastal states/union territories of the country (Krishnamurthy et al. 1987; Untawale 1987). Sundarbans accounts for over 10% of the mangrove forests in the world, and a little less than half of the total area under mangroves in India (Ramesh et al. 2009) covering about 12,000 km2 of mangrove forest and water (of which 1/3 lies in West Bengal, India, and rest occurs along the Bangladesh coast). This area is restricted within the latitudes 21°31′N and 22°30′N and longitude between 88°10′E and 89°51′E. The eco-geography of this area is totally dependent on tidal effect. The tidal action of the sea inundates the whole of Sundarbans to varying depths, pushing back silt to the channels and depositing there. Sundarbans delta is one of the dynamic estuarine deltas of the world. It is crisscrossed by water channels that bring with them tons of sediments from terrestrial sources and play a major role in the eroding and accreting nature of this deltaic estuary (Gurmeet 2009). The lower delta plain is sensitive to change in hydrodynamics, riverine input, and relative sea level. The Sundarbans consists of low flat alluvial plains in the active delta region. The sediments of this area show limited grain-size variation (clay to coarse sand) and firmness (soft to firm) (Banerjee 1998). The soil pH averages 8.0 (Christensen 1984), and the mean elevation of Indian Sundarbans above sea level is about 3.30 m, and the mean highest high water level (MHHW) and mean lowest high water level (MLHW) are 5.94 and 0.94 m, respectively (Untawale 1987).
Hooghly estuary, the first deltaic offshoot of the Ganges is located along latitude 21°40′N and longitude 87°47′E (Fig. 1). It is also known as the Hooghly–Matlah estuarine system and lies in the state of West Bengal. The Hooghly River, after traversing a considerable distance inland, passes through the city of Kolkata that has a port located on its banks. Further down, the estuary passes through Diamond Harbor from where it fans out to about 4 km into a funnel-shaped water body that spreads out to more than 20 km before merging into Bay of Bengal (Estuaries of India 2002). The Hooghly is a globally significant estuary and drains a catchment of 6 × 104 km2. The depth of the water column varies from 10 to 15 m (Mukhopadhyay et al. 2006). Hooghly is a well-mixed estuary because of shallow depth (average 6 m) and intense tidal mixing (Sadhuram et al. 2005). The estuary gets semidiurnal tides with a maximum range of 5.5 m at spring and minimum 1.8 m at neap.
Despite the fact that Hooghly estuary has large tidal amplitude, considerable mixing, dilution, and flushing a gradual accumulation of toxic wastes and progressive reduction in the freshwater flow over a period of years have resulted in the deterioration of aquatic environment. Sundarbans mangroves receive increased quantity of agricultural runoff; domestic sewage and industrial effluent from northern parts and through many large and small channels of Hooghly. In Hooghly estuary, the potential sources of trace metals are mainly, port activity (Haldia and Kolkata port), semi-treated wastes from varied industries (tanneries, jute mills, pulp and paper mills, pesticide manufacturing plants, thermal power plants, Kilns, rubber, fertilizer, soap factories, antibiotic plants), and oil refinery (Haldia) (Gopal and Chauhan 2006; De et al. 2010). These industries are considered to be the major sources of metals like Cu, Zn, Cr, Co, Ni, Pb, Cd, Fe, and Mn to the system.
The estuary lies adjacent to the Sundarbans mangroves, but is largely separated from it by urbanization and development along its banks. The exception is the lower part of the estuary that is associated with mangrove patches on the outskirts of Sundarbans. This is significant in terms of this study because it allows a direct comparison between two environments broadly impacted by identical freshwater and marine end members but with significantly different levels of human impact. The geology, climate, geomorphology, soil type of the Hooghly estuary are similar to the adjacent Sundarbans mangrove forests.
Materials and methods
Sampling
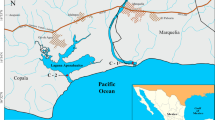
Four sediment cores were collected from Sundarbans mangroves (S1, S2, S3) and Hooghly estuary (H1) (Fig. 1) in June 2008 during low tide, in order to determine the variation of mass accumulation of sediments and vertical profile distribution of trace metals. Average maximum corer penetration of 33 cm was possible due to sediment and root compaction beyond this point (S-1, 34 cm; S-2, 32 cm; S-3, 28 cm; H-1, 36 cm) (Table 1).
The sampling locations were selected based on differences in hydrological conditions, varied anthropogenic pressure, and feasibility of sampling. (a) S1 and S2 stations receive substantial input from Hooghly and Matlah through channels and also have marine (Bay of Bengal) influence, (b) S3 region (Sundarbans—core mangrove) receives input from Matlah river and other upstream rivers and have less tidal influence, (c) H1 is situated in a high energy non-mangrove region of Hooghly with minimal marine influence. This area is under direct anthropogenic influence of Kolkata city and Haldia. Figure 2 shows the sampling techniques and processing of samples for different analyses.
Determination of trace metals, organic carbon, 210Pb and 226Ra in sediments
Analytical methods of trace metals, organic carbon, 210Pb and 226Ra in sediments are mentioned in Table 2. For trace metal analysis, Sediment T (NOAA/7) standard as reference material along with analytical grade (MERCK, Germany) chemical standards for each element was used to check the accuracy and precision of the instrument. Good to excellent recoveries ranging from 93 to 101% were obtained for the studied metals (Zn = 98%; Cu = 95%; Cr = 99%; Ni = 99%; Co = 93%; Fe = 99%; Mn = 98%; Pb = 100%; Cd = 101%) indicating an overall good accuracy of the analysis.
Sediment accumulation rate model
For the present study, a simpler mathematical model was used to calculate sediment accumulation following Krishnaswamy et al. (1971) and Joshi and Ku (1979). Provided that the sedimentation rate, S(cm2 year−1) and the activity of the excess 210Pb added to the surface sediments, Co, C(dpm gm−1) are constant in time the distribution of excess 210Pb in undisturbed sediments is governed by the relationship
where Co, C, activities of excess 210Pb at the surface, and depth D, respectively; λ radioactive decay constant for 210Pb (0.693/22.26 year−1); S sedimentation rate.
Statistical analysis
The data were analyzed by multivariate statistical methods, including Pearson correlation matrix. Statistical analysis was performed using statistical software SPSS version 16.0 for Windows. The results were analyzed to establish the natural processes and to identify the sources of pollution.
Results and discussion
Mass accumulation and sedimentation rates
210Pb dating method has been widely used to estimate the age of sediments and sedimentation rates for modern sediments (Appleby and Oldfield 1978; Towler and Smith 2000; Gonneeaa et al. 2004). Figure 3 depicts the unsupported 210Pb activities of the four cores (S1, S2, S3, and H1), which were measured by excess 210Pb method. Sediment accumulation rates were determined from 210Pbxs activities by subtracting the supported levels of 210Pb activity from the total 226Ra measured.
The sediment cores exhibited a typical 210Pbxs profile characterized by three distinct zones: a surface mixed layer (SML), a middle inclined zone with exponential radioactive decay, and a constant low activity in the bottom layer. The activity profile of 210Pbxs appears to decrease monotonically with depth and the irregular decrease in the middle layers, which is influenced by physical mixing and bioturbation (Fig. 3). The activity concentration in all cores ranges between 0.27 and 3.85 dpm g−1, which is similar to that in the Indian coastal deposits (Subramanian and Mohanachandran 1997; Ramesh et al. 2002). Assuming that sediment mixing is restricted to the surface mixed layer, i.e., the mixing coefficient is zero below the surface mixed layer (Nittrouer et al. 1984), a linear sediment accumulation rate was calculated from the gradient of 210Pbxs activity with depth in the sediment column (Krishnaswamy et al. 1971; Guinasso and Schink 1975; Nittrouer et al. 1979). In this calculation, it was also assumed that a steady-state supply of 210Pb to bottom layer and SML coincided with the depth of 210Pb activity.
Sediment accumulation rates were highest in the core site S2, 4.8 mm year−1, and all along the Sundarbans mangroves and Hooghly estuary, ranging from 3.0 to 4.8 mm year−1 (Table 3). This value is consistent with previous studies in the same area by Stanley and Hait (2000). At core site S3, 210Pbxs activities are very low, which suggests that there has been low net accumulation of sediment at this site over the past several decades. Such scatter in 210Pbxs may be expected here, since this site receives comparatively less quantity of sediment load compared to other mangrove sites as it is far from the main source region and located in the back mangrove region. Whereas H1 core site located in the Hooghly estuary that receives large quantity of sediments brought by Ganges River showed comparatively low sedimentation rate (3.2 mm year−1) due to high freshwater discharge, active shipping, and dredging.
The mass accumulation rates ranged between 0.41 and 0.72 g cm−2 year−1 indicating little vertical mixing in the core site H1. Observations of mass accumulation rates from the four core sites (Table 3) indicate small-scale mixing processes (core site H1) to large-scale sediment (core site S2). In addition, the high mass accumulation rate is due to the frequent exposure to tidal inundation and flocculation under high saline conditions. The sediments at these sites remain as slurry for a longer period compared to that of rear mangrove and estuarine regions. The present study indicates that the sedimentation and mass accumulation rates of Sundarbans mangroves and Hooghly estuary are in general comparable to the observations made for other mangrove ecosystems worldwide (Table 4).
Vertical distribution of organic carbon in sediments
Estuarine and coastal areas are characterized by high productivity and account for about 80% of the organic carbon being buried in recent sediments annually (Ittekot 1993). The amount and origin of organic carbon in mangrove sediments are mainly influenced by both physical (e.g., tidal amplitude) and biological (e.g., consumption, removal, degradation) factors (Bouillon et al. 2003). Vertical profile distribution of organic carbon (OC) was studied in sediment cores collected from Hooghly estuary and Sundarbans mangroves. Average values of sediment organic carbon (% dry weight) are shown in Table 5, and the observed concentration of OC in estuarine core (H1 = 0.49%) was lowest when compared to that observed in mangrove region (S1, S2, and S3) that ranges from 0.70 to 0.85% (Fig. 4). The values of OC obtained in this study area are comparatively lower than those reported (2.78%) for various tidal and estuarine regions of southeast coast of India (Achyuthan et al. 2002). Relatively low concentration of OC in sediments is due to the nature of marine sedimentation and mixing process in sediment–water interface and also due to high microbial activity in this region, which use the organic matter as nutrient source and removes it from the system as CO2 (Canuel and Martens 1993; Subramanian et al. 2001; Chatterjee et al. 2007). All the cores collected showed relatively low OC values in the top layers (0–6 cm) due to regular inundation by tidal activities (Chatterjee et al. 2007). The cores collected from Sundarbans mangroves (S1, S2, and S3) showed an increasing trend of OC in the middle layers (8–20 cm) due to restricted microbial activity in this anoxic or sub-oxic region and also due to partial decomposition of leaf litters, root exudates, and their preservation as burial in the sediments (Jayaprakash et al. 2008). Overall asynchronous distribution of OC with depth was observed in both Sundarbans and Hooghly cores due to mangrove root penetration at various depths coupled with bioturbation activities and physical mixing processes (Chatterjee et al. 2007, 2009).
Vertical distribution of Fe and Mn
The geochemical behavior of Fe and Mn is coupled as both the metals are highly redox sensitive (Lacerda et al. 1999; Mortimer and Rae 2000; Marchand et al. 2006). Average concentrations and standard deviations of Fe (%) and Mn (mg kg−1) obtained from mangrove and estuarine core sediments are shown in Table 5. Average Fe concentration was found to be higher in mangrove sediments (3.75%) when compared to estuarine sediments (2.86%). It could be due to the differences in hydrodynamics, churning, bioturbation, and navigation activities. Vertical profile of Fe and Mn (Fig. 5a, b) in S2, S3, and H1 core sites show similar trends and fairly close distribution pattern with OC clearly indicates Fe–Mn oxy-hydroxides plays a major role in accumulating trace metals from the overlying water column (Abu-Hilal and Badran 1990; Chatterjee et al. 2007). However, S1 core did not show the similar trend of OC with Fe and Mn due to relatively high sedimentation and partial decomposition of pollens and spores, which are associated with coarse-grained sediment with less surface area and less trace element adsorption ability.
Vertical distribution of Cu, Zn, Cr, Co, Ni, Pb, and Cd
The average concentrations and standard deviations of Cu, Zn, Cr, Co, Ni, Pb, and Cd (mg kg−1) obtained from mangrove and estuarine core sediments are shown in Table 5. In Sundarbans mangroves and Hooghly estuary, the average metal concentration in the cores was in the following sequential order; Fe > Mn > Zn > Ni > Cr > Cu > Pb > Co > Cd and Fe > Mn > Zn > Cr > Ni > Pb > Cu > Co > Cd, respectively. The concentrations of trace metals did not show pronounced variations between the metals and between sites. The maximum average concentration values for Cu, Cr, Pb, and Cd (mg kg−1) were observed in S1 core site. Core site S1 and S2 showed similar concentrations of trace metals, whereas mangrove site S3 showed comparatively low concentrations due to poor sediment accumulation, and H1 located in high energy system showed the least concentrations. In the present study, the elevated concentrations of Zn and Cu (Fig. 5a, b) from bottom to top in the all four cores could be attributed to the increase in natural weathering of soil materials and inputs from rapid industrial development and urbanization. Generally, increased surface runoff correlates with the accumulation of rock-forming metals, like Fe. No significant increase was observed in the concentration of Fe in the sediment profile. It may be concluded that the increase in trace metals in the core sediments mainly come from extensive fishing activity, public boat services, domestic sewage release from nearby areas, and other industrial waste discharge (Ip et al. 2004; Gopal and Chauhan 2006; Chatterjee et al. 2007). In surface layer (0–8 cm), an increase in concentrations of Pb at S3 site (Fig. 6f) showed exchangeable fraction and in H1 core sites (4–18 cm) (Fig. 6f) showed gradual increase due to additional Pb precipitation around the redox boundaries (Lee and Cundy 2001 and Janaki-Raman et al. 2007).
a Vertical profiles of Zn (mg kg−1) in S1, S2, S3, and H1 cores. b Vertical profiles of Cu (mg kg−1) in S1, S2, S3, and H1 cores. c Vertical profiles of Cr (mg kg−1) in S1, S2, S3, and H1 cores. d Vertical profiles of Ni (mg kg−1) in S1, S2, S3, and H1 cores. e Vertical profiles of Co (mg kg−1) in S1, S2, S3, and H1 cores. f Vertical profiles of Pb (mg kg−1) in S1, S2, S3, and H1 cores. g Vertical profiles of Cd (mg kg−1) in S1, S2, S3, and H1 cores
Statistical approach
To study the inter-elemental associations, Pearson correlation coefficients of the trace metals were computed for four cores separately (Table 6A, B). In all the cores studied, trace metals and their inter relationship showed strong correlation with Fe and Mn and insignificant correlation with OC. Due to relatively low concentration of OC in sediments, it does not act as a carrier of metals, and therefore, shows asynchronous distribution pattern with trace metals. Adsorption of trace metals is associated with Fe–Mn oxy-hydroxides precipitation, which plays a major role in these systems (Buckley et al. 1995; Zwolsman et al. 1996; Srinivasalu et al. 2008). Strong inter-element relationships suggest that one common process determines the sediment composition for all trace metals. The strong positive correlation between all the metals in S1, S2, and H1 cores reveals that these metals are carried by industrial discharges, fishing activities, and constant movement of fishing and commercial boats into the nearby environment (Chatterjee et al. 2007). In S2 core, the poor association of Mn with other metals suggests that Mn oxide may only be a minor host phase for these metals in the mangrove environment (Marchand et al. 2006). In S3 and H1 cores, Cr showed no significant correlation with other metals possibly due to different processes like biological effects and external inputs operating in the mangrove and adjacent estuarine sediments. The differences in correlation among the above metals reflect differential behaviors, which require further examination. Significant correlations of Cd with Ni, Pb, and Cu in S1 and S3 indicate the industrial contribution of these metals into the system.
Enrichment factor (EF)
Enrichment factor (EF) of individual metal is highly variable depending upon the trace metal of interest and place of study. The mean trace metal EF (EFm) provides fine fingerprints of anthropogenic activities over a period of time. It is an essential part of geochemical studies to differentiate the sources of trace metals whether from human activities or from natural weathering. Several authors have successfully used Fe to normalize heavy metal contaminants in estuarine and mangrove sediments (Schiff and Weisberg 1999; Neto et al. 2000; Mucha et al. 2003). In the present study, Fe was used as a lithogenic reference to differentiate between natural and anthropogenic inputs. Enrichment factor was calculated for each metal, as per Ergin et al. (1991).
Wedepohl (1995) shale values were used as background for the calculation of EFs as pristine areas of Sundarbans mangroves lie in the inaccessible Tiger Reserve. Previous studies conducted in this system have also used Wedepohl shale values instead of pristine background values (Chatterjee et al. 2007, 2009). Metals with EF values significantly higher than 1 can be considered to be attributed to long transportation phenomena from natural and/or anthropogenic sources (Nolting et al. 1999) and not from crustal background. The enrichment of trace metals (Table 7) over the crustal values are in the following order for mangrove cores (S2, S1, and S3) Cd > Pb > Co > Cu > Ni > Mn > Zn > Cr and for estuarine core (H1) Cd > Pb > Co > Mn > Ni > Cu > Cr > Zn indicating that the high values (EF > 1) are due to the anthropogenic addition of metals to the system. All the cores studied showed high enrichment of Cd, Pb, Co, and Cu. High enrichment of Cd suggests the presence of domestic effluent discharge or dredging activity along the international shipping zones (Batley and Brockbank 1990). An overall higher EF value for Pb shows atmospheric deposition of Pb from power plant operation, fly ash, and other industries and also from constant movement of fishing boats (Chatterjee et al. 2007; Stephen-Pichaimani et al. 2008). High values of Co could be due to the steel industry discharges and harbor activities (Jonathan and Ram Mohan 2003). Enrichment of Cu in the system could be from rain washed impervious surfaces (combustion of diesel and lubricating oils) into the riverine system. Ganges River carries effluents from upstream industrial belt (mainly rubber industry), which also contains considerable quantity of Cu (Rice et al. 2002). In the present study, it is observed that some metals such as Ni, Mn, Zn, and Cr show enrichment factors less than unity. This indicates that these metals are depleted relative to crustal abundance. The depletion may be driven by the biological uptake of these metals (Chatterjee et al. 2007; Chatterjee et al. 2009).
Conclusion
The sediment cores from selected locations of Sundarbans mangroves and Hooghly estuary reveal a wide range in sedimentation rates (3.0–4.5 mm year−1) and mass accumulation rates (0.41–0.78 g cm−2 year−1) reflecting their respective locations. At core site S2 located within the Sundarbans mangroves, radionuclide inventories imply that sediment is effectively focused, resulting in significantly elevated mass accumulation rates. It is due to the frequent exposure to the tidal inundation and flocculation under high saline conditions. The sediment cores collected from four sites provide an ongoing and historical record of pollutant loading and anthropogenic impacts both in mangroves and estuarine regions. The trace metals mobilized from the sub-oxic reduced sediment zone re-adsorbed onto Fe oxides, and at greater depths they co-precipitated with Fe–Mn oxy-hydroxides. This process plays a greater role in trace metal adsorption from these systems than organic matter. Cd, Pb, Co, and Cu were enriched in the sediments of both estuarine and mangrove ecosystems, which have deleterious effects on these sensitive ecosystems. These metals also pose eco-toxicological risk through biomagnification ultimately causing severe health risk to humans. The results of our study suggest that an effective environmental management is required to safeguard the health of the ecosystems.
References
Abu-Hilal, A. H., & Badran, M. M. (1990). Effect of pollution sources on metal concentration in sediment cores from the Gulf of Aqaba (Red Sea). Marine Pollution Bulletin, 21(4), 190–197.
Achyuthan, H., Richardmohan, D., Srinivasalu, S., & Selvaraj, K. (2002). Trace metals in the sediment cores of estuary and tidal zones from northern part of Southeast coast of India. Indian Journal of Marine Science, 31, 141–149.
Appleby, P. G., & Oldfield, F. (1978). The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena, 5, 1–8.
Banerjee, A. (1998). Environment, population and human settlements of Sunderban Delta (1st ed.). New Delhi: Concept Publishing Company.
Batley, G. E., & Brockbank, C. I. (1990). Impact of ocean disposal of dredged sediments from the RTA Glebe Island bridge site—A coastal sediment survey. CSIRO report, Center for Advanced Analytical Chemistry–Division of Coal and Energy Technology, Report FT/IR 050.
Bouillon, S., Dahdouh-Guebas, F., Rao, A. V. V. S., Koedam, N., & Dehairs, F. (2003). Sources of organic carbon in mangrove sediments: Variability and possible ecological implications. Hydrobiologia, 495, 33–39.
Buckley, D. E., Smith, J. N., & Winters, G. V. (1995). Accumulation of contaminant metals in marine sediments of Halifax harbour, Nova Scotia: Environmental factors and historical trends. Applied Geochemistry, 10, 175–195.
Canuel, E. A., & Martens, C. S. (1993). Seasonal variations in the sources and alteration of organic matter associated with recently-deposited sediments. Organic Geochemistry, 20, 563–577.
Chatterjee, M., Massolo, S., Sarkar, S. K., Bhattacharya, A. K., Bhattacharya, B. D., Satpathy, K. K., et al. (2009). An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environmental Monitoring and Assessment, 150, 307–322.
Chatterjee, M., Silva Filho, E. V., Sarkar, S. K., Sella, S. M., Bhattacharya, A., Satpathy, K. K., et al. (2007). Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environmental International, 33, 346–356.
Christensen, B. (1984). Ecological aspects of the Sundarbans (p. 42). FAO: TCP/BGD/2309 (Mf). Rome: FAO.
De, T. K., De, M., Das, S., Ray, R., & Ghosh, P. B. (2010). Level of heavy metals in some edible marine fishes of mangrove dominated tropical estuarine areas of Hooghly River, North East Coast of Bay of Bengal, India. Bulletin of Environmental Contamination and Toxicology, 85, 385–390.
Defew, L. H., Mair, J. M., & Guzman, H. M. (2005). An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Marine Pollution Bulletin, 50, 547–552.
Dong, D., Nelson, Y. M., Lion, L. W., Shuler, M. L., & Ghiorse, W. C. (2000). Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides. Water Research, 34, 427–436.
Ergin, M., Saydam, C., Basturk, O., Erdem, E., & Yoruk, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chemical Geology, 91(3), 269–285.
Estuaries of India, State-of-the-Art Report. (2002). ENVIS Publication Series: 1/2002.
Flynn, W. W. (1968). The determination of low-levels of polonium-210 in environmental materials. Analytica Chemica Acta, 43, 221–227.
Gaudette, H., Flight, W., Toner, L., & Folger, D. (1974). An inexpensive titration method for the determination of organic carbon in recent sediments. Journal of Sedimentary Petrology, 44, 249–253.
Gonneeaa, M. E., Paytana, A., & Herrera-Silveira, J. A. (2004). Tracing organic matter sources and carbon burial in mangrove sediments over the past 160 years. Estuarine, Coastal and Shelf Science, 61, 211–227.
Gopal, B., & Chauhan, M. (2006). Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquatic Science, 68, 338–354.
Guinasso, N. L., & Schink, D. R. (1975). Quantitative estimates of biological mixing rates in abyssal sediments. Journal of Geophysical Research, 80, 3032–3043.
Guo, T., DeLaune, R. D., & Patrick, W. H., Jr. (1997). The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environment International, 23, 305–316.
Gurmeet, S. (2009). Nutrient dynamics in the buffer zones of the Sundarban mangrove ecosystem, India. PhD thesis submitted to Jawaharlal Nehru University.
Hussain, Z., & Acharya, G. (Eds.). (1994). Mangroves of the Sun-darbans. Volume Two: Bangladesh (p. 257). Bangkok, Thailand: IUCN—The World Conservation Union.
Ip, C. C. M., Lia, X. D., Zhang, G., Farmer, J. G., Wai, O. W. H., & Li, Y. S. (2004). Over one hundred years of trace metal fluxes in the sediments of the Pearl River Estuary, South China. Environmental Pollution, 132, 157–172.
Ittekot, V. (1993). Abiotically driven biological pump in the ocean and short-term fluctuations in the atmospheric CO2 contents. Global Planetary Change, 8, 17–25.
Janaki-Raman, D., Jonathan, M. P., Srinivasalu, S., Amstrong-Altrin, J. S., Mohan, S. P., & Ram-Mohan, V. (2007). Trace metal enrichments in core sediments in Muthupet mangroves, SE coast of India: Application of acid leachable technique. Environmental Pollution, 145, 245–257.
Jayaprakash, M., Jonathan, M. P., Srinivasalu, S., Muthuraj, S., Ram-Mohan, V., & Rajeshwara-Rao, N. (2008). Acid-leachable trace metals in sediments from an industrialized region (Ennore Creek) of Chennai city, SE coast of India: An approach towards regular monitoring. Estuarine, Coastal and Shelf Science, 76, 692–703.
Jonathan, M. P., & Ram Mohan, V. (2003). Heavy metals in sediments of the inner shelf off the Gulf of Mannar, Southeast coast of India. Marine Pollution Bulletin, 46, 263–268.
Joshi, L. U., & Ku, T. L. (1979). Measurement of 210Pb from a sediment core off the coast of California. Journal of Radio Analytical Chemistry, 52, 329–334.
Krishnamurthy, K., Choudhury, A., & Untawale, A. G. (1987). Status report: Mangroves in India (pp. 150). New Delhi: Ministry of Environment and Forests, Govt of India.
Krishnaswamy, S., Lal, D., Martin, J. M., & Meybeck, M. (1971). Geochronology of lake sediments. Earth and Planetary Science Letters, II, 407–414.
Ku, T. L., & Lin, M. C. (1976). 226Ra-distributions in the Antarctic Ocean. Earth Planetary Science Letters, 31, 236–248.
Kuehl, S. A., Levy, B. M., Moore, S. W., & Allison, M. A. (1997). Subaqueous delta of the Ganges–Brahmaputra river system. Marine Geology, 144, 81–96.
Lacerda, L. D., Ribeiro, M. G., & Guiros, B. B. (1999). Manganese dynamics in a mangrove, mud flat tidal creek in SE Brazil. Mangroves Salt Marshes, 3, 105–115.
Lee, S. V., & Cundy, A. B. (2001). Heavy metal contamination and mixing processes in sediments from the Humber Estuary, eastern England. Estuarine, Coastal and Shelf Science, 53, 619–636.
Lichtfouse, E., Schwarzbauer, J., & Robert, D. (2005). Environmental chemistry, green chemistry and pollutants in ecosystems. 1. Analytical Chemistry. 2. Toxic Metals. 3. Organic Pollutants. 4. Polycyclic Aromatic Compounds. 5. Pesticides. 6. Green Chemistry.7. Ecotoxicology (p. 780). Springer.
Liu, Y., Tam, N. F. Y., Yang, J. X., Pi, N., Wong, M. H., & Ye, Z. H. (2009). Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Marine Pollution Bulletin, 58, 1843–1849.
Loring, D. H., & Rantala, R. T. T. (1992). Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth Science Review, 32, 235–283.
Lynch, J. C., Meriwether, J. R., McKee, B. A., Vera-Herrera, F., & Twilley, R. R. (1989). Recent accretion in mangrove ecosystems based on 137Cs and 210Pb. Estuaries, 4, 284–299.
MacFarlane, G. R., & Burchett, M. D. (2002). Toxicity, growth and accumulation relationships of copper, lead and zinc in the Grey Mangrove Avicennia marina (Forsk.) Veirh. Marine Environmental Research, 54, 65–84.
Marchand, C., Lallier-Verges, E., Baltzer, F., Alberic, P., Cossa, D., & Baillif, P. (2006). Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Marine Chemistry, 98, 1–17.
Milliman, J. D., & Meade, R. H. (1983). World-wide delivery of sediment to the oceans. Geology, 91, 1–21.
Milliman, T. D., Rutkwashk, C., & Meybeck, M. (1995). River discharge to the sea: a global river index (p. 125). Texel: Glori, Nioz.
Mortimer, R. J. G., & Rae, J. E. (2000). Metal speciation (Cu, Zn, Pb, Cd) and organic matter in oxic to suboxic salt marsh sediments, Severn Estuary, Soutwest Britain. Marine Pollution Bulletin, 40, 377–386.
Mounier, S., Lacerda, L. D., Marins, R. V., & Bemaim, J. (2001). Copper and mercury complexing capacity of organic matter from a mangrove mud flat environment, Sepetiba bay, Brazil. Environmental Contamination and Toxicology, 67, 519–525.
Mucha, A. P., Vasconcelos, M. T. S. D., & Bordalo, A. A. (2003). Macrobenthic community in the Doura estuary: Relations with trace metals and natural sediment characteristics. Environmental Pollution, 121(2), 169–180.
Mukhopadhyay, S. K., Biswas, H., De, T. K., & Jana, T. K. (2006). Fluxes of nutrients from tropical River Hooghly at the land–ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. Journal of Marine Systems, 62, 9–21.
Neto, J. A. B., Smith, B. J., & McAllister, J. J. (2000). Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environmental Pollution, 109(1), 1–9.
Nittrouer, C. A., DeMaster, D. J., Kuehl, S. A., McKee, B. A., & Thorbjarnarson, K. W. (1984). Some questions and answers about the accumulation of fine-grained sediment in continental margin environments. Geo-Marine Letters, 4(3–4), 211–213.
Nittrouer, C. A., Sternberg, R. W., Carpenter, R., & Bennett, J. T. (1979). The use of Pb-210 geochronology as a sedimentological tool: Application to the Washington continental shelf. Marine Geology, 31, 297–316.
Nolting, R. F., Ramkema, A., & Everaarts, J. M. (1999). The geochemistry of Cu, Cd, Zn, Ni and Pb in sediment cores from the continental slope of Banc d’ Arquin (Mauritani). Continental Shelf Research, 19, 665–691.
Preda, M., & Cox, M. E. (2002). Trace metal occurrence and distribution in sediments and mangroves, Pumicestone region, southeast Queensland, Australia. Environment International, 28, 433–449.
Ramesh, R., Purvaja, R., Lakshmi, A., Newton, A., Kremer, H. H., & Weichsel-gartner, J. (2009). South Asia basins: LOICZ global change assessment and synthesis of river catchment—coastal sea interaction and human dimensions (p. 134). LOICZ Research & Studies No. 32. Geesthacht: GKSS Research Center.
Ramesh, R., Purvaja, R., Ramesh, S., & James, R. A. (2002). Historical pollution trends in coastal environments of India. Environmental Monitoring and Assessment, 79(2), 151–176.
Ramesh, R., Subramanian, V., & Pillai, K. C. (1988). Sediment and elemental accumulation rates in the Krishna river basin India. The Science of the Total Environment, 72, 221–226.
Ravichandran, M. (1994). Investigations on the sediment chronology and trace metal accumulation in Sabine-Neches estuary, Beaumont, Texas. M.S. Thesis, Texas A&M University, Galveston, Texas.
Rice, K. C., Conko, K. M., & Hornberger, G. M. (2002). Anthropogenic sources of arsenic and copper to sediments in a Suburban Lake, Northern Virginia. Environmental Science and Technology, 36, 4962–4967.
Saad, S., Husain, M. L., Yaacob, R., & Asano, T. (1999). Sediment accretion and variability of sedimentological characteristics of a tropical estuarine mangrove: Kemaman, Terengganu, Malaysia. Mangroves and Salt Marshes, 3, 51–58.
Sadhuram, Y., Sarma, V. V., Ramana Murthy, T. V., & Prabhakar Rao, B. (2005). Seasonal variability of the physicochemical characteristics of the Haldia channel Hooghly estuary. Journal of Earth System Science, 114, 37–49.
Sarkar, S. K., Franciscovic-Bilinski, S., Bhattacharya, A., Saha, M., & Bilinski, H. (2004). Levels of elements in the surficial estuarine sediments of the Hugli River, northeast India and their environmental implications. Environment International, 30, 1089–1098.
Schiff, K. C., & Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in Southern California coast shelf sediments. Marine Environmental Research, 48(2), 161–176.
Smoak, J. M., & Patchineelam, S. R. (1999). Sediment mixing and accumulation in a mangrove ecosystem: Evidence from 210Pb, 234Th and 7Be. Mangroves and Salt Marshes, 3, 17–27.
Srinivasalu, S., Thangadurai, N., Jonathan, M. P., Armstrong-Altrin, J. S., Ayyamperumal, T., & Ram-Mohan, V. (2008). Evaluation of trace-metal enrichments from the 26 December 2004 tsunami sediments along the Southeast coast of India. Environmental Geology, 53, 1711–1721.
Stanley, D. J., & Hait, A. K. (2000). Holocene depositional patterns, neotectonics and Sundarban mangroves in the western Ganges–Brahmaputra Delta. Journal of Coastal Research, 16(1), 26–39.
Stephanie, K. R. (2007). Suspended sediment transport in the Ganges–Brahmaputra river system, Bangladesh. Thesis submitted to Texas A&M University, USA.
Stephen-Pichaimani, V., Jonathan, M. P., Srinivasalu, S., Rajeshwara-Rao, N., & Mohan, S. P. (2008). Enrichment of trace metals in surface sediments from the northern part of Point Calimere, SE coast of India. Environmental Geology, 55, 1811–1819.
Stevenson, J. C., Kearney, M. S., & Pendleton, E. C. (1985). Sedimentation and erosion in a Chesapeake Bay brackish marsh system. Marine Geology, 67, 213–235.
Subramanian, S., Eswaramoorthi, S., Periakali, P., Saravana-Kumar, K., & Lakshmi-Narasimhan, C. L. (2001). Major and trace elements (Fe, Mn, Al, Cu, & Hg) in Pichavaram Mangrove sediments, Tamil Nadu, east coast of India. Journal of Applied Geochemistry, 3, 6–12.
Subramanian, V., & Mohanachandran, G. M. (1997). Deposition and fluxes of heavy metals in the sediments of three major peninsular estuaries on the east coast of India. In K. R. Dyer & R. J. Orth (Eds.), Changes in fluxes in estuaries (pp. 97–106). Denmark: Olsen and Olsen Publ.
Syvitski, J. P. M., Vörösmarty, C. J., Kettner, A. J., & Green, P. (2005). Impact of humans on the flux of terrestrial sediment to the global coastal ocean. Science, 308, 376–380.
Tam, N. F. Y., & Wong, Y. S. (2000). Spatial variation of heavy metal in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110, 195–205.
Towler, P. H., & Smith, J. D. (2000). Studies of the influence of graded storm layers on 210Pb and heavy metals profiles in Great Barrier Reef sediments. Environmental Science and Technology, 34, 2947–2952.
Untawale, A. G. (1987). Conservation in Indian mangroves a national perspective. In: T. S. S. Rao, R. Natarajan, B. N. Desai, G. Narayanaswamy, & S. R. Bhat,(Eds.), A special collection of papers to felicitate Dr. S. Z. Qasim on his sixtieth birthday (pp. 85–104).
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1232.
Weis, D. A., Callaway, J. C., & Gersberg, R. M. (2001). Vertical accretion rates and heavy metal chronologies in wetland sediments of the Tijuana Estuary. Estuaries, 24, 840–850.
Woodroffe, C. D. (1990). The impact of sea-level rise on mangrove shorelines. Progress in Physical Geography, 14, 483–520.
Yuan, X., Chen, Y., Li, B., & Siegel, D. I. (2010). Source of sediments and metal fractionation in two Chinese estuarine marshes. Environmental Earth Sciences, 60, 1535–1544.
Zhang, J. E., Liu, J. L., Ouyang, Y., Liao, B. W., & Zhao, B. L. (2010). Removal of nutrients and heavy metals from wastewater with mangrove Sonneratia apetala Buch-Ham. Ecological Engineering, 36, 807–812.
Zwolsman, J. J. G., Van Eck, G. T. M., & Berger, G. W. (1996). Spatial and temporal distribution of trace metals in sediments from the Scheldt estuary, south-west Netherlands. Estuarine, Coastal and Shelf Science, 43, 55–79.
Acknowledgments
The authors extend profound thanks to Ministry of Environment & Forests (MoEF), Government of India, for all the assistance provided for sampling and analysis. They also express their sincere appreciation to Mr. R. Elumalai for helping with radiochemical analysis. The authors also thank to Dr. S. Srinivasalu, Department of Geology, Anna University, Chennai, and Dr. V. Kannan, Former Director of ESL, Kalpakkam, for providing valuable suggestions. Authors are thankful to anonymous referees for their thoughtful and constructive comments on the earlier manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, K., Senthilkumar, B., Purvaja, R. et al. Sedimentation and trace metal distribution in selected locations of Sundarbans mangroves and Hooghly estuary, Northeast coast of India. Environ Geochem Health 34, 27–42 (2012). https://doi.org/10.1007/s10653-011-9388-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-011-9388-0