Abstract
This study aimed to measure spatial and temporal distributions of total phenolic compounds and their ecological and health hazards using UV-vis spectrophotometers as a low-cost, fast, simple method in water and sediments collected from Timsah Lake, Suez Canal, Egypt, 2022. Also, assessing highly adaptive fungal species associated with contamination is designed. Due to human and environmental activities and industrial waste discharges, Timsah Lake is increasingly threatened by all kinds of pollutants. The results indicated that the seasonal concentration means of the phenolic compounds were winter (0.229) > spring (0.161) > summer (0.124) > autumn (0.131) mg/l and winter (3.08) > summer (2.66) mg/g in water and sediment samples, respectively. The result has shown that the phenol concentrations in all stations were more than 0.005 and 0.1 mg/l for Egyptian National Standards and World Health Organization (WHO) for drinking water but less than the limits of 1 mg/l for wastewater. Notably, the fungi recorded the highest counts during spring, totaling 397 colonies/100 ml of water and 842 colonies/gram of sediment. Four isolates of fungi were identified and deposited in the GenBank database by Aspergillus terreus, Aspergillus terreus, Penicillium roqueforti, and Penicillium rubens under accession numbers OR401933, OR402837, OR402878, and OR424729, respectively. Moreover, ecological risk (RQ) for the total phenolic compounds was > 1 in all investigated stations for water and sediments. The hazard quotient is HQ < 1 in all seasons in water and sediments except winter. The hazard index (HI) in water and sediments for children is higher than for adults. It can be concluded that the low-cost, fast, simple method for determining phenolic content in water and sediment samples, using UV-vis spectrophotometry, was useful for predicting the reactivates of a wide variety of phenol and their derivatives. Furthermore, it can be concluded that Periodic assessments of water quality and strict regulations are necessary to safeguard this vital resource from pollution and ensure the well-being of future generations. Finally, policymakers and water treatment specialists might use the information from this research to reduce these chemical pollutants in Egypt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds constitute a diverse group of organic compounds ubiquitous in nature. Their occurrence can stem from natural sources, such as decomposing organic matter and vegetation, and anthropogenic activities, including industrial processes, agricultural runoff, and domestic waste. In aquatic ecosystems, the presence and concentration of phenolic compounds serve as critical indicators of environmental health and quality (Apostică et al. 2018; Brusseau et al. 2019; Mainali 2020; Hanafi and Sapawe 2020; Xu et al. 2021; Gziut and Thomas 2022).
Water and sediment serve as sinks for phenolic compounds, with sediments acting as long-term reservoirs that can release these compounds into the water column under certain conditions. The accumulation of phenolic compounds in sediment can have far-reaching effects on ecosystem dynamics, including altering nutrient cycling, sediment stability, and potentially bioaccumulating aquatic organisms. These pollutants threaten marine organisms and harm human health (Mi et al. 2019; Hu et al. 2020; Feng et al. 2020) due to their solubility, persistent, toxicity, fast prevalence, and accessible transportation in water and their consequences on the food chain and human health as their neurotoxicity, carcinogenicity, endocrine-disrupting and mutagenicity and bio-accumulative properties (Sun et al. 2022). Regulatory bodies such as the United States Environmental Protection Agency (USEPA) and the European Union (EU) classify phenolic compounds as priority pollutants due to their harmful effects on humans and animals (Eletta et al. 2020; Chen et al. 2021; Prabhu et al. 2021). Therefore, it is crucial to develop effective and affordable methods for their detection that do not require expensive laboratory equipment and are time-efficient (Zhao et al. 2018; Rahimi-Mohseni et al. 2019).
The presence of phenolic compounds in surface waters is devoted by various studies, emphasizing their toxicity and their risks to human health and aquatic organisms in multiple regions around the world (Wang et al. 2020; Chen et al. 2021; Ramos et al. 2021; El-Naggar et al. 2022). More research is needed to evaluate the phenol content using spectrophotometers from water and sediment in Suez Canal lakes (Timsah Lake) (El-Sawy et al. 2023).
The prevalence of polluted microorganisms (including bacteria, fungi, and algae) in lakes relies on specific contamination sites encompassing water and sediment areas (Barathi et al. 2023). Fungi’s presence in these tainted sites contributes to the degradation of these pollutants, a process known as mycoremediation. Various microorganisms are universally distributed globally and can be harnessed to degrade pollutants (Singh et al. 2020). Moreover, they are remarkably able to adapt and persist across diverse conditions. This adaptability extends to various fungal species employed for remediating pollutants, including heavy metals, hydrocarbons, pesticides, and radioactive compounds (Kottb et al. 2019; Singh et al. 2021).
Understanding and monitoring the impact of total phenolic compounds on water and sediment are crucial for maintaining ecological balance, preserving biodiversity, safeguarding water quality, and ensuring the health and sustainability of aquatic ecosystems. Management strategies aimed at mitigating the sources of phenolic compounds and reducing their concentrations play a vital role in preserving these delicate environments (Ramos et al. 2021). It is frequently used in environmental decision-making because it is a foundation for creating safeguards. Several research studies have evaluated the ecological danger of phenolic compounds in surface waters from various places (Yuan et al. 2017; Zhong et al. 2018).
Timsah Lake, located in Egypt as a critical part of the Suez Canal Lakes, is an essential aquatic ecosystem with significant biodiversity and environmental balance. It is a crucial component of the canal system, acting as a substantial water reservoir along the maritime route between the Mediterranean Sea and the Red Sea (Donia 2011; Mohamedein et al. 2019). Like many water bodies worldwide, it faces environmental challenges due to anthropogenic activities and environmental change. Pollution from industrial effluents, agricultural runoff, and urban activities threatens water quality and the overall ecosystem health (El-Serehy et al. 2018; Hassan and Kandil 2022).
This study aims to examine total phenolic compounds using a rapid spectrophotometric method in Timsah Lake to understand the lake’s health and contamination. Moreover, highly adaptive fungal species were assessed for pollution in Timsah Lake. Furthermore, a risk assessment will evaluate the potential ecological and human health risks associated with the observed phenolic compound levels in adults and children.
Materials and methods
Study area
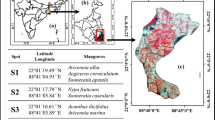
Timsah Lake, in Ismailia City, is the most significant water body, with a surface area of 14 km2. It is a salty shallow water basin between Suez and Port-Said at 30° 35046.55″ N and 32° 1930.54″ E. The lake’s coastline is uneven and trapezoidal; it has a surface area of about 8 km2 with long sides ranging around 5 km east to west and 4.5 km north to south. The lake’s most excellent depth surpasses 25 m near the Suez Canal navigational channel (El-Azim et al. 2018; El-Serehy et al. 2018; Hassan and Kandil 2022). The region has three basins: Timsah Lake, the western lagoon, and the Suez Canal pathway. The lake takes saline water from the Suez Canal, mainly from the south. It also obtains fresh water from the outlet Ismailia Canal at its eastern side; El-Forsan drains from the North middle and drains via the western Lagoon (Dar et al. 2021).
Timsah Lake’s bottom is primarily silt, with deeper sections dominated by anaerobic black mud and shallower portions dominated by aerobic gray mud. The coastlines, shallow waterways, and dredged regions are sandy, with occasional rocky outcrops. The lake gets saltwater mostly from the Suez Canal, with freshwater coming from various sources, including the Ismailia Canal, seasonal streams, and sewage outflows. This mixture of saltwater and freshwater inputs causes salinity stratification in the lake water (El-Azim et al. 2018; El-Serehy et al. 2018; Dar et al. 2021) (Table 1 and Fig. 1).
Sampling collection and storage
Water
Surface water samples in triplicate were collected seasonally from 12 stations during 2022 (Table 1). Clean bottles were submerged below the water surface to collect the samples and then raised. Specific locations were chosen to cover the areas predicted to be pollution-related. To inhibit microbial growth in transit, 1 L of water was acidified to a pH of 2 with 10% HCl. After collection, the samples were sent to the lab, where they were filtered via 0.45 m pore-size Millipore glass-fiber filters. The filtered samples were kept at 4 °C until analysis. The analysis followed standard procedures as described by (IOC/UNEP 1991).
Sediment
In 2022, sediment samples weighing 1 kg each were collected from the same water collection stations two times/year from Timsah Lake. Triplicate samples were taken from each station to ensure reliability. The samples were collected using a Van-Veen grab sampler, explicitly targeting the middle portion of the grab by removing stones and other heavy particles. Large non-clay fractions were removed from the clay. After collection, the samples were carefully wrapped in pre-cleaned aluminum foil and stored at a freezing temperature of − 20 °C to preserve their integrity until analysis. The storage method was based on established protocols outlined by (Amini Ranjbar 1998; Readman et al. 2002).
Procedure
One of the key challenges researchers face when measuring phenolic compounds is the expensive and time-consuming extraction process and advanced measuring instruments using HPLC and GC-mass techniques (Xu et al. 2021). At the same time, spectrophotometric techniques have been summarized for the trace-level determination of phenolic compounds, which often utilize reagents like 4-amino antipyrine and potassium ferricyanide. The UV–visible spectrophotometer technique is commonly used because of the ability of phenolic compounds to absorb UV light and exhibit absorption features in the visible region. Additionally, this method is suitable for analyzing various water samples, including surface waters, saline waters, and domestic and industrial wastes. It allows for the measurement of phenolic compounds present in the aqueous phase without solvent extraction (Rahimi-Mohseni et al. 2019).
Water
The total phenolic content was analyzed using a modified form of the traditional method described by Martin (1949), following the guidelines outlined in the EPA 420.1 Manual 1978. The analysis used a spectrophotometric technique, specifically a UV–Vis Model 6800 instrument. The proposed method for phenol determination is based on the reaction between phenol and 4-amino antipyrine in alkyl media.
where TPC (total phenolic compounds) is in mg/l, and Abs is the reading of phenol established from the calibration curve.
Sediment (ultrasonic extraction)
The standard of EPA method 3550 C carried out the initial extraction from the sediments (Ziyaadini et al. 2016) so that 1 g of the dried sediment samples was added to 5.0 mL of dichloromethane-n-hexane (50:50, v/v), and the mixture was shaken by sonication for 15 min using an ultrasonic extractor.
where TPC in sediment samples (mg/g), Cs is the concentration of phenol sample established from the calibration curve in mg/g, Cs is the concentration of phenol standard established from the calibration curve in mg/g, and conc., of St. is the concentration of standard used.
Quality control (QC)
Experiment chemicals were from reliable sources, including Merck, BDH, and HPLC grade, and all had high purity. All of the experiments were conducted using double-distilled water to exclude any chance of contamination. The standard phenol stock solutions were made by dissolving the required phenol in an acceptable solvent until a concentration of 1000 mg/l was reached. The stock solution was then diluted with deionized water under strict conditions to produce standard solutions of varying concentrations. Analyses of aqueous solutions at different concentrations of the analysts of interest were used to generate a standard calibration curve. From the calibration curve, we calculated an r-value of 0.999, which indicates a very high degree of linearity between the response signal and the concentration of the analysts. The calibration sensitivity is shown by the slope of the calibration curve, which shows how much the signal from the analysts’ responses changes for each unit of concentration. Indicating that the sensitivity was stable over different concentrations of analyzers, the calibration curve was linear.
Microbiological analysis
The microbiological analysis involves isolating fungal specimens from water–sediment samples and conducting morphological and genetic identification of the most abundant species. For sediment samples, sand samples were mixed with sterile distilled water, spread on agar plates, and incubated at 28 °C for 3–4 days. Colonies were then transferred and identified based on their characteristics. For water samples, serial dilutions were made, spread on Sabouraud agar plates, and incubated at 28 °C for 7 days. Fungal colonies were counted and identified based on their morphology. All isolates exhibited similar growth on Czapek’s dextrose agar plates at 28 °C, with most colonies reaching 3–4 cm diameters after 48 h of incubation (Mbata 2008; Souza et al. 2017; Glukhova et al. 2018).
Four well-isolated fungal colonies were randomly selected based on their growth characteristics and morphology. Fungal DNA was extracted and isolated from these colonies. The ribosomal rRNA gene was then amplified using universal fungal primers (ITS1 and ITS4) and subjected to PCR under specific conditions. The PCR products were purified, and their molecular sizes were determined using agarose gel electrophoresis. Subsequently, the purified PCR products were sequenced in both forward and reverse directions using ITS1 and ITS4 primers. The obtained ITS sequences were analyzed using the BLAST search program to identify species in the GenBank. This methodology is similar to the rapid fungal species identification methods described by Alwakeel. Phylogenetic analysis was performed by aligning the nucleotide sequences and constructing a phylogenetic tree using Mega 7 software (Gontia-Mishra et al. 2014; Chen et al. 2015; Alwakeel 2017).
Ecological risk assessment
The US Environmental Protection Agency’s (EPA) ecological risk assessment approach serves as the foundation for the environmental risk evaluation of phenolic compounds in this research (Li et al. 2016, 2017; Chakraborty et al. 2016; Zhang et al. 2018). To evaluate the potential danger posed by these phenolic chemicals, researchers used the risk quotient (RQ) technique. RQ is calculated using the formula:
MEC is the detected environmental concentration (mg/l), and PNEC water is the predicted no-effect concentration (mg/l). RHOsolid is the density of the solid phase, which is 2500 kg/m3; RHOsusp is the density of wet suspended matter, which is 1150 kg/m3; F water − susp is the volume fraction of water in suspension, which is defined as 0.9 m3/m3; F solid − susp is the volume fraction of solid in suspension, which is defined as 0.1 m3/m3; Koc is the partition coefficient of organic carbon–water (L/kg); Foc − susp is the mass fraction of organic carbon in suspension, which is assigned as 0.1 kg/kg; (EPA 2015). The ecological risk was categorized into three classes, which depended on RQ values: RQ > 1 indicated a great ecological danger, 0.1 < RQ < 1 indicated a medium risk, and RQ < 0.1 indicated a low environmental risk (EPA 2015; Wang et al. 2019a, b).
Human health risk assessment
Non-carcinogenic risk to human health is evaluated using the hazard quotient (HQ) in the following way (USEPA 2012):
Table 2 from USEPA (2017) and IRIS provide the Oral Reference Dose (RfD) for phenol (0.6 mg/kg/day); C. The concentration of the contaminant (mg/l), DR daily consumption rate for water (200 L/day), EF exposure frequency (day/year), ED exposure duration (Year), BW body weight (Kg), and AT averaging time (day). The overall score is known as the hazard index (HI):
A high HQ value is associated with an increased risk of non-cancer, whereas a low HQ value is associated with a decreased risk of non-cancer.
Data analysis
The contour plot technique was utilized with the internal preferences map to represent Timsah Lake visually, highlighting clusters where preferences increased and others where preferences decreased (Busing et al. 2010). Furthermore, to analyze phenol-fungal persistence across different stations in the lake, Hierarchical cluster analysis was employed based on the approach proposed by Qadir et al. (2008). A one-way ANOVA test with Fisher multiple comparisons was conducted to determine the significance of the recorded data. Additionally, to establish a relationship between the abundance of fungal species and environmental variables, particularly phenol concentration, Canonical Correspondence Analysis (CCA) was developed, as outlined by Bodaghabadi et al. (2011). All previous studies were implemented using the R-4.2.1 program, following the methodology introduced by Matloff (2011).
Results and discussions
Occurrence of physical parameters
Physical parameters in Timsah Lake occur due to a combination of natural and anthropogenic factors influencing the lake’s physical characteristics. These parameters play a crucial role in shaping the lake’s environment. Table 3 summarizes the physical parameters (water temperature, pH, salinity, and transparency) concentrations in water samples annual and seasonal variation from Timsah Lake during 2022. Temperature is essential for determining water quality and evaluating aquatic ecosystems. Water temperature ranged between 17.35 °C in winter and 29.65 °C in summer, with an average mean of 23.65 ± 8.70. At the same time, annual water temperature ranged between 23.92 and 24.77 °C at stations 6 and 9, respectively. It substantially impacts numerous chemical and biological processes and influences aquatic organisms’ distribution and living conditions (Zaghloul et al. 2023). The pH level is another critical chemical parameter with biological significance in natural water systems. Changes in pH can affect the toxicity of certain substances in water (Zaghloul et al. 2023). The present study recorded slightly alkaline mean values for pH, with 7.98 for minimum and 8.99 for maximum values, respectively. At the same time, pH values ranged between 8.06 and 8.67 at stations 11 and 1, respectively. pH of Timsah Lake and the western lagoon lies on the alkaline side in all the studied stations; the lowest pH value was (affected by the Mahsama drain). This drain, primarily agricultural but also carrying domestic waste from various areas, contains significant amounts of salts, nutrients, and biological pollutants. The large nutrient and pollution from urban and industrial centers can lead to organic over-enrichment, eutrophication, and associated health problems. These issues negatively impact biological communities and lead to the lowest pH (Abukila 2015; Khairy and Ghany 2021). Salinity levels ranged between 1.10‰ during winter and 42.58 during summer, averaging 24.41 ± 29.33‰. Variations in salinity were the driving force behind all variations in the lake’s water quality, in which annual variation ranged between 1.20 and 38.05‰ at stations 12 and 6, respectively. The lowest salinity in the western site may be due to freshwater discharged from sewage treatment systems, untreated sources, and nonpoint source runoff (EL-Mahsama drain and Abu Gamous drain). On the other hand, the highest salinity was recorded on the eastern side of Timsah Lake, which receives municipal water from a covered conduit and a minor quantity of fresh water from the Ismailia Canal (El-Azim et al. 2018). Transparency in water is caused by various sources, including natural sources, sewage waste, surface runoff, and industrial wastewater (WHO 2017). Transparency concentration is generally higher in areas exposed to agricultural and urban pollution than in non-residential areas (Zaghloul et al. 2023). Values of transparency ranged between 20.00 and 200.00 cm. Transparency recorded near the southwestern side is very high due to the outfall of domestic and industrial wastewater. Finally, Timsah Lake suffers from increased human population density, associated with decreased water resources and increased organic contamination in the lake’s water (El-Azim et al. 2018).
Occurrence of phenolic compounds
Natural and synthetic mono-aromatic phenol compounds are a significant pollutant in many ecosystems (Abarian et al. 2019). Depending on the volume and composition of industrial and municipal discharges, phenol concentrations in Timsah Lake’s surface water varied widely. The strong correlation between concentrations of phenolic compounds and solubility in water is possibly due to organic chemicals having a greater propensity to dissolve in water (Zhou et al. 2017). Timsah Lake’s water and sediment concentrations, including annual and seasonal fluctuations, are listed in Table S1 and Figures (S1–S4).
The annual mean of total phenolic concentration in Timsah Lake varied from (0.124–0.286) mg/l and (0.043–0.425) mg/g with an average value of (0.161 ± 0.042) mg/l and (0.229 ± 0.043) mg/g for water and sediment samples, respectively. The highest values in water and sediment were at station 12, while the lowest values in water were at station 8 and station 6 sediments, respectively. The high concentrations are mainly due to anthropogenic discharges. The highest pollution occurred, especially at station 12, a very shallow semi-closed zone with no circulation in the lake, nearly 1.5 m deep. It is located near wastewater discharge; pesticide application may induce the pollution of phenolic compounds in aquatic environments by irrigation, agricultural, domestic, and industrial sources with high land activities (Zhou et al. 2017; Etchegoyen et al. 2017). Also, due to low water currents, which are not thoroughly mixed, high-density organic materials exist in sewage water (Amteghy 2022). On the other hand, the lowest phenol concentration in water was recorded at station 8, located farther away from pollution sources and connected to freshwater sources, with dilution occurring in this station (El-Azim et al. 2018). At the same time, the lowest phenol concentration in sediment was recorded at a station (6), which is far away from pollution sources besides the Suez Canal pathway (Donia 2011).
The seasonal mean of total phenolic concentration in Timsah Lake varied from (0.169–0.377), (0.080–0.417), (0.084–0.244), and (0.102–0.147) mg/l with average values (0.229 ± 0.048), (0.161 ± 0.088), (0.124 ± 0.043), and (0.131 ± 0.016) mg/l for winter, spring, summer, and autumn, respectively, and from (0.048–0.450), and (0.037–0.400) mg/g dw in sediment with average mean values (0.246 ± 0.117) and (0.213 ± 0.109) for winter and summer, respectively. Seasonal variation in water follows the order of winter > spring > summer > autumn and winter > summer for sediment. The highest seasonal variations mean of the total phenolic compound was recorded during winter in Timsah Lake (0.229 mg/l and 0.246 mg/g) for water and sediment samples, respectively. This season represents the period with the highest phenol abundance in the lake. The high levels of phenol in Timsah Lake can be attributed to the discharge of untreated sewage and agricultural effluents into the western lagoon. Other factors include the effects of the Ismailia Canal, the dockyard of the Suez Canal, and sewage discharge from Ismailia City and nearby villages restricted to these stations (El-Azim et al. 2018; Hassan and Kandil 2022). In contrast, the lowest total phenolic water concentrations were observed in Timsah Lake during summer (0.161 mg/l). This may be due to high temperatures accelerating the breakdown of organic pollutants due to increased microbial activity (Soliman et al. 2017; Ezz El-Din et al. 2023). Furthermore, Egypt’s climate, photolysis, volatilization, and biological impact occur quickly (Amteghy 2022).
By comparing our result in this study with other studies, phenol content in water samples with acceptable limits of USEPA 2018 for wastewater and higher than the limits of WHO 2003 for drinking water. Moreover, the recorded limits are within range for drinking water sources in Nigeria (Otitoju et al. 2023), the coastal waters of Alexandria (El-Naggar et al. 2022), Aqueous media Alexandra Georgiana (Apostică et al. 2018), and Lake Maryut, Egypt, (Khairy 2013). At the same time, they lie within the limit in sediment with (Lake Marriott), Egypt (Khairy 2013; El-Naggar et al. 2022) (Table 4).
Finally, it noted that the phenol concentrations observed in the study have yet to exceed the hazardous limits. They were higher than the WHO’s (2003) and Egyptian National Standards requirements for potable water but lower than the USEPA’s (2018) limits for wastewater.
Microbiological analysis
Forty-eight water and sediment samples were inoculated on Sabouraud agar plates; Table 5 shows the total colony counts, frequencies, and percentages of genera and species that grew on the phenol-Czapek’s agar. The number of fungi in the spring was higher than in other seasons by 397 cells/100 ml of water and 842 cells /100 gm of sediment. The number of fungi in the sediment was 669, 735, and 595 cells/100 gm in the summer, autumn, and winter, respectively. Meanwhile, the overall number of fungi in the water was 246, 312, and 177 cells/100 ml in the same seasons. Similar isolates were selected in terms of morphology and were identified as Aspergillus, Penicillium, and Fusarium, the main groups. Four fungal isolates were chosen for genetic identification. The amplified fungal sequences were used as BLAST queries against the NCBI database. Table 5 shows the fungi isolated from the Timsah Lake compared with closely related fungi in the GenBank. Most of the fungi we isolated had 97–99% similarity with the associated fungi recorded in the bank. Nucleotide sequences of the ITS1 region of 18 s rRNA genes of isolated fungal species were deposited in the GenBank database by Aspergillus terreus, Aspergillus terreus, Penicillium roqueforti, and Penicillium rubens under accession numbers OR401933, OR402837, OR402878, and OR424729. Figures (S5 and S6) represent the phylogenetic tree of identified strains.
According to the result, the relationship between fungal abundance and phenolic compounds is complex and influenced by factors like fungal species, phenolic type/concentration, and environmental conditions. Fungi can metabolize phenolics, with some specializing in breaking down complex phenols, while others tolerate high phenolic levels. Some phenolics can inhibit fungal growth, affecting their presence. In the water, the stations with the least fungal counts were stations four, five, and seven, with counts of 11, 9, and 12, respectively, over the four seasons. Conversely, phenol concentrations in these stations were relatively high compared to others. In the sediment, station nine had the lowest fungal count by 0.150 mg/g which was relatively low compared to other stations. On the contrary, fungal counts and phenol concentrations were high in the other stations. From these results, it can be inferred that fungal counts are positively or negatively affected by phenol concentrations, as observed in some stations. Similar findings to those of this study were noted in previous research by Boonmee et al. (2020), Khalil et al. (2021), Liu et al. (2023), and Matúš et al. (2023), which suggested the potential of fungi to reduce or grow on various phenolic compounds in water and soil. Environmental factors, like soil conditions, impact phenolic availability and fungal abundance. Fungal responses to phenolics vary due to their biochemical diversity and adaptations (Ezz El-Din et al. 2023).
Sabouraud agar is a commonly used medium for fungal culture due to its ability to support the growth of various fungal species. The pronounced fluctuations in fungal abundance across seasons underscore the dynamic character of fungal populations within the environment (Brazhnikova et al. 2022). The more fungi observed in the spring than in other seasons suggests that environmental factors, such as temperature and moisture, shape fungal growth patterns. The specific numbers provide quantitative insights into these variations.
The variation in fungal counts between seasons indicates the influence of temperature and other seasonal factors on fungal proliferation (Table 5). Similarly, the lower fungal counts in water during winter (14.75) compared to springer (33.08) could be attributed to environmental conditions impacting fungal growth and dispersion as shown in Table 5 (Chen et al. 2020). Morphological identification is a preliminary step in fungal taxonomy, providing insights into the diversity of fungal species. Aspergillus, Penicillium, and Fusarium groups are well-known genera with diverse ecological roles, including decomposition, pathogenesis, and symbiosis (Yadav et al. 2022). The genetic identification of fungal isolates adds a molecular dimension to the study. Using amplified fungal sequences as BLAST queries against the NCBI database, a comparative analysis can be performed between their isolates and established fungal sequences. This procedure plays a pivotal role in verifying the identity of the isolates and elucidating their connections with fungi that have been previously documented. Most isolated fungi (97–99%) are similar to related fungi in the GenBank database. This similarity suggests that the isolated fungi belong to established taxonomic groups and reinforces the accuracy of the identification process.
Ecological risk assessment
The ecological risk assessment was calculated depending on the RQ values for the total phenolic compounds surface water and sediment samples, and in comparing the seasonal mean of RQ, values of water samples ranged from (2.11–4.22), (1.00–5.22), (1.05–3.05), and (1.28–1.83) during winter, spring, summer, and autumn, respectively (Table S2 and Figures S7, 2, S8 and 3). In contrast, RQ in sediment ranged from (0.60–5.63) and (0.46–5.00) for winter and summer, respectively. The higher concentration RQ was recorded (2.86) during winter > spring (2.01) > summer (1.55) > autumn (1.64), whereas seasonal ecological risk variation in sediment showed a higher concentration during winter (3.08) > summer (2.66). The annual environmental risk means of water ranged between 1.56 and 3.58 with an average mean value of 2.01 ± 0.53. At the same time, the ecological risk of sediment samples ranged between 0.53 and 5.31 with an average mean value of 2.87 ± 1.41. Environmental risk (RQ) for the total phenolic compounds was > 1 in all investigated stations for water and sediment samples, reflecting the high ecological risk of these compounds in water and sediments. In addition, station 12 recorded the highest RQ in water during all seasons, especially spring and sediments, and was classified as a significant ecological danger. The relative areas with the greatest risk of phenol contamination in water and sediments may affected by the movement of current and different types of waste; as a result, more attention should be devoted to the ecological concerns presented by the sediment in this impactive impact on agriculture, stockbreeding, or generating further pollution in water via sediment desorption (Zhou et al. 2017). In contrast, stations (2 and 6) in sediments RQ < 1 reflect medium risk. Finally, total phenolic compound RQ risk > 1 in water and sediments indicated a great ecological danger, and 0.1 < RQ < 1 indicated a medium risk (EPA 2015; Wang et al. 2019a, b).
Human health risk assessment
Human risk assessment of phenolic compounds was performed by calculating non-carcinogenic risk. Non-carcinogenic risk assessment is dependent on a nonlinear model. Even though chemicals’ carcinogenic and non-carcinogenic risks cannot be wholly distinguished (Zhou et al. 2017). Given this study, it was proven that the dose threshold of the chemicals, any risk less than the dose threshold, was expected to have no risk to human health.
The hazard quotient (HQ) was used to assess the non-carcinogenic risk in this study. Table 6 shows the values of the pollutants of total phenolic compound in water were < 1 in almost all stations except the winter season and in sediment samples < 1. Hazard quotient HQ calculated for mutagenic and other non-cancer health effects ranged in water samples between (8 × 10−1 − 1.6 × 10+1), (3.8 × 10−1 − 2.0 × 10+1), (4.0 × 10−1 − 1.2 × 10+1), and (4.9 × 10−1 to 7 × 10−1) for adult, respectively, and from (1.90 × 10+1 − 3.75 × 10+1), (8.90 × 10−1 − 4.60 × 10+1), (9.33 × 10−1 − 2.71 × 10+1), and (1.13 × 10+1 − 1.63 × 10+1) for the child during winter, spring, summer, and autumn, respectively, whereas HQ risk in sediment samples ranged from (3.45 × 10−2 − 3.21 × 10−1) and (2.64 × 10−2 − 2.86 × 10−1) for adults and from (1.61 × 10−1 − 1.50 × 10+1) and (1.23 × 10−1 − 1.33 × 10+1) for child during winter and summer, respectively. HI in children was more than in adults in water and sediment samples. Elevated HI value (> 1) recorded in water and sediment samples points to a raised risk of non-cancer disease incidences in their lifetime. This implies that most examined samples may pose little or no mutagenic (non-cancer) health effects on consumers.
Data analysis
The analysis of the reference map with the contour map illustrates the influence of fungal abundance and phenol concentrations at different stations. This analysis relies entirely on examining principal components and Agglomerative Hierarchical clustering (AHC), merging their results into a unified analysis. The analysis of AHC resulted in the identification of nine clusters. The first cluster encompasses phenol concentrations in water during the summer, while the second cluster involves phenol concentrations in water during autumn. The third cluster represents phenol concentrations in winter, and the fourth cluster pertains to phenol concentrations in spring. The fifth cluster encompasses soil phenol concentrations during both summer and winter seasons. The sixth cluster pertains to fungal density in water during the summer, while the seventh cluster concerns fungal density in water during autumn and spring. The eighth cluster represents fungal density in water during the winter, and the ninth cluster involves fungal density in soil across all seasons. These nine clusters exhibited varying degrees of impact attributed to different stations, as depicted in (Figs. 4 and 5). The second, fourth, fifth, and ninth clusters exhibited the most pronounced effects. Consequently, this region displayed the highest impact percentage in the contour map, ranging from 80 to 90%.
On the other hand, the percentage of satisfied assessors for each station varied across the stations according to the results. The third, fourth, and ninth stations exhibited the lowest concentrations, while the second and eighth stations showed an 11% concentration. The fifth station had a concentration of 22%, the sixth and seventh stations had a concentration of 44%, the first station had a concentration of 67%, the tenth and eleventh stations had a concentration of 78%, and the twelfth station had a concentration of 100%. The analysis reveals that the presence of fungi is influenced by the price pollutants, including different phenol concentrations, across the different stations.
Considering the analysis of variables and their interdependencies, the impact of fungi on phenol concentrations and vice versa was examined through a canonical analysis (Fig. 6). The results of the analysis can be summarized as follows: there is no significant effect of phenol concentrations in water during the summer, autumn, and winter seasons on the presence and types of fungi. However, during other seasons, the presence of fungi was positively influenced. This suggests that the fungal population increases with higher concentrations of phenols and their derivatives during the winter and summer. The increase in phenolic compounds is attributed to elevated industrial discharge, including petroleum pollution, which occurs during these periods of the year.
An ANOVA analysis was conducted, as shown in Tables (5 and S1) to analyze the differences in the values of variables during this study. Regarding the phenol concentration in water and soil during different seasons, the twelfth station exhibited the highest variability according to the Shapiro constant. Following the twelfth station in terms of variability were the tenth and fifth stations in water during the summer season, the ninth and fifth stations in water during the autumn season, the eleventh station in water during the winter season, and the first station in water during the spring season. The first and tenth stations showed the most variability in soil during the summer, and the first station showed the most variability during the winter.
As for the presence of fungi in different seasons, the twelfth and eleventh stations were also the most influential stations according to the Shapiro constant. The increased impact is attributed to the accumulation of a large human population and various activities in these two stations, leading to a direct or indirect increase in pollutants, making the area suitable for the growth of various microorganisms, including fungi, the subject of the study.
Conclusion
The findings showed that the highest seasonal variations mean of the total phenolic compound was recorded during winter in Timsah Lake for water and sediment samples, respectively. Also, the total phenolic compounds in the water were above the maximum the World Health Organization and the Egyptian National Standards allowed. Furthermore, the number of fungi in the spring was higher than in other seasons by 397 cells/100 ml of water and 842 cells/100 gm of sediment. The most dominant detected fungal isolates in water and sediment samples are Aspergillus sp., Penicillium sp., and Fusarium sp. The RQ found that phenol levels in the water at the stations studied were consistently high throughout all four seasons. In addition, significant ecological concerns were associated with the total phenolic chemicals at all stations studied, regardless of the time of year. HQ were < 1 in almost all stations for water and sediment samples. HI values are higher in water and sediment samples in children than in adults. A significant summertime non-cancer risk was seen among regular users across all investigated stations, as determined by HI.
This work evaluated the distribution of total phenolic compounds, primary pollution sources, and their relation to the occurrence of the highly adaptive fungal species. Also, it assessed the risks of total phenolic compounds in the water and sediment of Timsah Lake. Finally, the authors offer a spectrophotometer for rapidly and accurately determining phenol concentrations in aqueous solutions with a cheap cost, short time, and ease of use, making this technique suitable for commercial labs.
References
Abarian M, Hassanshahian M, Esbah A (2019) Degradation of phenol at high concentrations using immobilization of Pseudomonas putida P53 into sawdust entrapped in sodium-alginate beads. Water Sci Technol 79(7):1387–1396
Abukila AF (2015) Assessing the drain estuaries’ water quality in response to pollution abatement. Water Science 29(1):1–18
Alwakeel SS (2017) Molecular identification of fungi isolated from coastal regions of Red Sea, Jeddah, Saudi Arabia. J Assoc Arab Univ Basic Appl Sci 24:115–119. https://doi.org/10.1016/j.jaubas.2016.10.001.
Amini Ranjbar GH (1998) Heavy metal concentration in surficial sediments from Anzali Wetland, Iran. Water Air Soil Pollut 104:305–312
Amteghy AH (2022) 4-amino antipyrine spectrophotometric and chromatographic gas determination of phenols in the water of southern Iraq along with the density functional theory study. Technology 12(2):539–545
Apostică AG, Ichim T, Radu VM, Bulgariu L (2018) Simple and rapid spectrophotometric method for phenol determination in aqueous media. Bull Polytech Inst Jassy, Constructions Archit Sect 64:9–18
Barathi S, Gitanjali J, Rathinasamy G, Sabapathi N, Aruljothi K, Lee J, Kandasamy S (2023) Recent trends in polycyclic aromatic hydrocarbons pollution distribution and counteracting bio-remediation strategies. Chemosphere 337:139396. https://doi.org/10.1016/j.chemosphere.2023.139396
Bodaghabadi MB, Salehi M, Martínez-Casasnovas JA, Mohammadi J, Toomanian N, Borujeni IE (2011) Using Canonical Correspondence Analysis (CCA) to identify the most critical DEM attributes for digital soil mapping applications. CATENA 86:66–74
Boonmee S, Atanasova V, Chéreau S, Marchegay G, Hyde KD, Richard-Forget F (2020) Efficiency of hydroxycinnamic phenolic acids to inhibit the production of ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum. Int J Mol Sci 21(1):8548
Brazhnikova YV, Shaposhnikov AI, Sazanova AL, Belimov AA, Mukasheva TD, Ignatova LV (2022) Phosphate mobilization by culturable fungi and their capacity to increase soil p availability and promote barley growth. Curr Microbiol 79:240
Brusseau ML, Matthias AD, Comrie AC, Musil SA (2019) Atmospheric pollution. PS (Third E). In: Brusseau ML, Pepper IL, Gerba CPBT-E. (eds) Environmental and Pollution Science. Academic Press, pp 293–309. https://doi.org/10.1016/B978-0-12-814719-1.00017-3
Busing FM, Heiser WJ, Cleaver G (2010) Restricted unfolding: preference analysis with optimal transformations of preferences and attributes. Food Qual Prefer 21:82–92. https://doi.org/10.1016/j.foodqual.2009.08.006
Chakraborty P, Khuman SN, Selvaraj S, Sampath S, Devi NL, Bang JJ, Katsoyiannis A (2016) Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: Occurrence, sources and Ecotoxicological risk assessment. Environ Pollut 219:998–1006
Chen H, Dou Y, Tang Y, Zhang Z, Zheng X, Niu X, Yang J, Yu X, Diao Y (2015) Isolation and genomic characterization of a duck-origin GPV-related parvovirus from Cherry Valley ducklings in China. PLoS One 10:e0140284
Chen J, Wang P, Wang C, Wang X, Miao L, Liu S, Yuan Q, Sun S (2020) A fungal community demonstrates stronger dispersal limitation and less network connectivity than a bacterial community in sediments along a large river. Environ Microbiol 22:832–849
Chen Y, Zhang J, Dong Y, Duan T, Zhou Y, Li W (2021) Phenolic compounds in water, suspended particulate matter, and sediment from Weihe River in north-west China. Water Sci Technol 83(8):2012–2024
Dar MA, Soliman FA, Mohamed SZ, Nasr RA, Hassan AR (2021) The occurrence of some carcinogenic metals in sediments and their effluences on some edible bivalves at Great Bitter and Timsah lakes, Egypt. Egypt J Aquat Biol Fish 25(4):119. https://doi.org/10.21608/ejabf.2021.186594
Donia NS (2011) Lake Temsah, Egypt’s water quality management, uses a geographical information system (GIS). Int J Environ Sci En 2:1–8
El Zokm GM, Ismail MM, El-Sayed AAEM, El-Shorbagi EK, Attia Sheradah M (2022) Mobility of carbohydrates, proteins, phenol and eutrophication assessment in a brackish Egyptian lake; predicted equations. Int J Environ Anal Chem 102(14):3316–3342
El-Azim A, Belal A, El-Salam A, Mourad F, Abo Elwafa S (2018) Water pollution by heavy metals in the western lagoon and its effect on Timsah Lake and Suez Canal. Catrina: Int J Environ Sci 17(1):71–76
El-Deeb FAA et al (2022) Ecological risk assessment and probability of human health risk of two phenolic compounds in different watercourses and Oreochromis niloticus. Egypt J Aquat Biol Fish 26(2):457–471
Ezz El-Din HM, Kelany MS, Hamed AM, El-Agroudy AN, Zaghloul YG (2023) Tracking of phenol bioremediation by two marine eco-friendly Aspergillus terreus MHG30 (ON649683) and A. terreus MHG60 (ON649704) strains at optimum conditions. Egypt J Aquat Biol Fish 27(4):579–595
Eletta OA, Tijani IO, Ighalo JO (2020) Adsorption of Pb (II) and phenol from wastewater using silver nitrate-modified activated carbon from groundnut (Arachis hypogaea L.) shells. West Indian J Eng 43(1):26–35
El-Naggar NA, Moawad MN, Ahmed EF (2022) Toxic phenolic compounds in the Egyptian coastal waters of Alexandria: spatial distribution, source identification, and ecological risk assessment. Water Sci 36(1):32–40
El-Sawy MA, Mohamedein LI, El-Moselhy KM (2023) Evaluation of arsenic, selenium, tin, and mercury in water and sediments of Bitter Lakes, Suez Canal, Egypt. Egypt J Aquat Res 49(2):137–143
El-Serehy HA, Abdallah HS, Al-Misned FA, Al-Farraj SA, Al-Rasheid KA (2018) Assessing water quality and classifying trophic status for scientifically based Management of the water resources of Lake Timsah, the lake with salinity stratification along the Suez Canal. Saudi J Biol Sci 25(7):1247–1256
EPA 420.1 Manual (1978) Phenolics (spectrophotometric, manual 4 AAP with distillation different watercourses and Oreochromis niloticus. Egyptian J Aquat Biol Fish 26(2):457-471
EPA U (2015) Update of human health ambient water quality criteria: penta-chlorophenol 87–86–5. OoW Office of Science and Technology, Editor
Etchegoyen MA, Ronco AE, Almada P, Abelando M, Marino DJ (2017) Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná basin. Environ Monit Assess 189:1–12
Feng T, Du H, Lin Z, Zuo J (2020) Spatial spillover effects of environmental regulations on air pollution: evidence from urban agglomerations in China. J Environ Manag 272:110998
Glukhova LB, Frank YA, Danilova EV, Avakyan MR, Banks D, Tuovinen OH, Karnachuk OV (2018) Isolation, characterization, and metal response of novel, acid-tolerant Penicillium spp. from extremely metal-rich waters at a mining site in Transbaikal (Siberia, Russia). Microb Ecol 76:911–924
Gontia-Mishra I, Tripathi N, Tiwari S (2014) A simple and rapid DNA extraction protocol for filamentous fungi efficient for molecular studies
Gziut T, Thomas SH (2022) International trends in systemic human exposures to 2, 4 dinitrophenol reported to poisons centers. Clin Toxicol 60:628–631
Hanafi MF, Sapawe N (2020) A review of the water problem associated with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater Today: Proc 31:A141–A150
Hassan HB, Kandil NM (2022) Estimation of water quality of El-Timsah Lake using artificial neural networks (ANN) model. Int J Basic Appl Sci 11(4):118–125
Hu X, Yang T, Liu C, Jin J, Gao B, Wang X, Zhan N (2020) Distribution of aromatic amines, phenols, chlorobenzenes, and naphthalenes in the surface sediment of the Dianchi Lake, China. Front Environ Sci Eng 14:1–13
IOC/UNEP (1991) Intergovernmental Oceanographic Commission/United Nations Environment program. Report of CEPPOL. Regional workshop on coastal water quality criteria and effluent guidelines for the Winder Caribbean. Caribbean Environment program Tech. Rep. No.8 UNEP, Jamaica, p 31–31
Khairy MA (2013) Assessment of priority phenolic compounds in sediments from an extremely polluted coastal wetland (Lake Maryut, Egypt). Environ Monit Assess 185:441–455. https://doi.org/10.1007/s10661-012-2566-4
Khairy WM, Ghany MBA (2021) Sustainable management of treated wastewater–case study: new El-Mahsama wastewater treatment plant in Sinai
Khalil DMA, Massoud MS, El-Zayat SA, El-Sayed MA (2021) Bioremoval capacity of phenol by some selected endophytic fungi isolated from Hibiscus sabdariffa and batch biodegradation of phenol in paper and pulp effluents. Iran J Microbiol 13(1):407
Kottb MR, El-Agroudy NA, Ali AE, Hamed MA, Ezz El-Din HM (2019) Biodegradation of some petroleum hydrocarbons by fungi isolated from Gulf of Suez. CATRINA 18(1):169–175
Li B, Liu R, Gao H, Tan R, Zeng P, Song Y (2016) Spatial distribution and ecological risk assessment of phthalic acid esters and phenols in surface sediment from urban rivers in Northeast China. Environ Pollut 219:409–415
Li R, Liang J, Gong Z, Zhang N, Duan H (2017) Occurrence, spatial distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Sci Total Environ 580:388–397
Liu L, Si L, Yang J, Peng L, Qiao S, Sun Y, Guo C (2023) Biodegradation and process optimization of phenol and formaldehyde by Aspergillus nomius SGFA1. Int Biodeterior Biodegradation 182(1):105630
Mainali K (2020) Phenolic compounds contaminants in water: a glance. Curr Trends Civ Struct Eng 4(4). https://doi.org/10.33552/CTCSE.2020.04.000593
Martin R (1949) Rapid colorimetric estimation of phenol. Anal Chem 21:1419–1420
Matloff N (2011) The art of R programming: a tour of statistical software design. No Starch Press
Matúš P, Littera P, Farkas B, Urík M (2023) Review on performance of Aspergillus and Penicillium species in biodegradation of organochlorine and organophosphorus pesticides. Microorganisms 11(1):1485
Mbata T (2008) Isolation of fungi in hypersaline Dead Sea water. Sudanese J Public Health 3:172
Mi L, Xie Z, Zhao Z, Zhong M, Mi W, Ebinghaus R, Tang J (2019) Occurrence and spatial distribution of phthalate esters in the Bohai and Yellow Seas sediments. Sci Total Environ 653:792–800
Mohamedein L, El-Sawy M, Ali Bek AbdelRhman M (2019) Sediment contaminants in northern egyptian coastal lakes. Handb Environ Chem. https://doi.org/10.1007/698_2018_281
Otitoju OB, Alfred MO, Ogunlaja OO, Olorunnisola CG, Olukanni OD, Ogunlaja A, Unuabonah EI (2023) Pollution and risk assessment of phenolic compounds in drinking water sources from South-Western Nigeria. Environ Sci Pollut Res 30(1):76798–76817. https://doi.org/10.1007/s11356-023-27622-w
Prabhu S, Molath A, Choksi H, Kumar S, Mehra R (2021) Classifications of polyphenols and their potential application in human health and diseases. Int J Physiol Nutr Phys Educ 6(1):293–301
Qadir A, Malik RN, Husain SZ (2008) Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab, Pakistan. Environ Monit Assess 140:43–59
Rahimi-Mohseni M, Raoof JB, Aghajanzadeh TA, Ojani R (2019) Rapid determination of phenolic compounds in water samples: development of a paper-based nanobiosensor modified with functionalized silica nanoparticles and potato tissue. Electroanalysis 31(12):2311–2318
Ramos RL, Moreira VR, Lebron YA, Santos AV, Santos LV, Amaral MC (2021) Phenolic compounds seasonal occurrence and risk assessment in surface and treated waters in Minas Gerais—Brazil. Environ Pollut 268:115782
Readman JW, Fillmann G, Tosoola I, Bartocci J, Villeneuve JP, Catinni C, Mee LD (2002) Petroleum and PAH contamination of the Black Sea. Mar Pollut Bull 44:48–62
Singh RK, Tripathi R, Ranjan A, Srivastava AK (2020) Fungi as potential candidates for bioremediation. In Abatement of environmental pollutants 1(1):177–191. https://doi.org/10.1016/B978-0-12-818095-2.00009-6
Singh M, Singh D, Rai PK, Suyal DC, Saurabh S, Soni R, Giri K, Yadav AN (2021) Fungi in remediation of hazardous wastes: current status and future outlook. Recent Trends in Mycological Research: Volume 2: Environmental and Industrial Perspective, pp 195–224. https://doi.org/10.1007/978-3-030-68260-6_8
Soliman NF, El Zokm GM, Okbah MA (2017) Evaluation of phosphorus bioavailability in El Mex Bay and Lake Mariut sediments. Int J Sedim Res 32(3):432–441
Soliman NF, Elkady EM, Younis AM (2020) Chemical fractions and ecological risk of metals in the Bitter Lakes, Egypt sediments. Egypt J Aquat Biol Fish 24(6):167–196
Souza HMDL, Barreto LR, Mota AJd, Oliveira LAd, Barroso HDS, Zanotto SP (2017) Tolerance to polycyclic aromatic hydrocarbons (PAHs) by filamentous fungi isolated from contaminated sediment in the Amazon region. 39(4):481–488. https://doi.org/10.4025/actascibiolsci.v39i4.34709
Sun X, Yan Z, Ke X (2022) Distributions, compositions, and ecological risk assessment of typical pollutants in surface sediment of Xihe River, China
USEPA (2012) Provisional peer-reviewed toxicity values for Di-n-octyl phthalate [CASRN 117–84–0]. Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development, US EPA, Cincinnati, OH 45268, EPA/690/R-12/023F
USEPA (2017) United States Environmental Protection Agency (USEPA). Integrated Risk Information System (IRIS). 16 June 2013. Available online: http://www.epa.gov/IRIS/. Accessed 22 Sept 2017
USEPA (2018) 2018 Edition of the drinking water standards and health advisories tables. Document# EPA 822-F-18–001; Office of Water, US Environmental Protection Agency Washington, DC. https://www.epa.gov/sites/production/files/201803/documents/dwtable2018.pdf
USEPA, IRIS (2002) Phenol. CASRN 108–95–2. Integrated risk information system (IRIS), chemical assessment summary. US EPA, National Center for Environmental Assessment
Wang J, Wei H, Zhou X, Li K, Wu W, Guo M (2019a) Occurrence and risk assessment of antibiotics in the Xi’an section of the Weihe River, northwestern China. Mar Pollut Bull 146:794–800
Wang L, Yang X, Zhang A, Bidegain G, Li R, Na G, Yuan X (2019b) Distribution patterns and ecological risk of endocrine-disrupting chemicals at Qingduizi Bay (China): a preliminary survey in a developing mariculture bay. Mar Pollut Bull 146:915–920
Wang J, Sui Q, Lyu S, Huang Y, Huang S, Wang B, Yu G (2020) Source apportionment of phenolic compounds based on simultaneous monitoring of surface water and emission sources: a case study in a specific region adjacent to Taihu Lake watershed. Sci Total Environ 722:137946
WHO (2017) Organization of drinking water parameter cooperation project. Support to the revision of Annex I Council Directive 98:83
World Health Organization (WHO) (2003) Chlorophenols in drinking water. background document for development of WHO guidelines for drinking-water quality. 2nd edn. vol. 2. WHO/WSH/03.04/47
Xu W, Hu Y, Wu M, Miao E, Zhou H, Zhang X, Zhan J (2021) Determination of phenolic compounds in estuary water and sediment by solid-phase isotope insulation coupled with liquid chromatography-high resolution mass spectrometry. Anal Methods 13(11):1404–1411
Yadav AN, Kour D, Kaur T, Devi R, Yadav A (2022) Endophytic fungal communities and their biotechnological implications for agro-environmental sustainability. Folia Microbiol 67:203–232
Yuan X, Yang X, Zhang A, Ma X, Gao H, Na G, Sun Y (2017) Distribution, potential sources, and ecological risks of two persistent organic pollutants in the intertidal sediment at the Shuangtaizi Estuary, Bohai Sea of China. Mar Pollut Bull 114(1):419–427
Zaghloul GY, Ghoniem DGH, Zaghloul AY, Hamed MA, El-Moselhy KM (2023) Impact of some trace elements on the quality of edible fish species and its human health risks from Timsah Lake, Egypt. Egypt J Aquat Biol Fish 27(5):225–248
Zhang ZM, Zhang HH, Zou YW, Yang GP (2018) Distribution and Ecotoxicological state of phthalate esters in the sea-surface microlayer, seawater, and sediment of the Bohai Sea and the Yellow Sea. Environ Pollut 240:235–247
Zhao C, Yang S, Liu J, Liu C, Hao F, Wang Z, Lim RP (2018) Linking fish tolerance to water quality criteria for assessing environmental flows: a practical method for streamflow regulation and pollution control. Water Res 141:96–108
Zhong W, Wang D, Wang Z (2018) Distribution and potential ecological risk of 50 phenolic compounds in three rivers in Tianjin, China. Environ Pollut 235:121–128
Zhou M, Zhang J, Sun C (2017) Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potentially polluted river basin in China. Int J Environ Res Public Health 14(10):1140
Ziyaadini M, Zahedi MM, Khosravinia S (2016) Ultrasound-assisted extraction combined with reverse phase-dispersive liquid-liquid microextraction as a new approach for preconcentration and spectrophotometric determination of total phenol in marine sediments of Chabahar Bay. Mar Pollut Bull 109(1):104–109
Author information
Authors and Affiliations
Contributions
Zaghloul, Y. Gh; lab work: data collection; calculation, writing, reviewing and editing; Mohamedein, I. L.: fieldwork, investigation; Kelany, S. M.: fieldwork, lab work, data collection, writing; statistical analysis and software; El Moselhy, M. Kh.: supervision, reviewing and editing; Ezz El-Din, M. H.: lab work; fieldwork; writing; reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaghloul, G.Y., Mohamedein, L.I., Kelany, M.S. et al. Impact of total phenolic compounds on ecological and health risks of water and sediments from Timsah Lake, Suez Canal, Egypt. Environ Sci Pollut Res 31, 45667–45682 (2024). https://doi.org/10.1007/s11356-024-34047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34047-6