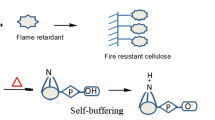
Abstract
A novel N–P ammonium salt 1,3-propylene glycol diphosphate ester (APGDPE) flame retardant was tersely synthesized under solvent-free condition to lower cotton fabric fire hazard. The APGDPE structure was characterized by 1H NMR, 13C NMR, 31P NMR and IR spectroscopy. The reactive P = O(NH4+)2 and PO(OH)2 groups of APGDPE were successfully grafted into cotton fabric to form P–O–C covalent bond. The limiting oxygen indexes (LOI) of treatment cotton with weight gain rate of 14.7%, 17.2% and 20.8% reach 40–44.5%. After 50 laundering cycles (LCs), the LOI value of treatment cotton with weight gain rate of 20.8% still maintains 27.3%. These results verified that the treated cotton has obtained outstanding flame retardancy and prominent durability. TG test indicates that the thermal stability and thermal oxidative stability of treated cotton are much higher than those of control cotton. TG-IR test displayed that treated cotton released less flammable volatile gases than those of control cotton. Cone calorimetry revealed that the PHRR and THR values of treated cotton decreased by 5.57% and 26.8%, respectively. XRD results suggest that the crystallization zone of cotton fabric before and after treatment hardly change. TG-IR and cone calorimetry tests verified the APGDPE condensation phase mechanism for treated cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural cotton fabrics contain many hydrophilic hydroxyl groups which have been widely applied in the textile industry(Xie et al. 2013), and possess the superior properties such as water-absorbing quality, breathability, acceptable physiological comfortableness and excellent mechanical properties which are used extensively in the manufacture of the soft and breathable textile e.g. underwear, hospital healthcare textiles, bedding and sleeping product, military and protective garments, etc. (Gao et al. 2015). However, cotton fibers have inflammable defect with 18% LOI (Abou-Okeil et al. 2013), which easily brings the life safety risk and the property losses once cotton fabrics catch fire. In order to figure out the key issue, many researchers have researched various flame retardants for cotton fabrics.
In the early research, the traditional halogen-containing flame retardant was widely applied for cotton fabrics, polyethylene terephthalate (PET) and nylon textiles etc. to obtain excellent flame retardancy. However, halogen-containing flame retardants can release cancerogenic dioxin, which is dangerous to human health and ecological environment (Sharma et al. 2018). Therefore, some halogen-containing flame retardants have been banned in many countries (Basak and Ali 2019). Phosphorus-containing and nitrogen-containing flame retardants have the advantages of low toxicity, high efficiency, and good durability, which have been proved to be an excellent substitute for halogen-based flame retardant (Ling and Guo 2020). Currently, N-P flame retardants which are widely used in the market mainly include "Proban" and " Pyrovatex CP "(Ali 2019). However, textiles treated with these flame retardants will release carcinogenic formaldehyde during finishing and use (Jia et al. 2017). Therefore, it is necessary to study the formaldehyde—free, halogen—free and durable flame retardant for cotton. In addition, in order to further enhance the fire resistance and durability of cotton fabrics, some effective strategies were implemented by coating (Zhang et al. 2019), grafting (Wang et al. 2020), layer-by-layer (LBL) self-assembly (Pan et al. 2015), plasma treatment (Lam et al. 2011) and sol–gel technology (Periyasamy et al. 2020) etc. which can efficiently enhance the cotton fabric fire resistance.
In recent years, along the global human desire for environmental friendliness, many researchers mainly focused on eco-friendly flame retardant by utilizing natural raw material (e.g. bio-based DNA coating(Luo et al. 2020), phytic acid/chitosan (Cheng et al. 2019), banana pseudostem sap (Kambli et al. 2018), casein (Xu et al. 2019), nacre (Xie et al. 2019), citric acid (Taherkhani and Hasanzadeh 2018), lignin (Shukla et al. 2019) and xylitol (Wang et al. 2019) etc.) as one of the components to synthesize no poisonousness flame retardant for combustible material (Paul et al. 2019). However, the dosages of natural resource materials are very limited. The excessive exploitation of natural resource will lead to environmental and ecological imbalance.
In this study, using the non-toxic chemical reagents 1, 3-propylene glycol and phosphoric acid as reactants, an efficient and environmentally friendly N–P synergistic flame retardant APGDPE was synthesized under solvent-free conditions. Compared with Proban and Pyrovatex CP, APGDPE is halogen-free and does not release formaldehyde. Moreover, the treatment cotton fabric with weight gain rate of 20.8% can still meet the flame retardant standard after 50 LCs. Therefore, APGDPE can also solve the durability problem of some biological macromolecules. APGDPE has the advantages of simple synthesis route, low raw material cost, environmental protection, high efficiency and it is easy to be applied in industry.
Experimental section
Materials
Plain cotton fabrics (100%, 133.27 g/m2) were purchased from the Chaotianmen Market in Chongqing, China. 1, 3-propylene glycol (98%) was bought from Aladdin Reagent Co., Ltd. (Shanghai, China). Phosphoric acid (85%) was purchased from Chongqing east Sichuan Chemical Co., Ltd. (Chongqing, China). Urea and dicyandiamide were supplied by Chengdu Kelong Chemical Reagent Co., Ltd. (Chengdu, China). All reagents were of analytical grade and were not further purified.
Synthesis of flame retardant APGDPE
1, 3-propylene glycol (0.100 mol, 7.765 g) and phosphoric acid (0.200 mol, 23.059 g) were added into a 250 mL beaker. The mixture was heated 130 °C for 3 h under magnetic stirring to obtain buff viscous liquid. The urea (0.200 mol, 12.012 g) was continuously added into the buff viscous liquid, and the mixture was further heated to 170 °C for 2.5 h under magnetic stirrer to obtain a crude product APGDPE, which was purified with absolute ethanol, then filtered and dried in oven at 60 °C to obtain white solid product. The yield is about 88%. The synthesis of APGDPE is shown in Scheme 1.
The structure of flame retardant APGDPE is characterized by NMR as follows:
1H NMR (D2O, 600 MHz) δ (ppm): 3.93 (t, 2CH2, H1), 1.87 (m, 1CH2, H2). 13C NMR (D2O, 600 MHz) δ (ppm): 67.67 (t, 2C, C1), 26.52 (t, 1C, C2). 31P NMR (D2O, 600 MHz) δ (ppm): −0.03.
Flame retardant finishing of cotton fabrics
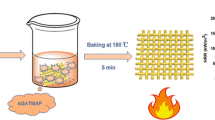
The 20%, 30% and 40% APGDPE solutions were prepared by dissolving APGDPE in distilled water. The dicyandiamide as catalyst was added to the APGDPE solution. Cotton fabrics were immersed in different concentrations of APGDPE solution with a bath ratio of 1:20 (the cotton weight versus the volume ratio of the solution) at 70 °C for 40 min. The cotton fabric was taken out and padded through a nip to obtain a wet pick up of 120%. The fabric was then cured by an automatic continuous backing machine at 180 °C for 5 min (Scheme 2). Finally, the treated fabric was rinsed with running water and dried in an oven at 60 °C for 1 h.
The weight gain (WG) of cotton fabrics is calculated by Eq. (1):
where, the W1 and W2 are corresponding to the masses of before and after cotton fabric grafting flame retardant, respectively.
Characterization
The LOI values of control cotton and reacted cotton were measured by M606B digital oxygen index apparatus (Qingdao Shanfang Instrument Co., Ltd. Shandong, China) according to the ASTM D2863-2000 standard.
The vertical flammability test of control cotton and treated cotton were tested on the YG815B vertical fabric flame retardancy tester (Nantong Sansi Electromechanical Science & Technology Co., Ltd. China) in accordance with the ASTM D6413-99 test standard. The sample (35 cm × 8 cm) was ignited by a 4 cm fire for 12 s.
The durability of treated cotton was tested by a soaping fastness tester at 49 °C according to the AATCC 61-2006 standard test (Roaches Co., England).
The nuclear magnetic resonance (1H NMR, 13C NMR and 31P NMR) spectra were measured on a Bruker AV III 600 MHz Spectrometer (USA) using D2O as a solvent or the TMS as the internal standard.
The chemical structures before and after cotton fabric treatment were measured by VERTEX80v Fourier transform infrared spectroscopy (FT-IR) (Germany Bruker) in a scanning range of 500–4000 cm−1 with a resolution of 4 cm−1. The samples were prepared using KBr pellets.
The thermal stability and thermal oxidative stability of control cotton and treated cotton were determined by a TG209F3 thermogravimetric (TG) (Germany Nets) in air and nitrogen atmospheres, respectively. The measurement range is 40–800 °C and the heating rate is 20 K/min.
The TG-IR was measured by TG209F3 thermogravimetric analyzer connected to the FTIR via a polytetrafluoroethylene pipe. The sample was heated at a rate of 20 K/min under nitrogen atmosphere in the temperature range of 40–800 °C.
Cone calorimetry was used to analyze the combustion behavior of square cotton samples (10 cm × 10 cm) in horizontal configuration in accordance with ASTM E 1354 standard under 35 kW/m2 of irradiative heat flux by using cone calorimetry (CCT, Mordis Combustion Technology Co., Ltd.). Record time to ignition (TTI, s), total heat release (THR, MJ/m2), heat release rate (HRR, kW/m2), peak of heat release rate (PHRR, kW/m2), total smoke release (TSR, m2/m2), the ratio of carbon dioxide to carbon monoxide (CO2/CO) and final residue (%).
The crystal structures of the control and treated cotton were measured by an XD-3 X-ray diffractometer (XRD) (Beijing Purkinje General Instrument Co., Ltd, Beijing, China) at a diffraction angle range of 5–60° at 36 kV and 20 mA.
The surface structures of control cotton and treated cotton were observed by a Hitachi S-4800 scanning electron microscope (SEM) (imaging beam voltage: 20 kV).
The tensile strengths of the samples were measured on an HD026N electronic fabric tension tester (Nantong Hongda Experiment Instruments Co., Ltd., China) in accordance with ASTM 5035–2006 standard.
The stiffness of the cotton fabric before and after treatment was measured on an LLY-01 automatic fabrics stiffness tester (Wenzhou Darong Textile Machinery Co., Ltd., Zhejiang, China) in accordance with ASTM D 1388–96 (2002) standard.
Air permeability of cotton fabric was determined using an YG (B) 461D-II air permeability tester in accordance with TS 391 EN ISO 9237 standard.
Results and discussion
FT-IR and XRD
The structures of C0 and FRC3 were characterized by FT-IR spectra in Fig. 1a. The absorption peaks at 3345 and 2900 cm−1 are considered as stretching vibrations of O–H and C-H bonds (from aliphatic hydrocarbon), respectively (Zhang and Wang 2013). Compared to control cotton, some new peaks appeared onto treated cotton. The absorption peak at 1697 cm−1 was assigned to the stretching vibration of C = O groups in which the O–H groups of cotton fabric were partially oxidized (Ortelli et al. 2019). The absorption peaks at 1234 and 838 cm−1 were severally attributed to stretching vibration of P = O and P-OH groups derived from APGDPE (Zhao et al. 2016). The absorption peak at 1004 cm−1 was assigned to the P-O-C bond of APGDPE reacting with the -OH group of cotton fabric (Liu et al. 2020). The results indicated that APGDPE is successfully grafted onto cotton fabric through P-O-C covalent bond to enhance the durability of treated cotton.
The crystal structures of the cotton fabric before and after treatment were analyzed by XRD. The crystal structures of control cotton and treated cotton were shown in Fig. 1b. The diffraction peaks of control cotton at 14.96°, 16.44°, 22.80° and 34.46° in accordance with crystal faces (1–10), (110), (200) and (004), respectively (Tian et al. 2019), which are typical characteristics of I-cellulose crystals. The crystal structure of treated cotton is much similar to control cotton. The diffraction peak intensity of treated cotton is slightly lower than that of control cotton. The results suggested that flame retardant APGDPE hardly effects on the crystal structure of treated cotton.
Surface morphology of cotton fabrics
The surface morphologies of C0 and FRC3 before and after combustion were observed by SEM in Fig. 2. The surface morphologies of control cotton (Fig. 2a and b) and treated cotton (Fig. 2c and d) are much similar, which is smooth and flat shape, fiber crimping and distinct fibrous structure. The surfaces of treated cotton are not obviously damaged and no obvious deposition. The results indicate that APGDPE flame retardant don't deposit onto the surface of cotton fiber, but it permeates into the interior of cotton fiber by grafting to generate P–O–C bond and no obvious damage for cotton surface. The surface of treated cotton after burning (Fig. 2e and f) substantially remains intact, and only some small bubbles appear on the fiber surface. The reason is that the APGDPE flame retardant contains phosphorus and nitrogen elements to trigger swell and arise many bubbles during combustion process. The small bubbles are attached onto the surface of the fiber. Furthermore, the EDS test indicated that the treated cotton contains 16.69% N, 1.99% P, 44.9% O and 36.42% C (Fig. 2g), and the control cotton retains only 52.63% C and 47.37% O (Fig. 2h). The results verify that the APGDPE flame retardant has been successfully grafted onto cotton fibers.
Flame retardancy and durability of treated cotton
The LOI values of cotton fabric treated with different weight gain rate are used to characterize the combustion properties of the cotton samples. The LOI value of control cotton is about 18.0%. Once the LOI value of the sample is higher than 26.0%, indicating the treated cotton fabrics possess flame retardancy. The higher the LOI value is, the better the flame retardancy is. The LOI value of FRC1 reaches 40.0%. After 30 LCs, the LOI value decreased to 26.6%, which could be used as semi-durable flame retardant cotton. The LOI value of FRC2 rises to 42.7%. After 30 LCs, the LOI value can hold 30.0%. After 50 LCs, the LOI value only was 25.5%, which can be used as the self-durable flame retardant cotton. The LOI value of FRC3 reaches 44.5%, and the LOI value was still 27.3% after 50 LCs. The results indicated that FRC3 could obtain durable flame retardant cotton. The higher the weight gain rate of cotton fabric is, the better the flame retardancy and durability of the fabric are.
According to Table 3, compared with other flame-retardant cotton, the APGDPE- treated cotton fabric has better flame retardancy and washability.
In order to further determine the flame retardancy of treated cotton, the vertical burning test was performed on control cotton and the treatment cotton with different weight gain. Figure 3 indicates that the results of vertical flame tests after the samples were ignited for 12 s. The relevant data are collected in Table 4. The control cotton burned violently during the ignition process. Even if the fire was removed, the control cotton still continued to burn. The after-flame time was 10 s, the fire gradually disappeared, and the after-glow continued for 5 s until the control cotton burned completely. No complete char structure was left and only turned into ashes. For treated cotton, after the fire source was removed, the fabric ceased to burning. No after-flame and after-glow were observed, and a complete and narrow carbon frame structure was left after the ignition. These results displayed that flame retardant APGDPE is very effective in preventing the spread of fire. The cotton fabrics with weight gain rate of 14.7%, 17.2% and 20.8% were fired for 12 s, the lengths of the char frames correspond to 74, 66 and 54 mm, respectively. After 50 LCs, the char length of FRC3 still leave over 95 mm after vertical burning test, and no after-flame and after-glow time once the fire source left. The experimental results further prove that the treated cotton already possess excellent flame retardancy and gratifying durability.
Cone calorimetry analysis
To further investigate the flame retardancy of cotton fabrics. The cone calorimetry test was used to simulate the combustion behavior of the sample under real fire conditions. The HRR and THR curves for control cotton and treated cotton were presented in Fig. 4, and the cone calorimetric data were listed in Table 5. TTI is an important parameter to characterize the flammability of materials. The TTI value of control cotton fabric is 9.0 s, while the treated cotton has not recorded the burning time, so APGDPE can effectively prevent the spread of fire. The HRR value is one of the effective parameters used to evaluate flame retardancy, and the lower HRR value represents better flame retardancy. From Fig. 4a, b and Table 5, the PHRR values of control cotton and treated cotton were 199.66 kW/m2 at 20 s and 11.13 kW/m2 at 24 s, respectively. The PHRR value of treated cotton significantly was lowered to 94.4% of control cotton. The decomposed mechanism of treated cotton is that at the initial stage, the treated cotton was promoted the degradation and carbonization to form char layer which prevents the heat transfer during the combustion. However, the treated cotton combustion releases non-flammable gases to dilute the combustible gases which reduce the intensity of the combustion reaction pyrolysis and the rate of heat release. Therefore, the THR value (0.78 MJ/m2) of treated cotton was much lower than 73.2% of control cotton (2.91 MJ/m2). Based on the HRR curve, the fire growth rate (FGR) can be used to assess the fire hazard of the material. The FGR can be calculated by Eq. 2:
In general, the lower the FGR value is, the more delayer of fire flashover time is, which allows sufficient time to evacuate and extinguish the fire. The FGR values of control cotton and treated cotton were 9.93 and 0.46 kW/ (m2/s), respectively, in which the treated cotton was reduced to 95.4% by contrast. The results indicated that the FGR value of treated cotton is greatly reduced, which can increase the escape time in actual accidents. CO and CO2 are the main components of fire gases. The lower CO2/CO ratio means the more CO converted to CO2, the combustion efficiency is much low. First, CO is produced during the pyrolysis of the fabric; then, when there is sufficient oxygen, the CO is oxidized to CO2 which releases into air. The CO2/CO ratio of treated cotton (36.54) was significantly lower of control cotton (75.21). Moreover, the SPR value of treated cotton is higher than that of control cotton, probably because the treated cotton fabric releases more H2O and other gases during the combustion process. And 34.67% residual amount of treated cotton was obtained, which was significantly higher than control cotton (2.22%). Besides, the APGDPE flame retardant can produce phosphoric acid or polyphosphoric acid during the combustion process to promote the formation of char, thereby increasing the residual amount of treated cotton after combustion. Generally, the char layer can reduce heat/mass transfer between the gas phase and the condensed phase, which limits the combustion of the underlying substrate. Therefore, the amount of flammable gases increase, and the amount of heat release is reduced, thereby lowering the PHRR value and the THR value.
TG analysis
Thermogravimetric analysis (TG) and derivative thermogravimetric analysis (DTG) are commonly used to test the thermal stability and thermal oxidative stability of samples under nitrogen and air atmospheres. The thermal and thermal oxidative degradation results of TG and DTG of control cotton, and 40% APGDPE treated cotton fabrics are shown in Fig. 5. The relevant characteristic data, such as the temperature at which the sample 5% mass loss (T5%), the temperature at which the sample mass loss 10% (T10%), the maximum degradation rate temperature (Tmax), mass loss rate (Rmax) at Tmax, and the residue percentage at 800 °C (%) are summarized in Table 6 The thermal stability of the sample was analyzed under a nitrogen atmosphere. In Fig. 5a and c, control cotton began to decompose at 307.3 °C (T5%, the mass at 95% was defined as the initial degradation temperature of the sample) and showed the maximum weight loss rate at 377.5 °C (Tmax). Because the glycosylation depolymerization of cellulose and the formation of coke, the weight loss during cellulose pyrolysis mainly occurs at 310–400 °C. As the temperature increases, the mass loss rate (MLR) of control cotton is significantly reduced, and the char residue is slowly decreased. The results indicate that the fragmented char formed at a lower temperature is further degraded to form a more stable residual char at a higher temperature. For the treated cotton, the initial degradation temperature (T5%) was 231.4 °C, which was significantly lower than that of control cotton. The lower thermal decomposition temperature can be attributed to the water absorption of flame retardant APGDPE, which releases the adsorbed water during the initial degradation. Besides, the phosphorus-containing group of APGDPE may produce phosphoric acid or polyphosphoric acid during thermal degradation in which can catalyze the thermal degradation of cellulose, resulting in a decrease in T5% of treated cotton. The temperature (T10%) at cotton mass loss was 10% (249.2 °C), which was lower than the T10% (327.2 °C) of control cotton. The Tmax and Rmax values of the treated cotton were 275.4 °C and 1.57%/oC, respectively, which were significantly lower than control cotton (Tmax: 377.5 °C, Rmax: 1.86%/oC). The residual amount of treated cotton (36.50%) at 800 °C was significantly increased compared to control cotton (3.89%). APGDPE produces phosphoric acid and polyphosphoric acid during thermal degradation to catalyze the thermal degradation of cotton fabrics, resulting in more char residue, which can protect the cotton fabric from heat/mass transfer, thus reducing the pyrolysis temperature of treated cotton. The appearance of more char residues prevented further combustion of the cotton fabric,hindered the rate of formation of volatiles and the ease of diffusion into the flame zone. These results indicated that APGDPE effectively improved the flame retardancy of cotton fabrics and increased thermal stability of cotton fabrics.
The TG analysis investigated the thermal oxidative stability of the samples in air. The results of the TG and DTG of the sample are shown in Fig. 5b and d. The relevant data are summarized in Table 6. The control cotton began to dehydrate at 236.3 °C (T5%) and the main decomposition stage was in the temperature range of 200–400 °C, due to the dehydration of cellulose to form aliphatic char and volatile products. Tmax at 352.3 °C, and the residual amount at Tmax temperature was 48.51%. As the temperature increases, the mass loss of control cotton gradually increases, and there is almost no residue at 800 °C because the formed aliphatic compound is converted into aromatic compounds and decomposition into CO2/CO was released. After APGDPE treatment, the thermal oxidative stability and catalytic char forming properties of the cotton fabric were greatly improved. The T5% (226.8 °C), T10% (246.5 °C), Tmax (272.3 °C) and Rmax (1.49%/oC) of the treated cotton were lower than those of control cotton, but at 800 °C, the char residue of the treated cotton was 2.59%, while the control cotton has almost no residual amount. The significant difference in decomposition temperature and char residue between the control cotton and the APGDPE treated cotton may be attributed to the phosphoric acid or polyphosphoric acid produced by APGDPE during the decomposition process to catalyze the dehydration of the cellulose. The results indicate that the APGDPE effectively increases the thermal oxidative stability of treated cotton fabrics.
TG-IR analysis
The gaseous volatile products of the samples during thermal degradation were analyzed by TG-IR. The TG-IR spectra of the pyrolysis products of treated cotton and control cotton at different temperatures are shown in Fig. 6a, b and c, respectively.
Figure 6a and b reveal that control cotton and treated cotton have different degradation rates at different temperatures. The maximum degradation rates of control cotton and treated cotton are at about 400 and 280 °C, respectively. The maximum degradation rate of control cotton was significantly higher than that of treated cotton which was consistent with the TG results. The volatile gases from control cotton and treated cotton at the maximum degradation rate were compared. The results indicated that the APGDPE grafting on the cotton fabric did not significantly change the characteristic absorption peaks of control cotton. The treated cotton fabric has almost no new volatile gas produced during the pyrolysis process. The TG-IR spectrum of control cotton was used to analyze the gaseous pyrolysis products. The absorption peak at 3562 cm−1 was attributed to the stretching vibration of -OH gaseous water (Ghanadpour et al. 2015). The absorption peaks at 2817 and 2356 cm−1 were attributed to the stretching vibration of the aliphatic C-H bond and CO2, respectively (Wang et al. 2018). The absorption peaks at 1746 cm−1 and 1077 cm−1 were attributed to the stretching vibration of C = O bond and C–O–C bond of ether (Chen et al. 2017). The peak values of the volatile gases released from control cotton at the maximum degradation rate is significantly higher than that of treated cotton. The results verified that treated cotton can release less volatile gases at the temperature of the maximum degradation rate.
To further investigate the flame retardant mechanism of cotton fabric treated with APGDPE, the maximum absorption strengths of flammable and non-flammable gases produced from control cotton and treated cotton at a maximum degradation rate temperature are shown in Fig. 7. The volatile gases containing non-flammable gases H2O and CO2 were displayed in Fig. 7a and b, respectively. The flammable gases such as aliphatic hydrocarbons in Fig. 7c, carbonyl in Fig. 7d and ether in Fig. 7e were produced during pyrolysis of the samples. The strengths of the volatile gases produced by treated cotton are much lower than those of control cotton. Furthermore, the absorption strengths of the flammable gases are much lower than those of control cotton. The results indicated that the treated cotton releases less flammable gases, resulting in a reduction in the "fuel" of the flame, thereby reducing the HRR and THR values. The phosphorus-containing APGDPE free radicals (P• and PO•) was generated in the gas phase which can capture the OH• and H• free generated during the pyrolysis process, thereby reducing the release of flammable volatile gases. In the condensed phase, the phosphorus-containing APGDPE decomposes to produce phosphoric acid or polyphosphoric acid, thereby promoting the formation of char, and the char residues can prevent the heat/mass transfer of cotton fabric into the burning zone, thereby increasing the flame retardancy of the cotton fabric. The flame retardant mechanism of treated cotton can be considered as both of gas phase and condensed phase flame retardant mechanism.
Mechanical properties analysis
The mechanical properties of cotton fabric before and after treatment were characterized. The data of tensile strengths, bending lengths and air permeability were listed in Table 7. It can be seen from Table 6 that the air permeability of the control cotton is 402.2 mm/s, while the air permeability of the cotton fabric treated by 40% APGDPE is 377.8 mm/s, which is only reduced by 6.46%, indicating that the air permeability of the cotton fabric treated by APGDPE is well maintained.
The tensile strength of the fabric is divided into warp and weft directions, and the tensile strength of the warp is significantly higher than that of the weft direction. The tensile strength of cotton fabric treated with APGDPE is reduced. The higher the treatment concentration is, the more severe the fabric tensile strength is. The tensile strengths of control cotton in warp and weft directions were 718 and 512 N, respectively. The tensile strengths in warp direction of C0, FRC1, FRC2 and FRC3 were 590, 510 and 480 N, respectively, and the tensile strengths in weft directions were 300, 289 and 234 N, respectively.
The stiffness of cotton fabric can be expressed in terms of the bending length, and the greater the value of the bending length of treated cotton fabric is, the worse the softness is. The bending length of the fabric is divided into warp and weft directions, and the warp bending length is significantly higher than the weft direction. As the weight gain rate of cotton fabric increases, the bending length increases. The bending lengths of control cotton in the warp and weft directions were 18.1 and 15.4 mm, respectively. The warp bending lengths of C0, FRC1, FRC2 and FRC3 were 21.5, 22.6 and 23.7 mm, respectively. The bending lengths in the weft direction were 17.2, 18.3 and 19.3 mm, respectively. After treatment, the bending length of the cotton fabric is increased to some extent, but it does not affect the hand feeling and softness of the cotton fabric. After 50 LCs, the breaking strength and bending length of treated cotton were restored. The flame retardant coating onto cotton fabrics can be reduced after soaping; therefore, the mechanical properties of the treated cotton are restored to some extent. These results suggest that flame retardant APGDPE has slight effect on the mechanical properties of control cotton, which hardly influences the practical application of treated cotton.
Conclusion
A phosphate ester ammonium salt (APGDPE) with reactive N-P groups was synthesized by solvent-free method. The synthesis process was simple and eco-friendly. The APGDPE treated cotton fabrics can achieve efficient flame retardancy and outstanding durability. The treatment cotton fabric with weight gain rate of 20.8% is used as durable flame retardant cotton. APGDPE flame retardant scarcely effects on the crystal structure of cotton fabrics. Cone calorimetry result displays that the PHRR and THR values of treated cotton are lower than control cotton. TG test manifests that the T5%, T10% and Tmax of treated cottons were much lower than those of control cotton. Comparing to control cotton, the better thermal stability and thermal oxidation stability of treated cotton have been achieved. The TG-IR results indicate that flame retardant cotton releases less flammable volatile gases, the APGDPE flame retardant possess excellent flame retardancy and sustainable durability for treated cotton. The mechanical properties of control cotton and treated cotton were almost unchanged.
References
Abou-Okeil A, El-Sawy SM, Abdel-Mohdy FA (2013) Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr Polym 92:2293–2298. https://doi.org/10.1016/j.carbpol.2012.12.008
Ali SBSW (2019) Wastage pomegranate rind extracts ( PRE ): a one step green solution for bioactive and naturally dyed cotton substrate with special emphasis on its fire protection efficacy. Cellulose 26:3601–3623. https://doi.org/10.1007/s10570-019-02327-x
Basak S, Ali SW (2019) Sodium tri-polyphosphate in combination with pomegranate rind extracts as a novel fire-retardant composition for cellulosic polymer. J Therm Anal Calorim 137:1233–1247. https://doi.org/10.1007/s10973-019-08034-w
Chen X, Wang W, Jiao C (2017) A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J Hazard Mater 331:257–264. https://doi.org/10.1016/j.jhazmat.2017.02.011
Cheng XW, Guan JP, Yang XH et al (2019) A bio-resourced phytic acid/chitosan polyelectrolyte complex for the flame retardant treatment of wool fabric. J Clean Prod 223:342–349. https://doi.org/10.1016/j.jclepro.2019.03.157
Gao WW, Zhang GX, Zhang FX (2015) Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose 22:2787–2796. https://doi.org/10.1007/s10570-015-0641-z
Ghanadpour M, Carosio F, Larsson PT, Wågberg L (2015) Phosphorylated cellulose nanofibrils: a renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromol 16:3399–3410. https://doi.org/10.1021/acs.biomac.5b01117
Jia Y, Lu Y, Zhang G et al (2017) Facile synthesis of an eco-friendly nitrogen-phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J Mater Chem A 5:9970–9981. https://doi.org/10.1039/c7ta01106g
Kambli ND, Samanta KK, Basak S et al (2018) Characterization of the corn husk fibre and improvement in its thermal stability by banana pseudostem sap. Cellulose 25:5241–5257. https://doi.org/10.1007/s10570-018-1931-z
Lam YL, Kan CW, Yuen CWM, Au CH (2011) Fabric objective measurement of the plasma-treated cotton fabric subjected to wrinkle-resistant finishing with BTCA and TiO2 system. Fibers Polym 12:626–634. https://doi.org/10.1007/s12221-011-0626-y
Li QL, Huang FQ, Wei YJ et al (2018) A phosphorus-nitrogen flame-retardant: synthesis and application in cotton fabrics. Medziagotyra 24:448–452. https://doi.org/10.5755/j01.ms.24.4.18606
Ling C, Guo L (2020) A novel, eco-friendly and durable flame-retardant cotton- based hyperbranched polyester derivative. Cellulose 27:2357–2368. https://doi.org/10.1007/s10570-019-02923-x
Liu S, Wan C, Chen Y et al (2020) A novel high-molecular-weight flame retardant for cotton fabrics. Cellulose 27:3501–3515. https://doi.org/10.1007/s10570-020-03020-0
Luo Q, Gao P, Zhou J et al (2020) Imparting flame resistance to citric acid–modified cotton fabrics using DNA. J Eng Fiber Fabr. https://doi.org/10.1177/1558925020922217
Ortelli S, Malucelli G, Blosi M et al (2019) NanoTiO 2 @DNA complex: a novel eco, durable, fire retardant design strategy for cotton textiles. J Coll Interf Sci 546:174–183. https://doi.org/10.1016/j.jcis.2019.03.055
Pan Y, Pan H, Yuan B et al (2015) Construction of organic-inorganic hybrid nano-coatings containing α-zirconium phosphate with high efficiency for reducing fire hazards of flexible polyurethane foam. Mater Chem Phys 163:107–115. https://doi.org/10.1016/j.matchemphys.2015.07.020
Paul S, Basak S, Ali W (2019) Zinc stannate nanostructure: Is It a new class of material for multifunctional cotton textiles? ACS Omega J. https://doi.org/10.1021/acsomega.9b02719
Periyasamy AP, Venkataraman M, Kremenakova D et al (2020) Progress in sol-gel technology for the coatings of fabrics. Materials (basel). https://doi.org/10.3390/MA13081838
Sharma V, Basak S, Rishabh K et al (2018) Synthesis of zinc carbonate nanoneedles, a potential flame retardant for cotton textiles. Cellulose 25:6191–6205. https://doi.org/10.1007/s10570-018-1962-5
Shukla A, Sharma V, Basak S, Ali SW (2019) Sodium lignin sulfonate: a bio-macromolecule for making fire retardant cotton fabric. Cellulose 26:8191–8208. https://doi.org/10.1007/s10570-019-02668-7
Taherkhani A, Hasanzadeh M (2018) Durable flame retardant finishing of cotton fabrics with poly(amidoamine) dendrimer using citric acid. Mater Chem Phys 219:425–432. https://doi.org/10.1016/j.matchemphys.2018.08.058
Tian P, Lu Y, Wang D et al (2019) Solvent-free synthesis of silicon–nitrogen–phosphorus flame retardant for cotton fabrics. Cellulose 26:6995–7007. https://doi.org/10.1007/s10570-019-02554-2
Wang D, Zhong L, Zhang C et al (2019) Eco-friendly synthesis of a highly efficient phosphorus flame retardant based on xylitol and application on cotton fabric. Cellulose 26:2123–2138. https://doi.org/10.1007/s10570-018-2193-5
Wang S, Du Z, Cheng X et al (2018) Synthesis of a phosphorus- and nitrogen-containing flame retardant and evaluation of its application in waterborne polyurethane. J Appl Polym Sci 135:1–10. https://doi.org/10.1002/app.46093
Wang X, Yu P, Zhang K et al (2020) Robust and durable polymer grafted cotton fabrics for sequential oil/water separation and heavy metal ions removal based on surface initiated ATRP. Polymer (guildf) 210:123002. https://doi.org/10.1016/j.polymer.2020.123002
Xie H, Lai X, Wang Y et al (2019) A green approach to fabricating nacre-inspired nanocoating for super-efficiently fire-safe polymers via one-step self-assembly. J Hazard Mater 365:125–136. https://doi.org/10.1016/j.jhazmat.2018.10.099
Xie K, Gao A, Zhang Y (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr Polym 98:706–710. https://doi.org/10.1016/j.carbpol.2013.06.014
Xu F, Zhong L, Xu Y et al (2019) Synthesis of three novel amino acids-based flame retardants with multiple reactive groups for cotton fabrics. Cellulose 26:7537–7552. https://doi.org/10.1007/s10570-019-02599-3
Yang Z, Wang X, Lei D et al (2012) A durable flame retardant for cellulosic fabrics. Polym Degrad Stab 97:2467–2472. https://doi.org/10.1016/j.polymdegradstab.2012.05.023
Yasemin A, Doğan M, Bayramlı E (2009) The effect of red phosphorus on the fire properties of intumescent pine wood flour – LDPE composites Yasemin. Finnish-Swedish Flame Days 2009:4B. https://doi.org/10.1002/fam
Zhang M, Wang C (2013) Fabrication of cotton fabric with superhydrophobicity and flame retardancy. Carbohydr Polym 96:396–402. https://doi.org/10.1016/j.carbpol.2013.04.025
Zhang Y, Tian W, Liu L et al (2019) Eco-friendly flame retardant and electromagnetic interference shielding cotton fabrics with multi-layered coatings. Chem Eng J 372:1077–1090. https://doi.org/10.1016/j.cej.2019.05.012
Zhao P, Liu S, Xiong K et al (2016) Highly flame retardancy of cotton fabrics with a novel phosphorus/nitrogen/silicon flame-retardant treating system. Fibers Polym 17:569–575. https://doi.org/10.1007/s12221-016-5316-3
Acknowledgments
The research was supported by Chongqing postgraduate education and teaching reform major project (No: yjg152022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
We declare that there is no financial or personal relationship between us and other people or organizations that may have an inappropriate impact on our work. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, F., Liao, Y., Chen, Y. et al. One-step green synthesis of eco-friendly novel N–P synergistic flame retardant for cotton fabric. Cellulose 28, 8205–8219 (2021). https://doi.org/10.1007/s10570-021-04049-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04049-5