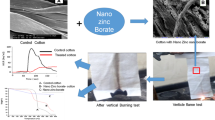
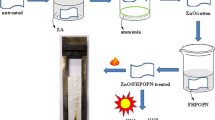
Abstract
For the first time, facile synthesized ZnCO3 nanoneedles have been integrated into cotton fabric at around neutral pH as well as alkaline treatment condition (using NaOH) by following padding method to obtain fire retardant efficacy of the fabric. Fire retardant properties of the treated fabrics were studied by measuring limiting oxygen index (LOI) and vertical burning test. It was found that the fabric treated with nano ZnCO3 in alkaline condition showed LOI value of 30 and a specific char length of 40 mm with more than 40% char mass retention at 600 °C. Degradation behavior of the control and the treated fabrics has also been revealed in detail by using Thermo-gravimetric and Differential Scanning Calorimetry analyzer. Char morphology and the interaction of the nanoneedles with the treated fabric also have been demonstrated by using SEM and FTIR analysis, respectively.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flame retardant textiles are in great demand for not only home textiles, but apparel and technical sectors as well. Phosphorous-nitrogen based chemicals and halogen based synthetic chemicals are most commonly available in the market as flame retardant agents (Horrocks 2011; Alongi et al. 2013c; Basak and Ali 2016). However, due to the environmental issues, (for releasing of dioxins and furanes) halogen based chemicals are strictly banned in the market (Horrocks 2011). From last one decade textile industries are mainly using pyrovatex (N-methylol dimethyl phosphonium chloride) with nitrogen based melamine for delivering durable fire retardant and toxic smoke suppressant cotton fabric (Basak et al. 2015; Coquelle et al. 2015). However, the above treatment process is not eco-friendly and the larger quantity of costly synthetic chemicals is being consumed. Moreover, acidic treatment condition and more add-on% used for the treatment hamper the hand value and the physical properties like tensile strength of the treated fabric (Shukla et al. 2016). Hence, there is increasing demand for inexpensive, eco-friendly fire retardant agents which after application to the fabric, would maintain its physical properties. In this direction, recently from last 5 years, researchers have invented various plant and protein based bio-macromolecules (banana pseudostem sap, coconut shell extract, pomegranate rind extract, DNA, casein, hydrophobin, whey protein, etc.) for making sustainable fire retardant textiles (Alongi et al. 2013a, b, 2014; Basak and Ali 2017; Teli et al. 2018). To this end, continuous search of the natural fire retardant sources is still going on all over the globe. Apart from the usage of the waste bio-macromolecules, plasma polymerization (by using different polymerizing gases), UV polymerization based treatment of the cotton textiles have also been emerged as different promising areas in the field of fire retardancy (Gupta and Basak 2010). Both of the aforementioned sustainable processes have positive aspect of maintaining the physical property of the fabric after finish. However, both the technologies are not very easy to apply uniformly on the fabric surface. In addition, durability of the aforementioned treatments after finishing are also not satisfactory (Teli and Pandit 2017; Basak et al. 2016; Basak and Ali 2018; Arputharaj et al. 2017). Therefore, from last the 5 years, a very few research groups in the world are involved to make the textiles fire retardant by using nano based formulations to obtain fire retardant efficacy with less add-on%. In this direction, researchers have used graphene, nano titanium dioxide, zinc oxide, silica, etc. for making fire retardant textile materials (Seshama et al. 2017; Bitnec et al. 2008; Shamsipur et al. 2013; Arputharaj et al. 2017; Samanta et al. 2017). Mostashari et al. (2005) have used zinc carbonate hydroxide for the flame retardant treatment of cotton and as per their report, in all the cases the add-on% is higher (more than 25%) and no char length has been observed. Pan and Wang (2015) has used nano zinc carbonate (particle) with the combination of antimony oxide for thermal stability of the synthetic polymer like polyvinyl chloride (PVC). Wang et al. (2012) has reported the synthesis mechanism of ZnO nano structures in a very systematic way. However, no application or engineering process has been reported in the concern article. Therefore, a continuous research is going on in this mostly unexplored direction to satisfy sustainability in fire retardancy as well as to fulfill the requirement of the end users of the textile products.
In this article the potential of nano dimensional zinc carbonate needles on the flame retardant effect of cotton fabric has been explored very systematically. In addition of the thermal degradation property of the nano zinc carbonate, the article deeply elucidated with the application of the nano zinc carbonate on the cotton fabric with detailed analysis of the thermal stability and the flammability parameters of the underlying material. Further, it also established the mechanism behind the fire retardancy action of nano zinc carbonate on the cellulosic material.
Materials and methods
Textile cotton fabric and chemicals
The chemical reagents used for the experiments are Zinc Sulphate (ZnSO4), Sodium Carbonate (Na2CO3), Ammonia (NH3) and Sodium Hydroxide (NaOH) which were laboratory grade chemicals and are extremely pure. These chemicals are directly used without further purification. 100% cotton fabric of density of 118 g/m2 has been used for the entire study.
Synthesis of ZnCO3 nanoneedles
Preparation of zinc carbonate (ZnCO3) nanoparticles was done by using direct precipitation reaction between aqueous solutions of zinc sulphate (ZnSO4) and sodium carbonate (Na2CO3). The reagents were accurately weighed and transferred into 100 ml beakers for making the stock solution of zinc sulphate (ZnSO4) and sodium carbonate (Na2CO3), followed by dissolving in 100 ml deionized water in order to produce homogenous solutions of 0.01 M zinc sulphate and 0.01 M Na2CO3. 100 ml of 0.01 M solution was added to 0.01 M ZnSO4 solution drop wise for around 2 h. The precipitate so-formed was filtered and washed twice with deionized water and transferred the precipitate into crucible followed by drying at 90 °C for 2 h in hot air oven.Reaction occurred can be represented as:
Characterization of synthesized nano dimensional ZnCO3
X-Ray diffraction patterns of synthesized nano zinc carbonate have been obtained by using Analytical Xpert Pro. The data was collected in the 2θ range from 10° to 80°, with a step size of 0.02° and a scan step size of 2. Anode material was CuKα1 with λ = 0.154 nm. Nanoparticles were also characterized by using Fourier Transform Infrared (FTIR) spectroscopy. Concerning the FTIR, The sample (nano zinc carbonate) was palletized with Potassium Bromide (KBr) and studied in the wavelength range of 500–4500 cm−1. Thermo-gravimetric analysis (TGA) was performed at a heating rate of 20 °C/min and within a temperature ranging from 50 to 900 °C under air atmosphere by using Thermo Gravimetric Analyzer (METTLER TOLEDO TG-50/MT5). Surface morphology and the size of the as-synthesized nano dimensional zinc carbonate were observed under Scanning Electron Microscope (SEM).
Treatment of cotton fabric with nano dimensional needles
Three different formulations (A, B, C) were prepared for application on to the cotton fabric. Solution A contained nano ZnCO3 (as it is, at neutral pH), solution B and C contain mixture of (nano ZnCO3 + NaOH) [by adjusting pH-10] and only NaOH, respectively. Three pieces of cotton fabric were immersed in the respective solutions at 80 °C for 1 h. Thereafter the treated fabrics were passed through padding mangle by maintaining the running speed of 15 m/min with a mangle pressure of 15 kgf/cm2. The padded samples were air-dried and then cured for 3 min at 140 °C.
Calculation of the add-on% of the treated fabric
Before testing, all the samples were conditioned in a standard atmosphere of 65% RH for 24 h, so that they are identically acclimatized. The percent add-on after the treatment (i.e., the increase in the sample weight relative to the original weight) was determined by the gravimetric method as follows:
where M1 and M2 are the oven dried weights of the control and the treated samples, respectively.
Sample prepared for the flammability test
The burning behavior of the control and the treated samples were evaluated as per the standard methods. For determination of Limiting Oxygen Index (LOI), an ignition time of 30 s was maintained as per the Indian standard IS 13501 [law resource.org1992]. In vertical flammability tests, the different parameters were measured as per the IS 1871 method A [law resource.org1986]. As per this method, the fabric sample (250 mm * 40 mm) was ignited with a flame of 38 mm height for 12 s.
Characterization of the treated cotton fabrics
Three different cotton fabrics treated with only nano ZnCO3 (as it is), nano ZnCO3 plus NaOH and with only NaOH were characterized by Thermo-gravimetric (TGA) and Differential Scanning Calorimetry (DSC) analyzer in both the air and nitrogen atmosphere. Thermo-oxidative degradation of the control and the treated cellulosic materials were drawn on a Thermo Gravimetric Analyser (METTLER TOLEDO TG-50/MT5) in air atmosphere at 2 ml/min flow rate and with a heating rate of 20 °C/min.
The FTIR-ATR analysis of the samples was carried out in Thermofisher Scientific (Model: Nicolet is50 FTIR) made FTIR analyzer over the wavelength of 500–4500 cm−1. Here an ATR transmittance mode was used with DLaTGS detector for 49 scans and 4 resolutions.
Changes in the surface morphology of the materials were studied with the help of a high resolution (up to 3 nm) Scanning Electron Microscope (SEM, ZEISS EVO 50) using SE detector. The samples were coated with a thin layer (100 angstroms) of conducting material (Gold/palladium) by using a sputter coater and examined under SEM with an accelerating voltage of 20 kV. The EDX analysis of the samples was carried out in a TM3000 tabletop microscope (Made by: HITACHI, Swift ED3000) to determine the different elements present on the surface, and was expressed in weight percent. Transmission Electron Microscope (TEM) [Made by: JEOL, JEM-1400 electron microscope) analysis of the nanoneedles has been performed by using a voltage of 120 kV.
Tensile strength of the fabric
Tensile strength of the control and the treated fabrics were evaluated by following the ASTM D5034, grab test method using tensile testing machine (Tinius Olsen, Model: H5KS). As per this process, all the samples were tested at a speed of 300 mm/min with the sample dimension of 200 mm * 50 mm.
Results and discussion
Synthesis of nano dimensional zinc carbonate (ZnCO3)
Zinc Carbonate (ZnCO3) nanoneedles have been successfully synthesized by direct precipitation reaction of aqueous solutions of zinc sulphate (ZnSO4) and sodium carbonate (Na2CO3). Another important part of this method is that along with ZnCO3, certain hydrated salts like Zn5(CO3)2(OH)6, Zn3CO3(OH)4.2H2O, etc. were also formed. These hydrated salts of nano zinc carbonate were also useful in the fire retardant treatment of the cotton fabric. Before application, precipitate was filtered and dried in hot air oven at 90 °C for 2 h.
Characterization of nano zinc carbonate (ZnCO3)
XRD analysis of nanoneedles
XRD curve of the synthesized nano dimensional ZnCO3 has been represented in Fig. 1. The observed XRD pattern, elucidated in the Fig. 1, matches with the ICDD-PDF 00-019-1458 and the formation of the Zinc carbonate hydroxide (Zn5(CO3)2(OH)6 has been confirmed (Pourmortazavi et al. 2015; Hingorani et al. 1993; Zhang et al. 2004). For further confirmation, FTIR analysis was also performed and the concern curve has been reflected in Fig. 2. FTIR spectroscopy was utilized to confirm the formation of ZnCO3. The absorption peaks observed between 700 and 1100 cm−1 in the spectrum are devoted to the lattice vibration of carbonate ion in bending mode. Peaks at 1383 and 1511 cm−1 are corresponding to the stretching mode of –CO32−peaks in ZnCO3 and thus confirmed the formation of zinc carbonate. The absorption peak also has been observed at 3396 cm−1 is due to the presence of hydroxyl group in the sample, corresponds to the stretching vibration of the hydrogen bonds and hence confirms the formation of the zinc carbonate in the basic form i.e., (Zn5(CO3)2(OH)6.
Surface morphology of nanoneedles
The surface image of the as-synthesized ZnCO3 was captured by Scanning Electron Microscope (SEM) and is represented in Fig. 1. The SEM analysis shows that the nanoneedles are having a homogenous size, having nano needle shape with a homogenous morphology distribution. However, some larger aggregates are also found in the images which are usual at the nano-scale range might be due to the inter-needle interactions.
Application of synthesized nano zinc carbonate on cotton fabric
Flammability analysis of the treated fabric
The synthesized zinc carbonate nanoneedles were applied on the cotton fabric in as it is (neutral pH) condition and at the alkaline pH (pH-10) condition. After application, the treated fabric was dried and conditioned by following the standard method. Thereafter, the treated fabric was tested for the fire retardant properties. It was found that like control cotton fabric (A), nano zinc carbonate (as it is at neutral pH) treated cotton fabric (B) was also burnt within 1 min with a flame. However, after burning of the control fabric afterglow was present but there was no evidence of the afterglow for the nano zinc carbonate treated cotton fabric. Nanoneedles were also applied on cotton fabric from alkaline pH (pH-10) condition and the treated fabric (D) showed more thermal stability compared to the nano zinc carbonate (as it is at neutral pH) treated cotton fabric as evidenced from high LOI value (30) and the specific char length (4 cm) after the vertical flammability test. Only alkali (pH 10) treated cotton fabric (C) showed the LOI value of 25 and the total sample was burnt out with a continuous afterglow and with a burning rate of 0.5 mm/s. Burning behavior of the control cotton fabric and the alkaline (pH 10) nano zinc carbonate treated cotton fabric has been depicted in Fig. 2. It showed that the control cotton fabric has been burnt with flashing and flame within 1 min and whitish fragile light weight char mass was remained after completion of the burning process. On the contrary, treated cotton fabric (D) showed no flame catch up and a specific char length has been observed after the completion of the burning cycle. Another interesting observation is that the add-on% of the application is also very less (9%) compared to the commercial treatment (which is likely 20–30%). Here add-on% for the (nano zinc carbonate + alkali) treated cotton fabric (D) is around 9 whereas only alkali treated cotton fabric (C) showed comparatively higher add-on% of 16. However, treated cotton fabric (D) showed more thermal stability (as observed from the burning rate, TG curve) compared to the only alkali treated (C) cotton fabric.
Actually, the prime aim of the present study was to achieve a fire retardant cotton fabric at low add-on% of the fire retardant agent (like nano dimensional zinc carbonate here). Concentration of nano zinc carbonate used for the experiment is 4% (w/v) only. If the concentration level of the zinc carbonate is less (i.e., 2%), the material could not pass the vertical flammability test because of the very low add-on%. On the contrary, by using higher dispersion concentration (i.e., 6%) of zinc carbonate in alkaline condition, add-on % has been increased slightly (12% add-on). However, char length has not much improved from 40 mm. Therefore, 9% add-on was found to be optimized concentration requirement for achieving adequate fire retardancy action. NaOH concentration was maintained 2% (w/v) throughout the treatment. It has been observed from Table 1 that when NaOH alone has been used for the treatment, the add-on % of the treated fabric was maintained 16%. However, in the presence of nano zinc carbonate, the add-on% has been reduced to 9% after the treatment. It means the quantity of the alkali deposition on the fabric surface has been hindered by the presence of nano zinc carbonate in the solution. On the contrary, it can be said that alkali increased the dispersion of the nano zinc carbonate in water media, facilitating the pick-up of the same on the fabric surface by creating more chain accessibility in the compact cellulosic structure. Indeed, alkali directly attacks the primary hydroxyl group (–CH2OH) of the cellulose and converts it to the soda cellulose (–CH2ONa). As a result, amount of the flammable gas (levoglucosan, furans, pyroglucosan, etc.) production has been reduced. Some researchers have also proposed that alkali increases the chain mobility in the amorphous zone of the cellulosic part due to the change in the supramolecular orientation and the disruption of the –H bonds which are facilitating the more amount of the nano zinc carbonate to penetrate inside the amorphous zone of the cellulosic structure. In addition of nano zinc carbonate, some amount of alkali also has been deposited on the fabric surface. As a result combined fire retardancy effect of alkali and nano zinc carbonate has been observed (Jordanov et al. 2010).
TGA analysis in air atmosphere
The TGA curve depicts the thermo-oxidative decomposition of the ZnCO3 as represented in Fig. 3a. The decomposition is responsible for the thermal degradation which occurs at a temperature range of 240–280 °C. The major mass loss happened at this temperature range, indicates the release of OH− and CO2− from the nano zinc carbonate. In addition to the releasing of carbon-di-oxide and water, zinc oxide (ZnO) could also be formed at this temperature range. From the TGA curve, it has been observed that the total percentage mass-loss in the thermal degradation of the zinc carbonate is approximately 23.96%. On the contrary, a very recent literature reports that the bulk ZnCO3 starts losing weight at around 240 °C (30% weight loss) and the total material decomposes at 350 °C (95% weight loss) (Jordanov et al. 2010). This phenomenon clearly indicates the more thermal stability of the nano dimensional ZnCO3 compared to the bulk ZnCO3.
Control cotton fabric (Fig. 3, curve B) showed sharp weight fall at around 380 °C and as a result depolymerisation of the cellulosic structure was occurred with the formation of different flammable gases (levoglucosan, pyroglucosan, etc.) and a less amount of char mass has been left at the higher temperature. Only nano zinc carbonate treated cotton fabric (curve C) showed 4.5% add-on and the weight fall peaks at around 400 °C (20 °C higher than the control cotton fabric). It means that nano zinc carbonate at neutral pH, cannot restrict the flammable gas formation. However, from the TGA curves (Fig. 4, D), it is clear that the degradation temperature becomes lowest (280 °C) in case of the (nano ZnCO3 + NaOH) incorporated sample which is blocking the formation of the levoglucosan and showing extensive dehydration of the treated fabric. TG curve of the only NaOH treated cotton fabric (Fig. 4, E) showed the major mass loss peak at around 330 °C which is 55 °C higher than the treated cotton fabric (D). It signifies the more catalyzing dehydration effect of the (nanoZnCO3 + NaOH) treated fabric compared to the only NaOH treated fabric. As a result, at higher temperature, the extent of char formation is maximum (40%) for the curve C compared to the only NaOH incorporated cotton fabric sample (E) which showed mass retention of only 30% at the same temperature. Table 2 compiled the data of the major mass loss peak and the quantity of the char mass remained at the various temperatures. It was found from Table 2 that nano ZnCO3 showed constant 70% mass retention even at higher temperature. Nano ZnCO3 treated fabric in alkaline condition also showed more than 40% mass retention at higher temperature (550 °C). In addition, the major mass loss peak was shifted to 280 °C which is 45 and 95 °C lower compared to the only alkali treated and the control cotton fabric, respectively. However, nano ZnCO3 treated cotton fabric (at neutral pH) showed only 20% mass retention at higher temperature and the major mass loss peak is 10 °C higher compared to the control cotton fabric. Total phenomena clearly indicates the fact that in alkaline condition, nano ZnCO3 is easily absorbed on the cotton fabric surface and as a result char mass retention and the fire retardant efficacy is very satisfactory compared to the only nano ZnCO3 treated cotton fabric.
DSC analysis
DSC analysis of nano ZnCO3 (Fig. 4A) showed one exothermic peak, indicates the extent of char mass formation. In the DSC curves of the only alkali treated (C) and the (nano ZnCO3 + NaOH) treated (D) fabric, the first peak observed at around 80 °C, corresponds to the removal of water molecules. In case of the curve D, the exothermic reaction is much higher in comparison with only NaOH incorporated cotton fabric (C) which suggest that there is an extensive dehydration in case of (nano ZnCO3 + NaOH) incorporated fabric. This phenomenon confirms the less formation of the levoglucosan (also concluded from the TG analysis) and other flammable gases and assists in the char mass formation. Moreover, the exothermic peak observed at (nano ZnCO3 + NaOH) incorporated fabric (at around 250 °C) is much higher than the only NaOH incorporated fabric which suggests superior fire retardant property of (nano ZnCO3 + NaOH) incorporated fabric compared to the only NaOH treated cotton fabric. Concerning, the DSC curve of the only nano ZnCO3 incorporated cotton fabric at neutral pH, there is no such exothermic peak, may be because of the very small add-on of the nano zinc carbonate on the treated fabric. As a result, the DSC curve of the only nano ZnCO3 treated cotton fabric is almost same like control cotton fabric. It only showed one clear endothermic pyrolysis peak which signifies the formation of different flammable gases with the depolymerisation of the cellulosic structure.
Hence by performing both the TGA and DSC analysis, it was confirmed that the nano ZnCO3 in the presence of alkali showed high dehydration potential with more amount of the carbonaceous char mass generation. In addition, the said treatment also restricts the flammable gas formation during burning.
Interaction of the zinc carbonate with the cotton fabric
Figure 5 represents the FTIR spectra of the nano zinc carbonate (A), (nano ZnCO3 + alkali) treated (B) and the control cotton fabric (C). FTIR curve of the synthesized nanoneedles showed peaks at around 1384 and 1512 cm−1 may be attributed with the peak of the zinc carbonate (Wahab et al. 2009; Gashti et al. 2012). Peak observed in between 700 and 1100 cm−1, assigned with the presence of the carbonate ion. Indeed, sharp peaks observed at 1046, 834 and 708 cm−1 are associated with the presence of the carbonate ion. Another sharp peak observed at 3396 cm−1 may be linked with the stretching vibration of the hydrogen bond (Hingorani et al. 1993; Jordanov et al. 2010; Mostashari et al. 2005). Control cotton fabric showed a clear peak at 1080 cm−1, assigned to the skeletal vibration of the C–O–C pyranose ring. Peaks observed at 1310–1360 cm−1 represents the C–C and C–O skeletal vibration. Broad peaks present at 3100–3600 and 2800–3000 cm−1 are attributed to the –OH and –CH stretching vibration, respectively. In addition to those aforementioned peaks, treated cotton fabric (B) also showed the band at around 1383 and 1508 cm−1, may be linked with the presence of the zinc carbonate deposition on the fabric surface. One sharp peak also was observed at 840 cm−1, attached with the presence of the carbonate ion. Sharp peak also observed at around 1600 cm−1, may be assigned with the Zn(OH)2 produced by the combination of the areal moisture and the treated ZnCO3 molecule. Another interesting observation is that one sharp peak has been observed at 3300 cm−1, in both the control and in the treated fabric (Curve B and C). However, intensity of this peak is more in case of the treated fabric (Curve B), may be assigned with the more stretching vibration of H-bond formation between zinc carbonate (–OH group of ZnCO3) and the –OH group of the cotton fabric.
Surface morphology and the elemental analysis
Surface morphology of the control cotton fabric (A) showed uniform, smooth and clean surface with the presence of convolutions as depicted in Fig. 6. Indeed, there is no sign of the presence of any particles on the surface of the control cotton fabric. On the contrary, (nano ZnCO3 + NaOH) treated cotton fabric showed uniform deposition of the nanoneedles on the surface of the fabric (Fig. 6B1). At larger magnification (Fig. 6B2), porous needle like nano dimensional deposition was observed on the cotton fabric surface. From the SEM analysis, depicted in Fig. 7, it is observed that the sizes of the nanoneedles are around (length: 90–100 µm, diameter: 100–300 nm). TEM image (Fig. 7b–d) substantiated the presence of the nano dimensional needles. Char morphology of the control and the (nano ZnCO3 +NaOH) treated cotton fabric are represented in Fig. 8. The char of the control cotton fabric (C) showed net like structure with the prominent capillary channels, observed throughout the structure. These channels usually assist to the heat propagation during burning. On the contrary, char morphology of the treated cotton fabric showed honey comb like coated intact fibrous structure with the presence of small cells throughout the body, depicted in Fig D1. At higher magnification (D2 and D3), a foamy, expanding coated structure of the cotton fabric surface (after complete combustion) is clearly visible. It means, at higher temperature, nanoneedles of zinc carbonate are dissociated into zinc oxide, which acts as an intumescent coating (foamy, voluminous insulated) on the fabric surface with the liberation of non flammable carbon dioxide and water.
Non flammable carbon dioxide and water formed during burning of the treated cotton fabric, restricts the flow of the flammable gases. EDX analysis of the control and the (nano ZnCO3 + NaOH) treated cotton fabric is represented in Fig. 9 and the concerning data has been shown in Table 3. It was found that the treated cotton fabric showed the presence of sodium, aluminum and zinc in addition to the presence of carbon and oxygen. The amount of carbon element presents in the treated fabric is around 41.36%. However, char mass of the same fabric showed the presence of carbon is around 58.8%. It means that the said treatment helps to dehydrate the structure and increase the amount of carbon containing char mass formation. The same phenomenon is also substantiated by DSC and TG analysis of the treated fabric as discussed earlier.
Mechanical strength of the treated fabric
Tensile strength of the control and the treated fabric has been measured and is represented in Fig. 10. From the figure, it is clear that the control cotton fabric showed the tensile strength of around 635 N whereas nano zinc carbonate treated cotton fabric showed the tensile strength of around 720 N. This phenomenon implies the fact that nano zinc carbonate treatment has no adverse effect on the strength of the treated fabric. It also clears the fact that the nano zinc carbonate is mainly deposited on the surface of cotton fabric might be with some accumulation in the amorphous zone of the fibre structure.
Conclusions
A newly conceived method has been demonstrated successfully for imparting fire retardancy to the cotton fabric (with less add-on% of 9) by exploring facile synthesized zinc carbonate nanoneedles. Needle like zinc carbonate has high surface area and aspect ratio compared to the nano particles and as a result it can provide fire retardancy at comparatively lower add-on % compared to the fire retardancy of nano particles embedded fabric. Nano zinc carbonate in presence of alkali showed sufficient pick up on the cotton fabric and as a result, treated fabric showed self-extinguishing behavior with a LOI value of 30 and specific char length of 40 mm. TG analysis of the (nano ZnCO3 + alkali) treated fabric revealed major mass loss peak at lower temperature and more amount of insulating char mass formation at higher temperature (500 °C) compared to the control cotton fabric. Char morphology of the treated cotton fabric illustrated foamy expand structure which is one common sign of the intumescent based fire retardant mechanism of the coated substrate. As the add-on% of the treated fabric is quite low, the technology can easily be used for making home textile, apparel fabric and for making non-permanent structures for outdoor applications.
References
Alongi J, Carletto RA, Bosco F, Carosio F, Blasio AD, Cuttica F (2013a) Intrinsic intumescent like properties of the DNA treated cotton fabrics. Carbohydr Polym 96:296–305
Alongi J, Carletto RA, Blasio AD, Carosio F, Bosco F, Malucelli G (2013b) DNA: a novel green natural flame retardant and suppressant for cotton. J Mater Chem A 1:4779–4785
Alongi J, Carletto RA, Bosco F, Carosio F, Blasio AD, Cuttica F, Antonucci V, Giordano M, Malucelli G (2013c) Caseins and hydrophobins as novel green flame retardant for cotton fabrics. Polym Degrad Stabil 99:111–117
Alongi J, Blasio AD, Cutica F, Carosio F, Malucelli G (2014) Bulk or surface treatments of ethylene vinyl acetate copolymers with DNA: investigation on the flame retardant properties. Eur Polym J 51:112–119
Arputharaj A, Vigneshwaran N, Shukla SR (2017a) A simple and efficient protocol to develop durable multifunctional properties to the cellulosic materials using insitu generated nano ZnO. Cellulose 24(8):3399–3410
Arputharaj A, Prasad V, Saxena S, Vigneshwaran N, Shukla SR (2017b) Ionic liquid mediated application of nano zinc oxide on cotton fabric for multifunctional properties. J Text Inst 108:1189–1197
Basak S, Ali SW (2016) Sustainable fire retardancy of textiles using bio-macromolecules. Polym Degrad Stabil 133:47–64
Basak S, Ali SW (2017) Leveraging flame retardant efficacy of pomegranate rind extract, a novel biomolecule on ligno-cellulosic materials. Polym Degrad Stabil 144:83–92
Basak S, Ali SW (2018) Fire resistant behavior of the cellulosic textile functionalized with wastage plant biomolecules: a comparative scientific report. Int J Biol Macromol 114:169–180
Basak S, Saxena S, Chattopadhyay SK, Narkar R, Mahangade R (2015) Banana pseudostem sap: a waste plant resource for making thermally stable cellulosic substrate. J Indus Text 46:1003–1023
Basak S, Samanta KK, Chattopadhyay SK, Pandit P, Maiti S (2016) Green fire retardant finishing and combined dyeing of proteinous wool fabric. Colour Technol 132:135–143
Bitnec M, Marinsek M, Orel ZC (2008) Preparation and characterisation of zinc hydroxide carbonate and porous zinc oxide particles. J Eur Chem Soc 28:2915–2921
Coquelle M, Duquensene S, Casetta M, Sun J, Gu X, Zhang S, Bourbigot S (2015) Flame retardancy of PA6 using a guanidine sulfamate/melamine phosphate mixture. Polym 7:316–332
Gashti MP, Alimohammadi F, Song G, Kiumarsi A (2012) Characterization of nanocomposite coatings on textiles: a brief review on microscopic technology. Curr Microsc Contrib Adv Sci Technol 22:1424–1437
Gupta D, Basak S (2010) Surface functionalization of wool using 172 nm UV Excimer lamp. J Appl Polym Sci 117:3448–3453
Hingorani S, Pillai V, Kumar P, Multani MS, Shah DO (1993) Microemulsion mediated synthesis of zinc oxide nanoparticles for varistor studies. Mater Res Bull 28:1303–1310
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stabil 96:377–392
Jordanov I, Mangovoska V, Tavcer PR (2010) Accessability of the mercerized, bioscoured and dried cotton yarn. Tekstil 59:439–447
Mostashari SM, Zanjanchi MA, Baghi O (2005) Burning of a cotton fabric impregnated by synthetic zinc carbonate hydroxide as a flame retardant. Combust Explos Shock Waves 41(4):426–429
Pan YT, Wang DY (2015) One-step hydrothermal synthesis of nano zinc carbonate and its use as a promising substitute for antimony trioxide in flame retardant flexible poly (vinyl chloride). RSC Adv 5(35):27837–27843
Pourmortazavi SM, Marashianpour Z, Karimi MS, Zadeh MM (2015) Electrochemical synthesis and characterization of zinc carbonate and zinc oxide nanoparticles. J Mol Struct 1099:232–238
Samanta AK, Bhattacharya R, Jose S, Basu G, Chaudhury R (2017) Fire retardant finishing of jute fabric using nano zinc oxide. Cellulose 24:1143–1157
Seshama M, Khatri H, Suther M, Basak S, Ali SW (2017) Bulk Vs nano ZnO: influence of fire retardant behaviour on sisal fibre yarn. Carbohydr Polym 175:257–261
Shamsipur M, Pourmortazavi SM, Hajimirsadeghi SS, Zahedi MM, Nasrabadi MR (2013) Facile synthesis of zinc carbonate and zinc oxide nanoparticles via direct carbonation and thermal decomposition. Ceram Inter 39:819–827
Shukla A, Basak S, Ali SW, Chattopadhyay R (2016) Development of fire retardant sisal yarn. Cellulose 24:423–434
Teli MD, Pandit P (2017) Novel method of eco-friendly single bath dyeing and functional finishing of wool protein with coconut shell extract biomolecule. ACS Sustain Chem Eng 5:8323–8333
Teli MD, Pandit P, Basak S (2018) Coconut shell extract imparting multifunction properties to ligno-cellulosic material. J Indus Text 47:1261–1290
Wahab R, Kim YS, Shin HS (2009) Synthesis, characterization and effect of pH variation on zinc oxide nanoparticles. Mater Trans 50:2092–2097
Wang W, Wang L, Liu L (2012) Morphology-controlled synthesis and growth mechanism of ZnO nanostructures via the NaCl nonaqueous ionic liquid route. Cryst Eng Commun 14(15):4997–5004
Zhang S, Fortier H, Dahn JR (2004) Characterization of zinc carbonate hydroxides synthesized by precipitation from zinc acetate and potassium carbonate solutions. Mat Res Bull 39:1939–1948
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sharma, V., Basak, S., Rishabh, K. et al. Synthesis of zinc carbonate nanoneedles, a potential flame retardant for cotton textiles. Cellulose 25, 6191–6205 (2018). https://doi.org/10.1007/s10570-018-1962-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1962-5