Abstract
A novel formaldehyde-free and bio-based flame retardant, ammonium salt of xylitol phosphoric ester acid (ASXPEA), was synthesized under moderate and solvent-free conditions. The structure of this flame retardant was characterized by 1H nuclear magnetic resonance (NMR), 13C NMR, and 31P NMR. The FT-IR spectrum confirms that the ASXPEA flame retardant was grafted on cotton fibers through P–O–C covalent bonds. The limiting oxygen index (LOI) values of cotton treated with 7% and 10% ASXPEA flame retardant reached 41.8% and 45.2%, and their LOI values after 50 laundering cycles were maintained at 29.6% and 30.2%, respectively, with outstanding durability. Results of vertical flammability and cone calorimetry suggest that the treated cotton fabrics achieved excellent flame resistance, and high residues were obtained after combustion by thermogravimetry (TG) analysis. TG-infrared analysis indicated that the treated cotton released fewer flammable gases than the control. Scanning electron microscopy and X-ray diffraction analyses of cotton before and after treatment verified that the fiber structure of the treated cotton had sustainable mechanical properties.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fatal accidents resulting from textiles have compelled researchers to study flame-resistant fabrics (Alongi et al. 2011). Flame-resistant fabrics provide more time for humans to escape in the event of fire and are the first line of defense against potential combustion injuries (Zope et al. 2017). Cotton is a popular natural textile fiber owing to its softness and breathability (Zhang et al. 2017); however, cotton fabrics burn extremely easily with a limiting oxygen index (LOI) value of only 18% (Chen et al. 2018). As a result, many researchers have investigated how to impart fire resistance to cotton fabrics for decades.
Various flame retardants have been used to modify the combustion characteristics of cotton fabrics. Flame-resistant elements include halogen, phosphorus, boron, and some metals. Because of the high toxicity of organic halogen flame retardants, these flame retardants have been phased out (Wang et al. 2018a, b). Boron-containing flame retardants can exclude heat and oxygen because the flame retardants release a substantial amount of nonflammable gas and form a coating during the thermal decomposition process (Li et al. 2010). Yet the flame retardancy of cotton fabrics treated with this flame retardant is unsatisfactory. Metal hydroxides such as aluminum, magnesium, and hydroxy carbonates can release water, which can absorb great heat under high temperature along with serving as smoke suppressants to adsorb soot particles (Laoutid et al. 2009). However, metal hydroxides cannot be used as efficient flame retardants (Alongi et al. 2011). Phosphorous-containing flame retardants are now widely applied in industry (Pethsangave et al. 2017). Traditional durable phosphorous flame retardants such as Pyrovatex CP new with reactive groups (N–CH2OH) and Proban with reactive groups (P–CH2OH) are two efficient flame retardants, which can be grafted onto cellulose through C–O–C covalent bonds. However, treated fabrics release formaldehyde during the serving process (Liu et al. 2018). Recently, some bio-based flame retardants (e.g., proteins and nucleic acids) containing flame-resistant elements such as phosphorous and nitrogen have been investigated as “green” flame retardants for cotton fabrics. These flame retardants may be good substitutes for traditional flame retardants, although their flame resistance and durability require further improvement. (Malucelli et al. 2014). Many studies have recently focused on two durable phosphoric flame retardants, namely triazine-based and olefin-based compounds. Triazine-based flame retardants are cyanuric chloride derivatives containing reactive chlorine atoms to react with the hydroxyl groups of cellulose to form covalent bonds, which endow the treated cotton with excellent durability (Li et al. 2015). Olefin-based flame retardants have been combined with cotton fabrics using UV-curable technology, exhibiting good durability (Xing et al. 2011). Yet the flame retardancy efficiency of cotton fabrics treated with these kinds of flame retardants was relatively poor. Meanwhile, the layer by layer assembly technique and sol–gel hybid coating also attract the attention of researchers. Layer by layer assembly technique is simple and versatile; various materials, such as polyelectrolytes and nanoparticles, can form multilayers on the fibers surfaces then endow them flame retardancy (Pan et al. 2018). The sol–gel technique can also be used to produce fire-retardant inorganic- or hybrid organic–inorganic coating, these coatings can endow cotton fabrics with good flame retardancy and other multi-functional properties (Liu et al. 2018). Up to date, some studies are committed to combining these two technologies, preparing the covalent layer by layer assembled coating thereby endow cotton durability (Jiang et al. 2018). However, these kinds of technologies are impractical due to its large number of processing steps.
In our earlier studies, the ammonium salt of ethylenediamine tetramethylenephosphonic acid (AEDTMPA) and hexamethylenediamineN, N, N′, N′-tetra (methylphosphonic acid) (AHDTMPA) were found to exhibit durability and high flame-retardant efficiency (Gao et al. 2015; Zheng et al. 2016) (Gao et al. 2015). This durability is related to reactive groups (–P–O−NH4+) that can combine with –OH groups of cellulose via P–O–C covalent bonds; the high efficiency is ascribed to the high phosphorous content. Formaldehyde is used during synthesis of flame retardants. Therefore, an ammonium salt of ethylene glycol diphosphoric acid (AEGDP) can be synthesized without formaldehyde. Additionally, this flame retardant can confer durability onto cotton fabrics by forming covalent bonds (Jia et al. 2017b). Nevertheless, every AEGDP molecule contains only two reactive phosphoric groups, resulting in unsatisfactory durability of AEGDP.
Xylitol, a natural alcohol with five reactive –OH groups found in many fruits, vegetables, and cereals, was extracted from plant material. It has been widely incorporated into commercial applications in the pharmaceutical, nutraceutical, and food and beverage industries (Dasgupta et al. 2017). In this study, the low-cost and natural compound xylitol was utilized to eco-friendly synthesize a novel ASXPEA flame retardant with high phosphorus content. The treated cotton fabrics possess high durability. This is owing to ASXPEA molecule containing five reactive phosphoric groups, and only all P–O–C bonds were hydrolyzed can the flame retardant molecule be washed off. In this paper, the ASXPEA flame retardant was facilely synthesized under moderate and solvent-free conditions and combined with cotton fabrics by forming P–O-C covalent bonds, thereby conferring excellent flame resistance and durability on cotton fabrics.
Experimental
Materials
Scoured and bleached cotton fabrics (100%; 124.35 g/m2, 482 × 298, 15.6 tex ×12.5 tex) were bought from Chongqing Chaotianmen Market, China. Phosphoric acid (85%) was supplied by Chongqing Chuandong Chemical Limited Company, China. Urea, dicyandiamide, and ethanol were purchased from Chengdu Kelong Chemicals Chemical Reagent Factory, China. Xylitol was obtained from Shanghai Jiuding Chemical Limited Company, China. All chemicals were used as obtained.
Synthesis of ASXPEA
Xylitol (1, 15.22 g, 0.1 mol) and phosphoric acid (2, 30.76 mL, 0.5 mol) were placed in a beaker (150 mL) equipped with a mechanical stirrer and a thermometer under heated conditions. The mixture was stirred and heated at 120 °C. The reaction continued for 3 h, producing xylitol phosphate ester acid (3, XPEA), a viscous and nut-brown liquid. Then, urea (4, 30.03 g, 0.5 mol) was added to the resulting liquid under mechanical stirring at 100 °C for 50 min, yielding a khaki-colored fluid. Next, the crude product was purified by precipitation using ethanol, and then dried in an oven at 60 °C. A light-yellow fluid, ASXPEA 5, was obtained with 94.37% yield. Scheme 1 shows the facile synthesis route of ASXPEA.
The structures of the synthesized flame retardant product 5 and XPEA 3 were characterized by nuclear magnetic resonance (NMR) spectra. Characterizations for different NMR nuclei of XPEA 3 were listed as followings:
-
1H NMR (D2O, 600 MHz) δ (ppm): 3.51 (s, CH2, H3), 4.09 (s, 2CH2, H2, H4), 4.38 (s, 2CH2, H1, H5), and 4.82 (s, deuterium oxide).
-
13C NMR (D2O, 600 MHz) δ (ppm): 54.17 (1C, C3), 55.45 (2C, C2, C4), and 59.25 (2C, C1, C5).
-
31P NMR (D2O, 600 MHz) δ (ppm): − 6.03 (P8), 0.21 (P7, P10), and 1.57 (P9, P6).
Characterizations for different NMR nuclei of ASXPEA 5 were listed as followings:
-
1H NMR (D2O, 600 MHz) δ (ppm): 3.91 (s, CH2, H3), 4.21 (s, 2CH2, H2, H4), 4.43 (s, 2CH2, H1, H5), and 4.71 (s, deuterium oxide).
-
13C NMR (D2O, 600 MHz) δ (ppm): 65.93 (1C, C3), 73.45 (2C, C2, C4), and 7 8.73 (2C, C1, C5).
-
31P NMR (D2O, 600 MHz) δ (ppm): − 9.85 (P8), − 5.69 (P7, P10), and 0.163 (P9, P6).
ASXPEA grafting onto cotton fabrics
Distilled water was used to dissolve ASXPEA to a certain concentration. Five percent of dicyandiamide was added as a catalyst to the solutions to promote a reaction between ASXPEA and the cellulose. Cotton fabrics were immersed into different ASXPEA solutions at 70 °C for 30 min at a 1:20 material-to-liquid ratio in a thermostat shaker bath; then padded through the nip to obtain cotton samples with 120% wet pickup, repeat two times. Next, the cotton samples were cured at 170 °C for 4 min in an automatic continuous shaping and baking machine. Then the finished cotton samples were washed with running water and dried in an oven to obtain the treated cotton fabrics. The grafting reaction of cotton fibers with ASXPEA is displayed in Scheme 2.
The weight gains (WGs) (wt %) of the cotton fabrics were calculated using Eq. (1):
where W0 and W1 represent the weight of the cotton samples before and after treatment, respectively.
Characterization
Fourier-transform infrared (ATR-FTIR) spectra were obtained by a Nicolet is50 FTIR/ATR spectrophotometer (Thermo Scientific Co., Germany) equipped with a diamond crystal. The ATR-FTIR spectra were within the range of 4000–500 cm−1 with a resolution of 4 cm−1.
Thermogravimetric (TG) analyses of the cotton samples were conducted with a Pyris 1 TG analyzer (Perkin-Elmer, USA), operated in the range of 40–800 °C at a heating rate of 20 K/min under a nitrogen and air atmosphere.
The NMR spectra of ASXPEA and XPEA were obtained at room temperature by a Bruker AV III 600-MHz spectrometer (USA) using D2O as a solvent according to the tetramethylsilane internal standard.
The surface morphologies of the control cotton fibers and char samples were observed using a scanning electron microscope (SEM; Hitachi S-4800, the Netherlands). All samples were coated with platinum using a sputter coater to clearly evaluate their surfaces.
The phase structures of cotton samples were studied with X-ray diffraction (XRD) using a Rigaku XD-3 wide-angle diffractometer with CuK radiation generated at 36 kV and 20 mV (Beijing Purkinje General Instrument Co., Ltd., Beijing, China). The diffraction angle was within the range of 5°–50° with a step size of 0.02° (λ = 0.154 nm).
The contents of phosphorus, carbon, oxygen and nitrogen of the surface for cotton samples before and after washing were obtained by energy-dispersive spectroscopy (EDS) (JEOL-6300F).
The TG-infrared (IR) analyses of the control and treated cotton fabrics were measured by a Netzsch STA 409PC thermal analyzer coupled with a Bruker Tensor 27 FTIR spectrometer through a transfer pipe. During testing, approximately 8 mg of the experimental samples were evaluated under a nitrogen atmosphere at a heating rate of 20 K/min in the temperature range of 40–600 °C. The wavelength range was 4000–600 cm−1 with a 4 cm−1 resolution and 32 scan times.
Properties measurement of treated cotton fabric
Cone calorimetry analysis was conducted according to the ASTM E 1354 standard. Samples measuring 100 mm × 100 mm were tested under an irradiation heat flux of 35 KW/m2. The following parameters applied: time to ignition (TTI, s); time to flame-out (TTF, s); total heat release (THR, kW/m2); heat release rate (HRR, kW/m2); peak of heat release rate (PHRR, kW/m2); ratio of CO2/CO; and final residue (%).
The LOI values of the cotton samples were measured using a digital display oxygen index apparatus (M606B; Qingdao Shanfang Instrument Co. Ltd., China) according to the ASTM D2863-2000 standard.
Vertical flammability was evaluated with a vertical fabric flammability tester (YG815B; Nantong Sansi Electromechanical Science & Technology Co. Ltd., China) in accordance with the ASTM D6413-99 standard.
The durability of cotton samples was measured by a soaping fastness tester (Roaches Co., England) according to the AATCC 61-2006 standard.
Tensile strength was measured with an electronic fabric tension tester (HD026 N; Nantong Hongda Experiment Instruments Co. Ltd., China) according to the ASTM 5035-2006 standard method.
The whiteness of cotton samples was measured by a Datacolor 650 (Datacolor Co., USA) according to the AATCC 110-2000 test method.
Cotton-sample stiffness was determined by an LLY-01-type computer-controlled stiffness tester according to the ASTM D1388-96 (2002) standard test method.
Results and discussion
ATR-FTIR spectra of cotton samples
The ATR-FTIR spectra of the control sample and cotton fabrics treated with 7% ASXPEA flame retardant are shown in Fig. 1. For the control and treated samples, the signal peak at 3340 cm−1 was assigned to O–H vibration absorption, and the 2910 cm−1 peak to C–H vibration absorption (Mortazavi et al. 2013). For the treated sample, except these signal peaks, new characteristic peaks appeared in the spectrum. The weak absorption peak at 1700 cm−1 was attributable to C=O vibration due to oxidation of the –OH groups during the finishing process (Zhang et al. 2014). The peak at 830 cm−1 was assigned to P–O–H vibration absorption (Jia et al. 2017b), and the peak at 768 cm−1 to P–N vibration absorption (Zheng et al. 2017). In addition, the strong peak at 1236 cm−1 was associated with P=O vibration and the P–O–C absorption peak at 1050 cm−1 (Wang et al. 2018a, b), indicating that ASXPEA flame retardants were grafted onto the cotton samples by P–O–C covalent bonds. The peak at 2355 cm−1 was ascribed to C≡C vibration because the xylitol was carbonized at a high temperature (Cataldo 1999). The ATR-FTIR results indicate that the ASXPEA molecules grafted onto cellulose via covalent bonds, endowing the treated cotton with excellent durability.
SEM morphology of samples
SEM was carried out to obtain sample morphology. Figure 2a–c demonstrate that the control fibers exhibited natural striations along with an applanate and smooth surface morphology. No thick coatings appeared on the surface of the treated fibers, and the treated cotton was slightly swollen, indicating that the synthesized hydrophilic ASXPEA flame retardant had penetrated and resided in the interior of the cotton fiber rather than being deposited on the surface (Fig. 2d–f). Additionally, the flame-resistant cotton fibers after burning are shown in Fig. 2g–i. The burned fiber structures were well maintained, and some small bubbles were discovered, implying the flame retardant contained little nitrogen.
EDS of samples
The surface elements of the cotton fabrics treated with different concentration ASXPEA flame retardant and after different laundering cycles (LCs) were measured by EDS analysis. The results are presented in Fig. 3. The phosphorus and nitrogen element contents of treated cotton increased significantly compared with those of control cotton, and gradually increased with the increase of concentration of ASXPEA flame retardant.
EDS spectral analyses of the surface of the control cotton (1), cotton treated by 10% (2), 7% (3), 5% (4), cotton treated by 10% after 30 LCs (5), cotton treated by 7% after 30 LCs (6), cotton treated by 10% after 50 LCs (7), cotton treated by 7% after 50 LCs (8), and cotton treated by 5% after 30 LCs (9)
The phosphorous and nitrogen weight percentages of the cotton fibers treated with 10% ASXPEA flame retardant accounted for about 4.81% and 16.77%, respectively [Fig. 3(2)]. After washing, the phosphorus and nitrogen contents of treated cottons gradually reduced. But even after 30 LCs, the cotton fibers treated with 5% ASXPEA flame retardant also possess the phosphorous and nitrogen weight percentage accounting for about 1.24% and 2.68%, respectively [Fig. 3(9)].
Thermal degradation behavior
Thermal degradation properties of the untreated cotton sample and the sample treated with 7% ASXPEA flame retardant were investigated by TG analyses in an air and nitrogen atmosphere. Generally, the thermal degradation of cotton fibers involves two competitive pathways, namely depolymerization (1) and dehydration (2). Depolymerization occurs at the crack of glycosidic chain bonds and generates volatile levoglucosan, which then decomposes further into small molecules (including flammable gases); competitive dehydration reactions promote thermally stable char, water, and CO2 (Malucelli et al. 2014). The acid catalyst tends to favor dehydration reactions, whereas alkaline media facilitate depolymerization reactions (Alongi and Malucelli 2015). The dehydration mechanism of cotton fabrics treated with phosphorus-containing flame retardants is that the flame retardants releasing phosphorus acid to promote cellulose dehydration, forming a stable char and preventing l-glucose generation (Dong et al. 2017).
The thermo-oxidative stability of cotton samples was evaluated as shown in Fig. 4a, c. When the temperature was below 100 °C, weight loss in all fabric samples was slight because of adsorbed water evaporation in the cotton fibers. For the control cotton, the initial decomposition temperature was 270 °C; in the decomposition stage of 270–365 °C, the cotton fabric demonstrated a weight loss of 73.4% with a maximum weight loss rate at 346 °C. For the treated cotton, the weight decomposition temperature occurred earlier than that of the control cotton: the treated cotton fibers began to decompose at only 240 °C, which may be ascribed to the decomposition of phosphate groups (Feng et al. 2017). In the first stage (240–298 °C), the weight loss of treated cotton was 38.7%. For the control and treated cotton samples, this stage involved two competitive pathways: yields of aliphatic char and volatile products. During the second stage (365–500 °C for the control cotton and 298–600 °C for the treated sample), some aliphatic char converted to aromatic char while some yielded CO and CO2 due to oxidation (Jia et al. 2017a). In the final stage (above 500 °C for the control sample and 600 °C for the treated sample), the remaining char was oxidized to CO2 and CO (Price et al. 1997). At 700 °C, the control exhibited essentially no remaining char, but a significant amount of residue was left on the treated sample (15.3%). These results suggest that the ASXPEA flame retardant can form phosphoric acid and/or polyphosphoric acid during the combustion process to catalyze the cotton dehydrate and form a char skeleton, hence limiting the spread of fire.
The thermal degradation of cotton samples in nitrogen is shown in Fig. 4b, d. For the control sample, the weight quickly declined in the range of 312–391 °C with an approximate weight loss of 82.43%. The main weight loss of the treated sample was in the range of 240–306 °C, reaching only 39.1%. The treated sample decomposed at a lower temperature compared with the control sample, presumably because the ASXPEA flame retardant released phosphoric acid and accelerated the thermal degradation of cotton fiber (Zheng et al. 2017). At 700 °C, the char yield of the treated sample (38.7%) was higher than that of the control sample (8.6%). The high-residue char implied that the ASXPEA flame retardant effectively conferred flame resistance on cotton fabric.
TG-IR analysis of cotton samples
The gaseous ingredients of the control cotton and cotton treated with 7% ASXPEA were evaluated by TG-IR tests. Figure 5 displays the three-dimensional (3D) FTIR spectra for gases produced during the thermal degradation of cotton samples under a nitrogen atmosphere. The TG-IR spectra of samples are presented in Fig. 6.
Figures 5 and 6 show that, compared with the control spectra, no new peaks appeared in the treated-cotton spectra. However, all absorption peaks of treated cotton fibers were weaker than those of the control. The signal from CO2 stretching vibrations was observed at 2352 cm−1; and–OH groups are clearly seen at 3587 cm−1 (Zhao et al. 2017). The peak at 2819 cm−1 was attributable to C–H vibration absorption derived from aliphatic series compounds. The sharp absorption peak at 1743 cm−1 was assigned to C=O vibration from carbonyl compounds, and the absorption peak at 1076 cm−1 was associated with the stretching vibration of ester (Chen et al. 2017). Additionally, the weak peak at 2184 cm−1 was assigned to the stretching vibration of CO, produced by continued char decomposition (Qian et al. 2017).
To further investigate the changes in typical pyrolysis products of the control and treated cotton samples, the intensity of the characteristic peaks of pyrolysis products (CO2, H2O, CO, aliphatic, carbonyl compounds, and ethers) is illustrated in Fig. 7. The absorption peaks of the treated cotton decomposition products were lower than those of the control cotton, presumably due to changes in the decomposition pathway of ASXPEA-treated cotton. The maximum absorption intensities of gaseous products in treated cotton were significantly lower compared with the control cotton, indicating that the amounts of released flammable gaseous volatiles in treated cotton decreased considerably and further limited burning.
Mechanical properties
Flame retardants can substantially decrease tensile strength and other mechanical properties (e.g., bending length); thus, it was essential to investigate the effects of the ASXPEA flame retardant on the mechanical properties of treated cotton fabrics. Data on tensile strength and bending length of the control and cotton fabrics treated with different ASXPEA concentrations are summarized in Table 1. Compared with the control cotton fabric, the tensile strength and elongation at rupture of treated cotton fabrics decreased slightly, this was resulted from a nearly neutral flame retardant solution and relatively low flame retardant concentration. The higher the ASXPEA concentration was, the lower the tensile strength of the treated cotton fabric was.
After LCs, all the tensile strength increased slightly. This may be because some of flame retardant was washed off from treated cotton fabrics, the linkage between flame retardant molecule and cellulose molecule decreased, the amorphous region of cotton fiber became more soft, and then the tensile strength recovered a little.
Stiffness corresponds to the handling of cotton samples. Stiffness was evaluated in this study by measuring the bending length of cotton samples. As shown in Table 1, after treatment, the stiffness of the cotton fabric increased steadily in line with the ASXPEA concentration and gradually decreased with the increase of LCs. However, even for cotton fabrics treated with the maximum ASXPEA concentration, the bending length of warp and weft only increased by 27.9% and 25.56%, respectively, indicating that the treated cotton fabric was still sufficiently soft.
The values of the CIE whiteness indexes of the control and treated cotton fabrics after different LCs are also showed in Table 1. The whiteness indexes were 87.0% for the control cotton, and 83.8%, 82.3%, 81.4% and 81.0% for the cotton treated with 3%, 5%, 7% and 10% ASXPEA, respectively. The increases of the ASXPEA mass concentrations just lead to the whiteness indexes of cotton samples a slight decrease, and the whiteness of the cotton samples gradually increases with the increasing LCs. Due to the ASXPEA is nearly neutral, the whiteness of treated cotton fabric is well sustained.
Flame resistance and fastness
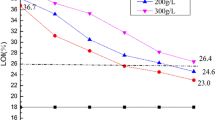
The flame resistance of cotton fabrics treated with ASXPEA after different LCs was estimated using LOI values; data are listed in Table 2. At flame-retardant-solution mass concentrations of 3%, 5%, 7%, and 10%, the LOI values of finished cotton fabrics were 32.5%, 36.5%, 41.8%, and 45.2%, respectively, significantly higher than that of the control cotton (17.6%); hence, ASXPEA appeared to be an efficient flame retardant. This result is attributable to the high phosphorous content of the ASXPEA. The LOI of cotton treated with 5% ASXPEA solution was reduced from 36.5 to 29.6% after 30 LCs; thus, the cotton fabric finished with 5% ASXPEA treatment could be used as a semi-durable flame retardant. After 50 LCs, the LOI values of cotton treated with 7% and 10% ASXPEA solution decreased to 29.6% and 30.2%, respectively, indicating high flame resistance and durability of these treated cotton fabrics. These findings indicated the synthesized flame retardant had successfully grafted onto the cotton fabric, and the two treated cotton fabrics could be used as durable flame-retardant fabrics.
Vertical flammability tests were employed to evaluate the flammability of the original and finished samples; post-burning results are displayed in Fig. 8, and the corresponding post-flame time, afterglow time, and char length are presented in Table 3. The control cotton fabric burned completely without any char formation at a post-flame time of 16 s and afterglow time of 15 s. In contrast, after 12 s of ignition, all treated samples stopped burning as soon as the ignition equipment was removed, and narrow chars remained at the end of combustion. Char lengths of cotton samples with 9.4%, 14.1%, 17.3%, and 21.2% add-ons measured 60, 56, 42, and 34 mm, respectively. Additionally, the cotton fabric with 21.2% add-ons after 50 LCs exhibited an intact 55-mm char, indicating that the finished cotton fabrics possessed good flame resistance and durability.
The above LOI and vertical flammability test results suggest that the ASXPEA flame retardant possessed good durability and high flame-retardant efficiency, as each hydroxymethyl could combine with a phosphoric group of xylitol; therefore, the ASXPEA flame retardant had high phosphorus content and high flame resistance. Because the ASXPEA flame retardant molecule contained five phosphoric groups, every ASXPEA flame retardant molecule could form several P–O–C covalent bonds with cellulose. Only all the phosphorus-containing groups were completely hydrolyzed, the ASXPEA flame retardant molecule could detach from the cotton fibers. Cotton fabrics treated with ASXPEA flame retardant thus exhibited excellent durability.
Flammability
A cone calorimeter was used to assess the combustion behavior of cotton materials, as corresponding data are key parameters in evaluating flame-resistant materials in a real fire scenario. The collected data are summarized in Table 4 and displayed in Fig. 9. The TTI value of the control cotton was 8 s, and the TTF was 26 s. The treated cotton could not be ignited, indicating that the treated cotton fabric possessed excellent flame resistance. Figure 9a showed that the THR of the control cotton was 2.80 MJ/m2 at the end of combustion versus 0.65 MJ/m2 for cotton treated with 7% ASXPEA flame retardant, much lower than that of the control. As demonstrated in Fig. 9b, the control cotton burned rapidly once ignited, producing a PHRR of 203 kW/m2; however, the PHRR of the treated cotton was 9.2 kW/m2, representing a 95% reduction compared with the control.
The ratio of CO2 to CO reflects combustion efficiency; the ratio for treated cotton was 15.2 versus 77 for the control cotton. The low CO2/CO ratio indicated inefficient material combustion (Dong et al. 2017).
XRD of cotton samples
XRD spectra of the control cotton and samples treated with 7% ASXPEA flame retardant are shown in Fig. 10. The XRD pattern of treated cotton was similar to that of the control; no new diffraction peaks appeared, indicating that the ASXPEA flame retardant did not significantly affect the crystal structure of cellulose. Both spectra of cotton samples exhibited four characteristic peaks at approximate 2θ values of 13.54°, 15.18°, 21.37°, and 33.24°, corresponding to the crystal faces (1–10), (110), (200), and (004) of cellulose I, respectively (Nam et al. 2011). For the treated cotton, diffraction peaks were slightly weaker compared with the control, possibly because the flame retardant entered the amorphous region and reacted with the cellulose of treated fibers during the finishing process.
Conclusions
A novel halogen-free and formaldehyde-free flame retardant, ASXPEA, was synthesized in an eco-friendly manner using low-cost, natural xylitol under moderate and solvent-free conditions. The ASXPEA flame retardant contained reactive groups (–P–O−NH4+) that could react with cellulose to form P–O–C covalent bonds. Findings show that cotton fabrics treated with flame-retardant ASXPEA exhibited excellent flame resistance and outstanding durability. Cotton treated with 7% and 10% ASXPEA flame retardant attained 41.8% and 45.2% LOI values. After 50 LCs, the LOI values were 29.6% and 30.2%, respectively. The PHRR, HRR, THR, TTI, and CO2/CO values of the treated fabrics were lower than those of the untreated fabrics. TG-IR results indicated that the amount of released flammable gaseous volatiles of the treated cotton decreased considerably compared with the control cotton. Lastly, the mechanical properties of the treated cotton fabrics were well maintained.
References
Alongi J, Malucelli G (2015) Cotton flame retardancy: state of the art and future perspectives Rsc. Advances 5:24239–24263
Alongi J, Tata J, Frache A (2011) Hydrotalcite and nanometric silica as finishing additives to enhance the thermal stability and flame retardancy of cotton. Cellulose 18:179–190
Cataldo F (1999) From dicopper acetylide to carbyne. Polym Int 48:15–22
Chen X, Wang W, Jiao C (2017) A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J Hazard Mater 331:257
Chen Z, Dong CH, Li Q, Pu Y, Lu Z (2018) Multifunctional, hydrophobic and flame-retarded cotton fabrics modified with liner piperzine/phosphorous/polysiloxane copolymer. Fibers Polym 19(4):861–867
Dasgupta D, Bandhu S, Adhikari DK, Ghosh D (2017) Challenges and prospects of xylitol production with whole cell bio-catalysis: a review. Microbiol Res 197:9–21
Dong C et al (2017) Preparation and properties of cotton fabrics treated with a novel antimicrobial and flame retardant containing triazine and phosphorus components. J Therm Anal Calorim 87:1–9
Feng Y, Zhou Y, Li D, He S, Zhang F, Zhang G (2017) A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr Polym 175:636–644
Gao WW, Zhang GX, Zhang FX (2015) Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose 22:2787–2796
Jia Y, Hu Y, Zheng D, Zhang G, Zhang F, Liang Y (2017a) Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 24:1159–1170
Jia Y, Lu Y, Zhang G, Liang Y, Zhang F (2017b) Facile synthesis of an eco-friendly nitrogen–phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J Mater Chem A 5:9970–9981
Jiang ZL, Wang CS, Fang SY, Ji P, Wang HP, Ji CC (2018) Durable flame-retardant and antidroplet finishing of polyester fabrics with flexible polysiloxane and phytic acid through layer-by-layer assembly and sol–gel process. J Appl Polym Sci. https://doi.org/10.1002/app.46414
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta JM, Dubois P (2009) New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng R 63:100–125
Li YC et al (2010) Flame retardant behavior of polyelectrolyte−clay thin film assemblies on cotton fabric. ACS Nano 4:3325
Li X, Chen H, Wang W, Liu Y, Zhao P (2015) Synthesis of a formaldehyde-free phosphorus–nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym Degrad Stab 120:193–202
Lin DM, Zeng XR, Li HQ, Lai XJ, Wu TY (2019) One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol–gel reaction. J Colloid Interface Sci 533:198–206
Liu XH, Zhang QY, Cheng BW, Ren YL, Zhang YG, Ding C (2018) Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 25:799–811
Malucelli G et al (2014) ChemInform abstract: biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv 4:46024–46039
Mortazavi SH, Pilehvar S, Ghoranneviss M, Hosseinnejad MT, Zargham S, Mirarefi AA, Mirarefi AY (2013) Plasma oxidation and stabilization of electrospun polyacrylonitrile nanofiber for carbon nanofiber formation. Appl Phys A Mater Sci Process 113:703–712
Nam S, Condon BD, Parikh DV, Zhao Q, Cintrón MS, Madison C (2011) Effect of urea additive on the thermal decomposition of greige cotton nonwoven fabric treated with diammonium phosphate. Polym Degrad Stab 96:2010–2018
Pan Y, Liu LX, Wang X, Song L, Hua Y (2018) Hypophosphorous acid cross-linked layer-by-layer assembly of green polyelectrolytes on polyester-cotton blend fabrics for durable flameretardant treatment. Carbohydr Polym 201:1–8
Pethsangave DA, Khose RV, Wadekar PH, Some S (2017) Deep eutectic solvent functionalized graphene composite as an extremely high potency flame retardant. ACS Appl Mater Interfaces 9:35319–35324. https://doi.org/10.1021/acsami.7b09587
Price D, Horrocks AR, Akalin M, Faroq AA (1997) Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in air. J Anal Appl Pyrolysis 40:511–524
Qian Y, Li S, Chen X (2017) Preparation of mesoporous silica-LDHs system and its coordinated flame-retardant effect on EVA. J Therm Anal Calorim 130:2055–2067
Wang C et al (2018a) Flame-retardant rigid polyurethane foam with a phosphorus–nitrogen single intumescent flame retardant. Polym Adv Technol 29:668–676
Wang DF, Zhong L, Zhang C, Zhang FX, Zhang GX (2018b) A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 25:5479–5497
Xing W, Jie G, Song L, Hu S, Lv X, Wang X, Hu Y (2011) Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim Acta 513:75–82
Zhang ZJ, Li LJ, Li F, He J, Gan ZQ (2014) Infrared analysis on pyrolysis products of flame retardant rigid polyurethane foam. Adv Mater Res 1033–1034:900–906
Zhang D et al (2017) Flame retardant and hydrophobic coatings on cotton fabrics via sol–gel and self-assembly techniques. J Colloid Interface Sci 505:892–899
Zhao B, Liu YT, Zhang CY, Liu DY, Li F, Liu YQ (2017) A novel phosphoramidate and its application on cotton fabrics: synthesis, flammability and thermal degradation. J Anal Appl Pyrolysis 125:109–116
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G (2016) A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 23:2211–2220
Zheng D, Zhou J, Wang Y, Zhang F, Zhang G (2017) A reactive flame retardant ammonium salt of diethylenetriaminepenta(methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose 4:1–11
Zope IS, Shini F, Seah D, Akunuri A, Dasari A (2017) Development and evaluation of a water-based flame retardant spray coating for cotton fabrics. ACS Appl Mater Interfaces 9:40782
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Zhong, L., Zhang, C. et al. Eco-friendly synthesis of a highly efficient phosphorus flame retardant based on xylitol and application on cotton fabric. Cellulose 26, 2123–2138 (2019). https://doi.org/10.1007/s10570-018-2193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2193-5