Abstract
A novel P–N based, flame retardant ammonium salt of pentaethylenehexamine octa (methylene-phosphoric acid) (APHOMPA) was synthesized in this study. The structure of the prepared material flame retardant contains eight reactive ammonium phosphorus groups and was characterized by 1H NMR, 13C NMR, 31P NMR, and (Fourier-transform infrared) FT-IR. The results showed that cotton fabrics finished with this flame retardant exhibited excellent flame retardancy and durability. The cotton fabrics finished with 30% APHOMPA solution had limiting oxygen index value 40.5%, and maintained 28.8% even after 50 laundering cycles. The FT-IR results showed that APHOMPA could be grafted on cellulose through P–O–C covalent bond. Vertical flammability, cone calorimetry, thermogravimetric (TG), and TG-IR results revealed the excellent flame retardancy of the finished cotton fabrics and suggested that the flame retardant mechanism of APHOMPA is a condensed phase process. Scanning electron microscopy results suggested the flame retardant entered the inner space of the cotton fabrics, and the X-ray diffraction results showed slight changes to the cotton crystal structure in the finishing process. The breaking strength, softness, and whiteness of finished cotton fabrics were sufficient, suggesting this retardant can be used to prepare treated cotton for multiple applications.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is one of the most important crops in the world, with high output and low production costs (Abdelraheem et al. 2019). As a renewable, decomposing, and sustainable resource, cotton is the most widely used natural fiber for textiles and apparel (Xie et al. 2013). However, cotton fabrics have a limiting oxygen index of only about 16–18%, make its stability very low under fire. To counter this limitation, cotton fabrics must be treated to provide flame retardancy. Many studies have examined how to effectively endow flame retardancy and durability to cotton fabrics (Liu et al. 2008; Pan et al. 2015). Because cotton fabrics is a natural material, endowing flame retardancy to cotton fabrics can only be performed by an after-finish method.
Halogen flame retardants were widely used in the past, but this kind of flame retardants is now restricted due to the high toxicity of these chemicals (Lecouvet et al. 2013; Qiu et al. 2018). Therefore, phosphorus-based flame retardants developed rapidly. Phosphorus flame retardants have only low toxicity by-products, low smoke yield, and provide excellent flame retardant performance (Reddy et al. 2005). The widely used phosphorus based flame retardants are tetramethylolphosphonium chloride (THPC) and N-hydroxymethyl-3-(dimethoxyphosphono) propionamide (Pyrovatex CP). The cotton fabrics finished with these two flame retardants have good flame retardancy and excellent durability, but do release formaldehyde during usage (Moon et al. 2009; Yang et al. 2012). As an alternative, researchers have proposed a series of novel reactive groups to replace the reactive –OH produced by formaldehyde. For example, triazine-based reactive groups were tested, but the flame retardancy of cotton fabrics finished with triazine-based flame retardants was not sufficient (Chang 2012; Easson et al. 2011; Nguyen et al. 2012). Another reactive group, olefin, was used to synthesize reactive flame retardant, but the cotton fabrics finished with this kind of flame retardant similarly have only low flame retardancy, because homopolymerisation of the flame retardant can easily occur in the finishing process (Edwards et al. 2015; Xing et al. 2011).
Some emerging after-finish technologies have been described, such as nano-adsorption (Pan et al. 2016; Shahidi and Ghoranneviss 2013), layer-by layer assembly (Liu et al. 2017), and microcapsulation (Liu et al. 2013), but these new technologies have not been thoroughly studied. In recent years, there have been reports on natural flame retardancy materials, such as nucleic acids (Bosco et al. 2017), phytic acid, and casein (Alongi et al. 2014; Carosio et al. 2014; Kumar Kundu et al. 2017; Lessan et al. 2011; Liu et al. 2016). However, cotton fabrics treated with these natural materials typically have poor durability, because the natural flame retardants lack reactive groups.
In this study, a novel flame retardant ammonium salt of pentaethylenehexamine octa (methylene-phosphoric acid) (APHOMPA) was synthesized. APHOMPA has eight P=O–O–NH4+)2 groups, which are flame retardancy groups and are also reactive groups that can react with hydroxyls on cellulose molecules. The high P and N contents of this flame retardant provide the high flame retardancy of this material. Because every APHOMPA molecule has eight reactive groups, after grafting on cellulose, all the P–O–C covalent bonds between APHOMPA molecules and cellulose would be hydrolyzed, and the flame retardant APHOMPAs would be washed away, so cotton fabrics finished with this flame retardant should have excellent durability.
Experimental
Materials
Pure cotton fabrics (100%; 124.89 g/m2) were purchased from the Chaotianmen Market (Chongqing, China). Pentethylenehexamine was purchased from Shanghai Macklin Biochemical Co., Ltd. Phosphorous acid (H3PO3). Urea and formaldehyde (37 Wt %) were obtained from Chengdu Kelong Chemical Reagents Co. Ltd. (Chengdu, China). Dicyandiamide was supplied by Aladdin Reagent Co. Ltd. (Shanghai, China). All reagents were used without further purification.
Synthesis of APHOMPA
Pentethylenehexamine (1, 10 g, 0.043 mol), phosphorous acid H3PO3 (2, 28.230 g, 0.344 mol), formaldehyde (3, 10.366 g, 0.344 mol), and 50 ml distilled water were added to a 250-mL flask equipped with a reflux condenser. The mixed solution was reacted at 110 °C for 3.5 h, and a viscous brown-yellow liquid was obtained (4). Urea (5, 20.672 g, 0.344 mol) was then added to the resulting liquid with mechanical stirring, and the reaction was maintained at 110 °C for 1.5 h to obtain APHOMPA (6). The crude APHOMPA was purified with ethanol. The synthesis reactions of APHOMPA are shown in Scheme 1.
The APHOMPA structure was characterized by 1HNMR (D2O, 600 MHZ): 1 h NMR (D2O, 600 MHz) :δ (ppm):2.51 (s, 2CH2, H17, H18), 2.71 (s, 2CH2, H15,H16), 2.87 (s, 2CH2, H13, H14), 2.97 (s, 2CH2, H11, H12), 3.13 (s,2CH2, H9, H10), 3.35 (s, 2CH2, H7, H8), 3.60 (s, 2CH2, H5, H6), 4.38 (s, 4CH2, H1, H2, H3, H4) and 4.76 (s, D2O), (s, 4CH2, H1, H2, H3, H4);
13C NMR (D2O, 600 MHz): δ (ppm): 40.28 (2C, C17, C18), 43.49 (2CH2, H15, H16), 46.55 (2CH2, H13, H14), 51.31 (2CH2, H11, H12), 52.73 (2CH2, H9, H10), 52.99 (2CH2, H7, H8), 53.42 (2CH2, H5, H6) and 57.56 (4C, C1, C2, C3, C4);
31PNMR (D2O) δ (ppm): − 9.91 (2P, P1, P2), − 5.18 (2P, P3, P4), 0.19 (2P, P5, P6), 2.60 (2P, P7, P8).
Flame retardant finishing of cotton fabrics
APHOMPA solutions of 20%, 25%, and 30% concentrations were prepared with distilled water. Dicyandiamide was added as a catalyst to the solution at a final mass concentration of 10%. Then, cotton fabrics were immersed into the different APHOMPA solutions and incubated at 75 °C for 60 min with a bath ratio of 1:20. Subsequently, the samples were padded using a nip to gain wet pickup of about 100%. Next, the samples were cured at 180 °C for 4 min in an automatic continuous baking machine. Finally, the samples were rinsed with distilled water and dried in an oven at 110 °C. The graft reaction is shown in Scheme 2.
The weight gains (WG) (wt %) of the cotton fabrics were calculated using Eq. (1) :
where W0 is the weight of the untreated cotton fabrics and W1 is the weight of the cotton fabrics treated with flame retardant.
Characterization
Limiting oxygen index (LOI) and durability
The LOI values of samples were measured at room temperature by an M606B digital oxygen index apparatus (Qingdao Shangfang Instrument Co., Ltd., Shangdong, China) according to ASTM D2863-2000 standard. The sample size was 5 cm × 14 cm.
The washing resistance of samples was estimated by a soaping fastness tester (Roaches Co., England) according to the AATCC-61-2006 standard. The sample was washed in a 49 °C wash solution for 45 min, rinsed with tap water, and dried. The detergent was sodium dodecylbenzene sulfonate.
Vertical flame test
Vertical burning tests were performed on an YG815B vertical fabric FR tester (Nantong Sansi Electromechanical Science & Technology Co., Ltd. China) according to the ASTM D6413-99 standard. Samples measuring 350 mm × 80 mm were placed in a vertical flame of 40 mm for 12 s.
Scanning electron microscope (SEM)
An energy dispersive spectrometer–scanning electron microscope (EDS-SEM, Phenom ProX, NED) was used to observe the surface morphologies of control, treated, and burnt samples (imaging beam voltage: 20 kV).
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectrometer (Thermo Fisher Scientific K-Alpha, USA) was used to detect the element content of the samples. The excitation source was Alka radiation (hv = 1253.6 eV), the voltage was 15 kV, the filament current was 10 mA, and the signal accumulation was performed for 5–10 cycles. The testing energy (Passing-Energy) was 50 eV, the step length was 0.05 eV, and the charge correction was performed with the binding energy of C1s = 284.80 eV as the energy standard.
Fourier transform infrared (FT-IR) spectroscopy
Fourier transform infrared (FT-IR) spectroscopy was performed on samples of the control and treated cotton fabrics, within the range of 500–4000 cm− 1 with a solution of 4 cm− 1 using a Spectrum GX spectrometer (PE Co., USA).
X-ray diffraction (XRD)
X-ray diffraction (XRD) data were obtained using a Rigaku XD-3 wide-angle diffractometer with Cu Ka radiation generated at 36 KV and 20 mA (Beijing Purkinje General Instrument Co. Ltd., Beijing, China). The XRD angle ranged from 5° to 60° with a step size of 0.02° (λ = 0.154 nm).
Cone calorimetry tests
The combustion process of samples were measured using a fire testing technology cone calorimeter (FTT 0007, UK), in conformance with the ASTM E1354 standard. Samples with size of 100 × 100 × 3 mm3 were exposed to a heat flux of 35 KW/m2 and measured.
Thermal stability
Thermogravimetric analyses of samples were carried out using the Pyris-1 thermogravimetric Analyzer (PerkinElmer, USA). The test was performed in nitrogen atmosphere, and the mass of cotton sample was about 8 mg (the heating rate was 10 K min− 1).
Mechanical property tests
Tests of breaking strength were performedusing an electronic tension tester (HD026N, Nantong Hongda Experiment Instruments Co., Ltd., China) according to the ASTM 5035 -2006 standard. Tests of bending length were conducted using an YG (B) 022D-type automatic fabric stiffness tester (Wenzhou Darong Textile Machinery Co. Ltd., Zhejiang, China).
Results and discussion
Flame retardancy and durability
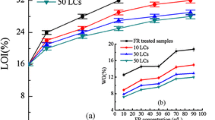
The flame retardancy of cotton fabrics treated with APHOMPA was evaluated using LOI. Generally, a higher LOI value corresponds to better flame retardancy (Cordner et al. 2013). Table 1 shows the relationship between the concentration of the flame retardant (APHOMPA) solution and the measured LOI values of the treated cotton fabrics. It can be seen from the data in Table 1 that from the initial LOI value of 18.2% for the untreated cotton fabrics, the LOI values of the cotton fabrics increased with increased concentration of the flame retardant. For concentrations of 20%, 25%, and 30%, the LOI values of the treated cotton fabrics samples were 33.8%, 37.5%, and 40.5%, and the WG values were 15%, 19.8%, and 22.1%, respectively. Overall, the results showed that the cotton fabrics finished with APHOMPA exhibited excellent flame retardancy due to the high P and N contents of the APHOMPA. The LOI value of the 25% APHOMPA-treated cotton fabrics decreased to 29.2% after 30 LCs, and the LOI value of the 30% APHOMPA-treated cotton fabrics decreased to 28.8% after 50 LCs. Thus, treatment of cotton fabrics with 30% APHOMPA was sufficient for use as durable flame retardant fabric.
The cotton fabrics finished with APHOMPA have excellent durability, due to the presence of eight P=O(–O–NH4+)2 reactive groups, with several P–O–C covalent bonds between APHOMPA molecules and cellulose. Only when all the P–O–C covalent bonds between the flame retardant molecules and cellulose were hydrolyzed, the flame retardant could be washed away from cotton fabrics. Then the flame retardant was difficult to be washed away. Therefore, the cotton fabrics finished with APHOMPA FR had excellent durability.
Vertical flame test results
As shown in Table 2; Fig. 1, the control cotton fabrics incinerated completely after ignition, leaving only a small amount of carbon residue. In contrast, all APHOMPA-treated cotton fabrics stopped burning immediately and formed intact chars after removal from the ignition source. The char lengths of the 20%, 25% and 30% APHOMPA-treated cotton fabrics after combustion were 56 mm, 52 mm, and 40 mm, respectively. After 50 LCs, the char length of the 30% APHOMPA-treated fabric was only 63 mm. Obviously, the cotton fabrics finished with APHOMPA had excellent resistance to burning and excellent durability.
Surface morphologies of the cotton fabrics
Figure 2 shows the SEM images of the pure cotton fabrics, cotton fabrics treated with 30% APHOMPA, and the combustion char of the finished cotton fabrics. Figure 2a–c present the SEM images of the control cotton fabrics; Fig. 2d–f exhibit the SEM images of the cotton fabrics treated with 30% APHOMPA; Fig. 2g–i show the char SEM images of the cotton fabrics finished with 30% APHOMPA. As shown, the control cotton fabrics surface was smooth, flat and curly. The APHOMPA-treated cotton fabrics surface showed no obvious change, suggesting that the APHOMPA infiltrated the interior of the fibers. After burning, the fiber surface shrank a little and some gas bubbles appeared, but the samples still maintained a complete structure. Gas bubbles formed on the char fibers due to the presence of nitrogen element in the flame retardant (Lu et al. 2018). The nitrogen decomposed at high temperature and released ammonia to create expansion
X-ray photoelectron spectroscopy (XPS) analysis
XPS was used to further analyze the surface chemical composition of control and treated cotton (Jiang et al. 2019). The spectra and surface chemical composition (atomic %) of the control and 30% treated cotton are shown in Fig. 3; Table 3. It can be seen from Fig. 3 that compared with the control cotton, the treated cotton has not only the peaks of C1s and O1s, but also some new peaks of N1s and P2p. These results suggested the flame retardant was grafted on cotton fabrics.
FT-IR analysis
The spectra of cotton fabrics before and after 30% APHOMPA-treated were obtained and are shown in Fig. 4. The peaks at 2903 cm− 1 and 3334 cm− 1 are attributed to the stretching vibration of C–H and O–H bonds, respectively (Hamideh Mortazavi et al. 2013). The peak at 1428 cm− 1 corresponds to the bending vibration of –CH2– (Horrocks et al. 2005). The peak of C–O–C corresponds to 1100 cm− 1 (Ghosh et al. 2011). Compared with the control cotton fabrics, the new absorption peak at 1240 cm− 1 is caused by the stretching vibration of P = O, and the peak at 1204 cm− 1 is attributed to P–O–C (Sun et al. 2012). The weak peaks at 775 cm− 1 and 824 cm− 1 are attributed to the stretching vibration of P–N and P–O–H, respectively (Zheng et al. 2016). These results indicated the successful grafting of APHOMPA onto the cotton fabrics through P–O–C covalent bond.
X-ray diffraction analysis
The XRD spectra of the control and 30% APHOMPA-treated cotton fabrics were determined and are presented in Fig. 5. As shown in Fig. 5, both the pure cotton fabrics and finished cotton fabrics exhibited diffraction peaks at 14.81°, 15.94°, 22.53°, and 34.23°, corresponding to the (1–10), (110), (200), and (004) planes of cellulose-I, respectively (French 2014). However, all the peaks weakened with treatment, likely due to two factors. First, the flame-retardant entered the inner space of cotton fabrics and reacted with cellulose to affect the crystal structure. Second, the weight gain of treated cotton fabrics was relatively high, and the content of cellulose in the treated cotton fabrics showed a slight decrease, corresponding to a weaker characteristic peak of cotton fabrics. Additionally, there was a new diffraction peak at 10.95°, which may be caused by the formation of a small number of crystal structures of the flame retardant grafted on the cotton fabrics (Gao et al. 2015).
Cone calorimetry tests
The cone calorimetry test data of control fabrics and 30% APHOMPA-treated cotton fabrics were obtained and are presented in Table 4; Fig. 6. According to Table 4, the peak heat release rate (PHRR) of the flame retardant cotton fabrics was 12.28 kW/m2, much lower than 201.40 kW/m2 measured for the control cotton. The total heat release (THR) 1.17 mJ/m2 of the flame retardant-treated cotton fabrics was also much lower than 2.51 mJ/m2 measured for the control cotton fabrics. These measurements indicated that the finished fabric had excellent flame retardancy. After the combustion of the treated cotton fabrics, there was more carbon residue than that of the control cotton. The CO2/CO value of the control cotton fabrics was 82.37, much higher than that of the flame retardant-treated cotton fabrics, only 23.21. All these data showed that APHOMPA treatment improved the flame retardant property of the cotton fabrics (Dong et al. 2017).
Analysis of the thermal cracking behavior of cotton fabrics
The TG curves of the original and 30% APHOMPA-treated cotton fabrics in air were determined and are shown in Fig. 7a. The decomposition of the FR finished cotton fabrics started at 229 °C, a much lower temperature than the 270 °C observed for the control cotton fabrics. In the range of 270–485 °C, the weight loss of the control cotton fabrics was 92.92%, probably reflecting the conversion of some aliphatic carbon to aromatic carbon, which produced CO and CO2 during carbonization and charring oxidation (Jia et al. 2017a, b). Above ~ 500 °C, the char and remaining hydrocarbons were further oxidized to CO and CO2 (Cheema et al. 2013). In the range of 229–300 °C, 32.9% of the finished cotton weight loss was due to the formation of char and some volatile gases, which resulted in a higher amount of char material above 300 °C. At about 600 °C, the treated cotton produced a high amount of char residue, while the control cotton burned completely. These results showed the flame retardant changed the decomposition path of the treated cotton fabrics (He et al. 2018).
The TG curves of the original and 30% APHOMPA-treated cotton fabrics in N2 were obtained and are shown in Fig. 7b. The main thermal decomposition range of the treated cotton fabrics was 230–310 °C, much lower than the 286–390 °C range of the control cotton fabrics, and the 30% weight loss of treated fibers was also much lower than the 73.9% weight loss of finished fibers. When the temperature increased to 600 °C, the amount of residual cotton in the control cotton was 11.9%, while that of treated cotton was 45.4%. These differences indicate the flame retardant decomposed to phosphoric acid and promoted the formation of carbon (Chang et al. 2011). The decomposition temperature of the treated cotton was lower than that of the control cotton, probably because the APHOMPA formed phosphorus or polyphosphoric acid during the decomposition process, which catalyzed the dehydration and carbonization of the cotton fabrics (Jia et al. 2017a, b). Overall, the TG results suggested that APHOMPA conferred good flame retardancy, consistent with a condensed phase mechanism.
The infrared total spectra of thermal pyrolysis gases of original and APHOMPA finished cotton fabrics were determined and are shown in Fig. 8. In the control cotton of Fig. 8a, large quantities of gases products were detected at 380 °C. The CO2 formed by pyrolysis exhibited a strong and wide absorption peak at 2350 cm− 1 (Li et al. 2017); The absorption peak at 3555 cm− 1 corresponds to the bending vibration of O–H in H2O, and the peaks at 2813 cm− 1 and at 2184 cm− 1 correspond to the vibration absorption of C–H in aliphatic compounds and CO vibration, respectively; The absorption peak at 1743 cm− 1 and at 1076 cm− 1 are vibrations of carbonyl (C=O) and C–O–C bonds, respectively (Chen et al. 2017). In the treated cotton of Fig. 8b, large quantities of gases products were detected at 320 °C. The absorption peak of CO2 (non-flammable gas) at 2350 cm− 1 is significantly enhanced, and the absorption peaks of C–H, C=O and C–O–C of the treated cotton are significantly weakened. The spectra of decomposition gases for the flame retardant-treated cotton fabrics revealed no new peak compared with the spectra data for the control cotton, suggesting that the flame retardant treated cotton fabrics did not release new gas at high temperature. The changes of absorption intensity of the representative functional groups of gas products released from thermal decomposition were also analyzed and the results are shown in Fig. 9. For the finished cotton, with increased heating time, the absorption peak strength of CO2 gradually increased, reaching maximum value at 300 °C, and then decreased gradually. For the control cotton, the maximum absorption peak strength of the control cotton fabrics occurred at 360 °C, consistent with the TG result. The maximum absorption intensities of the gaseous product functional groups of the flame retardant-treated cotton fabrics were much lower than those of the control cotton fabrics, indicating the release of a much lower amount of gas products by the flame retardant cotton fabrics compared to that of the control cotton fabrics. Additionally, the amount of combustible gases were greatly reduced, again consistent with excellent flame retardancy of the finished fabrics.
Mechanical properties
Table 5 shows the tensile strength and stiffness of pure and finished cotton fabrics. As presented in Table 5, with increased concentration of flame retardant, the warp and weft breaking strengths of the treated cotton fabrics gradually decreased. Additionally, the downward trend of warp strength was greater than that of weft strength. After treatment with 30% APHOMPA, the tensile strengths decreased by 29.7% in the warp direction and 21.7% in the weft direction; Additionally, the elongation at rupture decreased by 9.7% in the warp direction and 25.0% in the weft direction. The results indicated there was some damage for the treated cotton fabrics.
Softness is an important parameter of cotton fabrics, and it is important that treated cotton fabrics maintained good softness after finishing. From Table 5, after treatment with 30% APHOMPA, the bending lengths of the treated cotton were 46.8 mm in the warp direction and 39.9 mm in the weft direction, slightly increased values than those for control cotton, which were 40.7 mm in the warp direction and 36.9 mm in the weft direction. These results indicate that the softness of treated cotton fabrics was maintained.
The whiteness indexes of the original and finished cotton fabrics were evaluated and are shown in Table 5. The whiteness index of the control cotton fabrics was 92.9%, and the whiteness index of the finished cotton fabrics was 88.2% after 20% APHOMPA treatment. The whiteness indexes of finished cotton fabrics decreased gradually with increased concentration of the flame retardant. At APHOMPA concentrations of 25% and 30%, the whiteness indexes of the finished cotton fabrics were 87.0% and 85.9%, respectively, indicated that the whiteness of the APHOMPA-treated cotton was well maintained.
Conclusion
A novel flame retardant ammonium salt of pentaethylenehexamine octa (methylene-phosphoric acid) (APHOMPA) was synthesized and applied to cotton fabrics. This phosphorus-based flame retardant grafted on cellulose through P–O–C covalent bond, and this flame retardant exhibited a condensed phase mechanism of flame retardancy. The cotton fabrics finished with this new material exhibit excellent flame retardancy and durability, the LOI value of the cotton fabrics treated with 30% APHOMPA was 40.5%, and 28.8% after 50 LCs, suggesting that cotton fabrics finished with APHOMPA could be used as durable fabrics. The PHRR, HRR, THR, and CO2/CO values were all lower than those of control cotton fabrics, again consistent with excellent flame retardancy of the finished cotton fabrics, without significant decreases in whiteness and softness. The breaking strength of the treated cotton fabrics had some damage.
References
Abdelraheem A, Esmaeili N, O’Connell M, Zhang J (2019) Progress and perspective on drought and salt stress tolerance in cotton. Ind Crop Prod 130:118–129. https://doi.org/10.1016/j.indcrop.2018.12.070
Alongi J et al (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stab 99:111–117. https://doi.org/10.1016/j.polymdegradstab.2013.11.016
Bosco F, Casale A, Gribaudo G, Mollea C, Malucelli G (2017) Nucleic acids from agro-industrial wastes: a green recovery method for fire retardant applications. Ind Crop Prod 108:208–218. https://doi.org/10.1016/j.indcrop.2017.06.035
Carosio F, Di Blasio A, Cuttica F, Alongi J, Malucelli G (2014) Flame retardancy of polyester and polyester-cotton blends treated with caseins. Ind Eng Chem Res 53:3917–3923. https://doi.org/10.1021/ie404089t
Chang SC (2012) Antiflammable properties of capable phosphorus–nitrogen-containing triazine derivatives on cotton. In: Acs Symposium, vol 243, pp 865–869
Chang S, Condon B, Graves E, Uchimiya M, Fortier C, Easson M, Wakelyn P (2011) Flame retardant properties of triazine phosphonates derivative with cotton fabric. Fibers Polym 12:334–339. https://doi.org/10.1007/s12221-011-0334-7
Cheema HA, El-Shafei A, Hauser PJ (2013) Conferring flame retardancy on cotton using novel halogen-free flame retardant bifunctional monomers: synthesis characterizations applications. Carbohydr Polym 92:885–893. https://doi.org/10.1016/j.carbpol.2012.09.081
Chen X, Wang W, Jiao C (2017) A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J Hazard Mater 331:257–264. https://doi.org/10.1016/j.jhazmat.2017.02.011
Cordner A, Mulcahy M, Brown P (2013) Chemical regulation on fire: rapid policy advances on flame retardants. Environ Sci Technol 47:7067–7076. https://doi.org/10.1021/es3036237
Dong C et al (2017) Preparation and properties of cotton fabrics treated with a novel antimicrobial and flame retardant containing triazine and phosphorus components. J Therm Anal Calorim 131:1079–1087. https://doi.org/10.1007/s10973-017-6604-x
Easson M et al (2011) Cyanuric chloride derivatives for cotton textile treatment-synthesis, analysis, and flammability testing. AATCC Rev 11:60–66
Edwards B, Hauser P, El-Shafei A (2015) Nonflammable cellulosic substrates by application of novel radiation-curable flame retardant monomers derived from cyclotriphosphazene. Cellulose 22:275–287. https://doi.org/10.1007/s10570-014-0497-7
French AD (2014) Idealized powder diffraction patterns for cellulose. polymorphs Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Gao W-W, Zhang G-X, Zhang F-X (2015) Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose 22:2787–2796. https://doi.org/10.1007/s10570-015-0641-z
Ghosh B, Chellappan KV, Urban MW (2011) Self-healing inside a scratch of oxetane-substituted chitosan-polyurethane (OXE-CHI-PUR) networks. J Mater Chem 21:14473. https://doi.org/10.1039/c1jm12321a
Hamideh Mortazavi S, Pilehvar S, Ghoranneviss M, Hosseinnejad MT, Zargham S, Mirarefi AA, Mirarefi AY (2013) Plasma oxidation and stabilization of electrospun polyacrylonitrile nanofiber for carbon nanofiber formation. Appl Phys A 113:703–712. https://doi.org/10.1007/s00339-013-7707-2
He P et al (2018) Preparation and flame retardancy of reactive flame retardant for cotton fabric. J Therm Anal Calorim 132:1771–1781. https://doi.org/10.1007/s10973-018-7057-6
Horrocks AR, Kandola BK, Davies PJ, Zhang S, Padbury SA (2005) Developments in flame retardant textiles—a review. Polym Degrad Stab 88:3–12. https://doi.org/10.1016/j.polymdegradstab.2003.10.024
Jia YL, Hu YW, Zheng DD, Zhang GX, Zhang FX, Liang YJ (2017a) Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 24:1159–1170. https://doi.org/10.1007/s10570-016-1163-z
Jia YL, Lu Y, Zhang GX, Liang YJ, Zhang FX (2017b) Facile synthesis of an eco-friendly nitrogen–phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J Mater Chem A 5:9970–9981. https://doi.org/10.1039/c7ta01106g
Jiang ZM, Li H, He YW, Liu Y, Dong CH, Zhu P (2019) Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl Surf Sci 479:765–775. https://doi.org/10.1016/j.apsusc.2019.02.159
Kumar Kundu C et al (2017) A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr Polym 166:131–138. https://doi.org/10.1016/j.carbpol.2017.02.084
Lecouvet B, Sclavons M, Bailly C, Bourbigot S (2013) A comprehensive study of the synergistic flame retardant mechanisms of halloysite in intumescent polypropylene. Polym Degrad Stab 98:2268–2281. https://doi.org/10.1016/j.polymdegradstab.2013.08.024
Lessan F, Montazer M, Moghadam MB (2011) A novel durable flame-retardant cotton fabric using sodium hypophosphite, nano TiO2 and maleic acid. Thermochim Acta 520:48–54. https://doi.org/10.1016/j.tca.2011.03.012
Li Z-F, Zhang C-J, Cui L, Zhu P, Yan C, Liu Y (2017) Fire retardant and thermal degradation properties of cotton fabrics based on APTES and sodium phytate through layer-by-layer assembly. J Anal Appl Pyrol 123:216–223. https://doi.org/10.1016/j.jaap.2016.11.026
Liu Y, Wang X, Qi K, Xin JH (2008) Functionalization of cotton with carbon nanotubes. J Mater Chem 18:3454. https://doi.org/10.1039/b801849a
Liu J, Liu C, Liu Y, Chen M, Hu Y, Yang Z (2013) Study on the grafting of chitosan-gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloids Surf B Biointerfaces 109:103–108. https://doi.org/10.1016/j.colsurfb.2013.03.040
Liu S-H, Chen W-J, Shen M-Y, Yang J-M, Chiang C-L (2016) Preparation, characterization and its flame retardance performance of microencapsulated ammonium polyphosphate/ bridged polysesquisiloxane polyurethane composites. J Polym Res. https://doi.org/10.1007/s10965-016-1094-2
Liu L, Pan Y, Wang Z, Hou Y, Gui Z, Hu Y (2017) Layer-by-layer assembly of hypophosphorous acid-modified chitosan based coating for flame-retardant polyester–cotton blends. Ind Eng Chem Res 56:9429–9436. https://doi.org/10.1021/acs.iecr.7b02303
Lu Y, Jia Y, Zhou Y, Zou J, Zhang G, Zhang F (2018) Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohydr Polym 201:438–445. https://doi.org/10.1016/j.carbpol.2018.08.078
Moon S, Ku B-C, Emrick T, Coughlin BE, Farris RJ (2009) Flame resistant electrospun polymer nanofibers from deoxybenzoin-based polymers. J Appl Polym Sci 111:301–307. https://doi.org/10.1002/app.29067
Nguyen TMD, Chang SC, Condon B, Slopek R (2012) Synthesis of a novel flame retardant containing phosphorus-nitrogen and its comparison for cotton fabric. Fibers Polym 13:963–970. https://doi.org/10.1007/s12221-012-0963-5
Pan H et al (2015) Construction of layer-by-layer assembled chitosan/titanate nanotubes based nanocoating on cotton fabrics: flame retardant performance and combustion behavior. Cellulose 22:911–923. https://doi.org/10.1007/s10570-014-0536-4
Pan Y, Wang W, Pan H, Zhan J, Hu Y (2016) Fabrication of montmorillonite and titanate nanotube based coatings via layer-by-layer self-assembly method to enhance the thermal stability, flame retardancy and ultraviolet protection of polyethylene terephthalate (PET) fabric. RSC Adv 6:53625–53634. https://doi.org/10.1039/c6ra05213d
Qiu X, Li Z, Li X, Zhang Z (2018) Flame retardant coatings prepared using layer by layer assembly: a review. Chem Eng J 334:108–122. https://doi.org/10.1016/j.cej.2017.09.194
Reddy PRS, Agathian G, Kumar A (2005) Ionizing radiation graft polymerized and modified flame retardant cotton fabric. Radiat Phys Chem 72:511–516. https://doi.org/10.1016/j.radphyschem.2004.03.015
Shahidi S, Ghoranneviss M (2013) Effect of plasma pretreatment followed by nanoclay loading on flame retardant properties of cotton fabric. J Fusion Energy 33:88–95. https://doi.org/10.1007/s10894-013-9645-6
Sun L, Qu Y, Li S (2012) Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J Therm Anal Calorim 111:1099–1106. https://doi.org/10.1007/s10973-012-2494-0
Xie K, Gao A, Zhang Y (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr Polym 98:706–710. https://doi.org/10.1016/j.carbpol.2013.06.014
Xing WY, Jie GX, Song L, Hu SA, Lv XQ, Wang X, Hu YA (2011) Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim Acta 513:75–82. https://doi.org/10.1016/j.tca.2010.11.014
Yang Z, Wang X, Lei D, Fei B, Xin JH (2012) A durable flame retardant for cellulosic fabrics. Polym Degrad Stab 97:2467–2472. https://doi.org/10.1016/j.polymdegradstab.2012.05.023
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G (2016) A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 23:2211–2220. https://doi.org/10.1007/s10570-016-0949-3
Acknowledgments
This work was financially supported by the Chongqing Natural Science Foundation of China (No: CSTC 2019 jcqy50749) and the Chongqing Postgraduate Education and Teaching Reform Major Project (No: yjg152022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, D., Liu, S. et al. A novel P–N-based flame retardant with multi-reactive groups for treatment of cotton fabrics. Cellulose 27, 9075–9089 (2020). https://doi.org/10.1007/s10570-020-03387-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03387-0