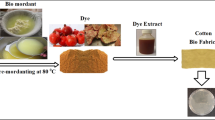
Abstract
Pomegranate rind (Punica granatum) extracts (PRE), a widely available wastage material, are explored as new multifunctional finishing agent (fire retardant, natural dye, mordanting and antimicrobial agent) on cellulosic textiles. PRE has been applied on the cellulosic cotton fabric at higher temperature (90 °C, 30 min) in different concentrations. 400 g/L PRE extract treated fabric showed 15 times lower burning rate as compared to the control cotton fabric. Thermo-gravimetry of the PRE treated fabric showed earlier dehydration phenomenon and more amount of char mass formation. The resulting fire retardancy of the treated fabric is the combined reaction effect of the acid source (carbamic acid, ammonium salt, hexacontanoic acid, etc.), carbon source (carbonic dihydrazide, nona hexacontanoic acid, 1 hydroxy 2 pentanone, sugar based material) and blowing agent [nitrogen containing bases like guaninidine, asparigine (amino acid of protein), di amino guanidine and ethanamine, piperidine] present in the PRE extracts as observed from the Gas Chromatography Mass Spectroscopy analysis. Antimicrobial efficacy of the PRE extract on cotton substrate has also been demonstrated successfully against both the Gram-positive and the Gram-negative bacteria because of its tannin and positively metal ion as well as amino acid contents. Furthermore, cotton fabric dyed from the PRE medium with anionic acid dye showed dark colour compared to the acid dyed cotton fabric from water medium, characteristics of the mordanting efficacy of the extract. Extract also has been explored as natural dye for the dyeing of the cotton textile substrates because of the presence of natural colouring material (betacyanin, coumarin, etc.) in it as observed from the phytochemical analysis of the extracts.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flame retardant textile material with attractive natural colour and hygienic antimicrobial property is in great demand for the home textile, home furnishing purpose as well as for using as apparel because of its fire safety and bioactive concern. Flame retardancy of the cotton fabric is an important aspect toward the end users as it mainly contains cellulosic backbone and very much prone to catch up fire (Alongi et al. 2013a; Basak and Ali 2017; Fawde et al. 2012). Reports unveil that lot of human life has been perished and different costly valuable materials, documents have been damaged due to the ignition of the cellulosic textile materials. In addition, afterglow, toxic smoke (mostly carbon monoxide) generated from the cellulosic material also equally harmful and life threatening to the living beings (Horrocks 2009). Since last five to six decades, researchers are tried to develop different flame retardant chemicals which can stop the combustion process of the cellulosic polymer or at least to delay the burning rate of the finished cotton fabric. Among them, phosphorus, nitrogen, sulphur based and the halogen based composition have been explored successfully by the researchers and the textile industries to make fire retardant cotton textile. Halogen based chemicals were also very popular when it has been used as back coating for the cellulosic textile material. However, due to the environmental issue (for releasing of dioxins and furanes) halogen based chemicals are strictly banned into the market (Camino 2009). To this end, from the last fifty years different flame retardant chemicals based on the composition of phosphorous, nitrogen (especially proban, pyrovatex based chemicals with combination of melamine resin) have emerged into the market for imparting flame retardancy. However, here also more amount of add-on % is required on the fabric surface and the larger quantities of toxic chemicals have been consumed. Therefore, getting an eco-friendly, cheap, widely available intumescent based flame retardant material from the wastage resource is still a big challenge and unmet needs. To this end, researchers have reported some of the protein and the plant based natural bio-molecules consist of different components (acid source, blowing agent and carbon source) which combinedly showed condensed phase fire retardant behaviour and also the application process is much more easier (can be apply in neutral pH) on the textile surface (Alongi et al. 2014; Basak et al. 2016; Malucelli et al. 2014). Protein based natural material like DNA, casein, whey protein, sulphur based hydrophobin, etc. has been explored successfully by the researchers on the cotton textile material. As per their report, DNA shows adequate flame retardancy at 10–15% add-on on the fabric surface (Malucelli et al. 2014; Carosio et al. 2014). Moreover, flame retardancy mechanism has also been reported clearly into the published manuscript. Previously, different plant based biomolecules like banana pseudostem sap, green coconut shell extract, spinach leaves extract have been used for making fire retardant cellulosic textile material (Basak et al. 2015, 2016a, 2016b; Mohammed et al. 2016). However, none of the previous published manuscript on the plant based biomolecule, have explained the detail fire retardancy mechanism (by analysing compounds of volatile species, char, etc.) of the cotton fabric imparted by plant based biomolecules. Moreover, in addition to the flame retardancy, a very few plant based extracts have been explored for the multifunctional finishing (antimicrobial, naturally dyed) of the cotton textile materials. Therefore, a continuous search is going on for the exploration of new plant based natural biomolecules for imparting multifunctional finishing to the cotton fabric. In this connection, pomegranate rind extract (PRE) (original pH − 4.5), an wastage widely available plant based bio-molecule, is a worthy alternative and has been explored for making combined multifunctional (fire retardant, antimicrobial, naturally dyed) cotton (cellulosic material) textile from one treatment bath. Previously, same research group have reported PRE for making fire retardant jute (lingo-cellulosic material) fabric. Connected to it, researchers have applied the extract in different pH condition and the detail fire retardancy property and the thermal kinetics have been established. It has been concluded that in alkaline pH PRE treated jute fabric has shown adequate flame retardancy. However, there is a large research gap exists on the mechanism behind the fire retardancy action of PRE on cellulosic substrate. In addition, in detail forced combustion behaviour, volatile species analysis, char analysis of the treated fabric are not revealed in the previous paper published by our research group. In the present article, PRE has been explored on the cellulosic material at different concentrations. Detail mechanism (volatile species, char analysis, PRE compound analysis) behind the flame retardancy, forced combustion behaviour of the treated fabric at definite heat flux has been established. Further, phytochemical analysis, multi-functionality (dyeing, antimicrobial efficacy) of the treated fabric has also been elucidated in the current context. PRE contains different nitrogen containing alkaloids, tannin, large molecular weight phenolic compounds, carbon sources, etc. which are responsible for the fire retardant behaviour of the treated cotton fabric by condensed phase, aromatised mechanism. Treated fabric showed earlier dehydration, less flammable gas formation and more carbonaceous char mass generation in thermal decomposition. Extract and the treated fabric also contains phenolic based acids, tannin, protein precipitation capability and the different positive metal ions, etc. which are responsible for the antimicrobial action against both the Gram-positive and the Gram-negative bacteria. PRE treated cotton fabric contains betacyanin, coumarin based phytochemicals which are responsible for the natural coloured effect on the treated cotton fabric. Thus, the present context reveals the multifunctional efficiency (fire retardancy, antimicrobial, mordanting and the natural dyeing efficacy) of the wastage pomegranate rind extract (PRE) on the cotton textiles in details.
Materials and methods
Material
A 150 g/m2 plain woven bleached cellulosic cotton fabric was used to make it flame retardant by using pomegranate rind extract (PRE) treatment. Wastage PRE, used for the study, has been collected from the local market of New Delhi, India.
Treatment of cotton fabric with PRE
The bleached cotton fabric was impregnated with three different concentrations of the pomegranate rind extract (PRE): 100 (as it is), 200 (1:2) and 400 (1:4) g/L with the help of a heater at 100 °C. Material to PRE ratio was maintained at 1:20 during the experiment. The fabric was treated into the solution of each concentration for 30 min at 90 °C, followed by drying at 110 °C for 5 min. Concentration of the PRE has been determined with the help of rotary evaporator machine which assist to separate the water from the solid particles present in the PRE.
Before the testing, all the samples were conditioned in a standard atmosphere of 65% RH for 24 h, so that they are identically acclimatised. The percent add-on after PRE treatment, i.e., the increase in the sample weight relative to the original weight was determined by the gravimetric method as follows:
where M1 and M2 are the oven dried weights of the control and the PRE treated samples, respectively.
Sample prepared for the flammability test
The burning behaviour of the control and the treated samples were evaluated as per the standard methods. For determination of Limiting Oxygen Index (LOI), an ignition time of 30 s was maintained as per the Indian standard IS 13501 (law resource.org 1992). In vertical flammability tests, the different parameters were measured as per the IS 1871 method A (law resource.org 1986). As per this method, the fabric sample (250 mm*40 mm) was ignited with a flame of 38 mm height for 12 s.
The cone calorimeter was used to test the 100 × 100 mm2 control and the PRE treated cotton textile in accordance with the procedure of ASTM international test method E 1354-10a. Specimens were tested at horizontal orientation with heat flux of 35 kW/m2 generated by cone calorimeter and to the direct application of the propane flame. Before testing all the samples were conditioned at 65% R.H and 27 °C. Samples were in horizontal condition during the combustion process. Data recorded included those specified in the ISO5660-1 standards. The parameters have been measured are total heat release (MJ/m2), heat release rate (kW/m2), Time to ignition, time to flame in and flame out, maximum average rate of heat emission (MARHE), peak heat release rate (PHRR) etc. In each case three replicates of the samples have been tested and the CV% value has been represented into the Table.
Characterisation techniques used for the experiment
Thermo-oxidative degradation of the dried PRE and the treated cellulosic materials were drawn on a Thermo Gravimetric Analyser (METTLER TOLEDO TG-50/MT5) in air atmosphere at 2 ml/min flow rate and heating rate of 20 °C/min. TG analysis of the control cotton material and the PRE treated cotton samples also have been performed in isothermal condition (350 °C, 20 min) at air atmosphere with heating rate of 20 °C/min, to understand the weight degradation phenomena with temperature and time.
The FTIR-ATR analysis of the samples was carried out in Thermofisher Scientific (Model: Nicolet is50 FTIR) made FTIR analyser over the wavelength of 500 to 4500 cm−1. Here an ATR transmittance mode has been used with DLaTGS detector for 49 scans and 4 resolutions.
Changes in the surface morphology of the materials were studied with the help of a high resolution (up to 3 nm) scanning electron microscope (ZEISS EVO 50) using SE detector. The samples were coated with a thin layer (100 angstroms) of conducting material (Gold/palladium) by using a sputter coater and examined under SEM with an accelerating voltage of 20 kV. The EDX analysis of the samples was carried out in a TM3000 tabletop microscope (Made by: HITACHI, Swift ED3000) to determine the different elements present on the surface, and was expressed in weight percent.
The component identification of the PRE, pyrolysate and the smoke of the control and the treated fabric was done by using Perkin Elmer Gas chromatography-mass spectroscopy (GC–MS) machine (Gas chromatography, model: Clarus 600 and for mass spectroscopy, model: Clarus 680). Samples were injected into a fused capillary silica column of 30 m*0.25 mm*0.25 µm by using helium as carrier gas. Connected with the analysis, injector and detector temperature of GC was maintained at 250 °C in the machine. Column temperature of the GC has been maintained at 60 °C, 3 min. 60 °C has been maintained constantly for 3 min dwell time and then the temperature of the column has been raised at heating rate of 5 °C/min and has been reached at 160 °C. This particular temperature again has been maintained for 2 min and again raised with gradient value 10 °C/min till the temperature has been reached to 300 °C. Thereafter the chromatography spectrum and the possible chemical compounds present (mass spectroscopy analysis) have been taken from the machine. Mass spectra have been acquired over a 40 to 500 amu range in EI mode with 35 min retention time. GCMS spectra of the PRE is represented in Fig. 1.
Dyes used for the experiment
Two different dyes (anionic and cationic) named Acid red 76 and Basic Methylene Blue [structure as shown in below in Fig. 2] have been used for examined the combine dyeing and the fire retardant finishing efficacy of the PRE.
Antimicrobial efficacy of the treated fabric
Different concentrations of the PRE solutions were screened for antibacterial activity by Agar well diffusion method with a cork borer of size 9 mm. For both the type of bacteria, broth cultures grown at 37 °C were used. An aliquot (0.1 ml) of the tenfold diluted inoculum was seeded into molten and cooled to 45 °C nutrient Agar buffs and overlaid on sterile nutrient Agar. The wells were then punched with exactly 0.1 ml of 5, 10, 15 and 20% of the PRE solution and introduced into the wells allowing 10 min for diffusion at 4 °C followed by an incubation period of 24 h at 37 °C. The antibacterial activity was evaluated against S. aureus (Gram-positive) and K. pneumonia (Gram-negative) by measuring the size of the zone of the inhibition of bacterial growth around the well.
For quantitative analysis, the percentage reduction in the control and the PRE treated cotton fabrics was calculated as per AATCC-100-2004 standard. In this method, the control and the treated swatches were inoculated with the test organism. After incubation, the bacteria were eluted from the swatches by shaking with a known amount of neutralising solution, serially diluted and plating out by standard plate count. The number of bacteria present in this liquid was determined and expressed in percent reduction as per the below formula
Where, A is the number of the bacteria recovered from the inoculated treated test specimen swatches immediately after inoculation (at “0” contact time) and B is the number of the bacteria recovered from the treated inoculated test specimen swatches incubated over the desired contact period. In this case, Staphylococus aureus (Gram-positive) and Escherichia coli (Gram-negative) bacteria were used.
Colour strength of the treated cotton fabric
PRE extract showed optical density absorbance value at a particular maximum absorbance wave length (maximum wave length = 510 nm) in a Hitachi-U-2000 UV–Vis-absorbance spectrophotometer.
It was observed that after application of the BPS in the cotton textile, its colour got changed from white to khaki and the colour parameters such as K/S, L, a and b were measured using a Perkin-Elmer double beam spectrophotometer, Lambda (35 model) equipped with an integrating sphere. The colour depth of the BPS treated fabrics was determined in terms of K/S from the reflectance data using the Kubelka–Munk equation as fo
where K is the absorption coefficient, S the scattering coefficient and R is the reflectance of the treated fabric at the wavelength of maximum absorption. The K/S was determined at 213 nm (λ max) of the respective dye. Other colour parameters such as L* (lightness-darkness), a* (redness-greenness) and b* (blueness-yellowness component) were measured using the Win lab software, delta-E 1976.
Phytochemical analysis of the PRE extracts
Tannin content of the extracts has been measured by adding 1 ml of the 5% ferric chloride solution into the 1 ml of the PRE extract. The blackish green colour of the resulting solution is the measure of the tannin content. Presence of the natural colour component coumarin in the extract has been identified by adding 1 ml of the PRE solution with the 1 ml of the 10% NaOH solution which creates yellow colour. 1 ml of the PRE solution is added with the 1 ml 2 N NaOH solution and the mixture was heated at 100 °C for 5 min. Yellow colour of the resulting solution proves the presence of the betacyanin based natural colour.
Tensile strength test of the treated fabric
Tensile strength of the control and the treated sisal yarns was evaluated following ASTM D5034, grab test method using tensile testing machine (Tinius Olsen, Model: H5KS). Here all the samples were tested at the speed of 300 mm/min with the sample dimension of 200 mm * 50 mm.
Results and discussion
Composition of the PRE extracts
To identify the composition inside the extract, GC–MS observation of the PRE extract has been carried out and the concerning spectra is represented in Fig. 1. Full mass spectra of the PRE extract provide a scenario of the nature of the chemical compound present in the PRE extract, depicted in Table 1. GCMS analysis showed chromatographic peaks at three different retention time (10.70, 11.35 and 12.46) span of the experiment, depicted in Fig. 1. Indeed, chromatogram showed peaks in respect of the different molecular weight component present in the PRE Peak observed at 10.69 s is mainly responsible for the compound 1, 3 amino guanidine, 1,4-dioxane-2,5-dione, 3 amino-1,2-propanediol, 1-propanol, 2-amino. Peak observed at 11.35 s is responsible for the compounds like tetrahydro-4H-pyran-4-ol, propanoic acid, 2-hydroxy, aziridin, 1- (methoxy methyl). Large peak observed at 12.46 s is attributed with the presence of 5-methyloxazolidine, 1,4-dioxane-2,5-dione, 3,6- dimethyl and 1,4-dioxane-2,5-dione, 3, 6-dimethyl- (3S CIS). Most of the components are aromatic based and the nitrogen containing alkaloids and has been reported in the Table 1 of the manuscript. There are some large molecular weight procarcinogenic compounds present in the PRE extract. However, the quantity of the material is less. From the mass spectra curves it has been clearly observed that the molecular weight of the higher amount of the material present in the PRE is in the range 17 to 100. Materials present in higher quantity in the PRE extract are 1, 3 aminoguanidine, hydrazine methyl, hydroxyl amine, glycidol, propanoic acid, alanine, carbamic acid, 5-methyloxadolizine, aziridine, etc. Most of these materials are nitrogen containing alkaloids, amino acids etc., However, some large molecular weight procarcinogenic compounds are also present in the extract but in less quantity.
Thermo-oxidative decomposition of PRE and the treated cotton fabric
Thermo-oxidative decomposition analysis of the dried PRE extract, control and the 400 g/L PRE treated cotton fabric has been represented in the Fig. 3. PRE extract showed major mass loss at 150 °C, may be due to the degradation of the nitrogen containing blowing agent (aminoguanidine, 1,3 diaminoguanidine, piperidine, etc. as found in the GC–MS analysis). During the degradation of the blowing agent present in the PRE extract, non combustible gases have been liberated. This gas liberation phenomenon is an endothermic (heat absorption) process and has been found in the differential scanning calorimetry (DSC) analysis of the PRE extract at 160 °C. Second weight loss of the PRE extract has occurred at around 500 °C, assigned to the oxidation of the carbonaceous char mass to carbon monoxide, carbon dioxide, etc.,. Connected to the textile material, control cotton textile showed one major mass loss stage at 375 °C, where more than 70% mass loss has been occurred. This temperature is commonly known as pyrolysis temperature where cellulosic cotton polymer has been depolymerised by the generation of aliphatic char and flammable gases like levoglucosan, carbon monoxide, pyroglucosan, etc. As a result of the depolymerisation of the cellulose polymer, at higher temperature only 5% char mass has been left out and the aliphatic char has been converted into the aromatic char mass (Shafizadeh and Bradbury 1979; Alongi et al. 2013a, b; Bourbigot et al. 2002). On the contrary, PRE treated cotton fabric showed mass loss in three stages. Initial mass loss (5%) occurred at 150 °C, may be due to the evaporation of the attached water molecule in the PRE treated fabric. 2nd mass loss peak has been observed at around 325 °C (50 °C lower than the control cotton cellulose) where only 25% mass loss has been observed. It clears the fact that the PRE treatment lowered the decomposition temperature and assist the dehydration of cellulose (more water liberation which has been proved by the GC–MS analysis of the pyrolysate of the treated fabric) at 325 °C and restricts the flammable gas formation (also has been proven from the GC–MS analysis of the pyrolysate of PRE treated fabric) and increase aliphatic char mass generation. Indeed PRE treatment helps to catalyze the dehydration of the cellulosic polymer by blocking the reactive primary—OH group of cellulose (auto-crosslinking phenomena) (Alongi et al. 2015). This primary—OH group of the gluco pyranose structure of cellulose is primarily responsible for the formation of flammable gas like levoglucosan. At 375 °C, i.e. at pyrolysis temperature, the control fabric shows 22% left over mass whereas the PRE treated fabric retains 50% mass. This phenomenon has been fortified with the dehydration tendency of the PRE treated cellulose polymer. At 450 °C, treated fabric showed strong peak, which signifies the aromatisation of the char mass. Concern peak is very much smaller for the control fabric. At higher temperature of 450 °C, control cotton fabric loss 80% of its mass whereas treated fabric lost only 60% mass in air atmosphere. However, at 500 °C not much difference has been observed in the comparison of the residual mass as char products have been oxidised at higher temperature in air atmosphere. Isothermal TG analysis of the control and the PRE treated cotton fabric has been carried out at 350 °C (pyrolysis temperature), 20 min in air atmosphere, depicted in Fig. 3. It showed that 350 °C temperature has been reached from 50 °C (temperature gradient 20 °C/min), after 15 min of thermo-oxidative decomposition,. Thereafter 350 °C temperature has been maintained constantly for 20 min. Results showed that once the temperature reaches at 350 °C, PRE treated cotton undergoes weight loss at lower rate compared to the control cotton fabric. Eventually, control cotton steeply lost 50% (90 to 40%) of its weight within 3 min (after reaching 350 °C) whereas treated cotton fabric lost 25% (75 to 50%) only at the said time. It clearly proves the weight Vs temperature stabilisation phenomena of the PRE treated cellulosic material.
Thermo-gravimetry in N2 atmosphere
Thermo-gravimetry of the control and the PRE treated (400 g/L) cotton fabric also has been performed in nitrogen atmosphere and represented in Fig. 4. It mainly signifies the pyrolysis behaviour and the extent of the char mass formation for the fabric samples (Alongi et al. 2013b). TG curve of the control cotton fabric shows two steps degradation. In the 1st step, glucosyl units of the cotton fabric has been decomposed to char at lower temperature and later in the 2nd step it has been depolymerised into volatile flammable products at higher temperature range (Alongi et al. 2015; Shafizadeh and Bradbury 1979). On the contrary, treated cotton fabric showed two small degradation steps at lower temperature (below 300 °C), may be linked with the degradation of the different component of PRE coating. Major mass loss peak of the treated cotton fabric (D) is almost 60 °C lower compared to the control cotton fabric as observed from the data represented in the curve. It signifies the catalysiation of the pyrolysis phenomena and reduction of flammable volatile generation for the treated fabric and as a result the amount of the char mass left at 450 °C is around 20% more compared to the control cotton fabric. Further, more amount of the char mass formation also can be understandable by both the TG curves.
Flammability property of the PRE treated cotton fabrics
Control and the PRE treated cotton fabrics have been examined in terms of the limiting oxygen index (LOI) value and the vertical flammability test and the results are reflected in Fig. 4.
In vertical flammability test, it was found that the (250 mm*40 mm) control cotton fabric has been burnt within 1 min (burning rate: 166 mm/min) with flame (60 s) and afterglow (30 s) whereas PRE treated cotton fabric (treated with various concentrations, 100, 200 and 400 g/L) of same dimension consume 7, 12 and 17 (burning rate: 31, 21, 14 mm/min) minutes for burning, respectively. All the concerning data of LOI and the vertical burning test has been presented in Fig. 5. To this end, Fig. 6 shows the comparison of the vertical burning behaviour of the control and the 400 g/L PRE treated fabric. Indeed, irrespective of all the concentration used for the experiment, PRE treatment showed potential flame stopping behaviour as large part of the treated samples have been burnt slowly only with afterglow. Intensity and the rate of the propagation of the afterglow has been decreased with increasing the concentration of the PRE treatment. It may be attributed with the chemical composition of the PRE and the gradual improvement of the add-on % (18, 24, 35%) of the treated cotton fabric with increasing the concentration of the PRE treatment. Connected with the oxygen consuming analysis, control cotton fabric showed limiting oxygen index (LOI) value of 18.5% whereas PRE treated fabrics (100, 200 and 400 g/L) showed the LOI value of 24, 28 and 32%, respectively. [Note: LOI is the Amount of oxygen consumed in the mixture of the oxygen and nitrogen for just flaming of the sample]. For a typical fire retardant textile material, LOI value is more than equal to 26% (Horrocks 2011). Concerning our research, it has been observed that the Oxygen index value of the treated sample is 32 which is higher LOI index for any cotton fabric compared to 18 Oxygen index of control cotton fabric. Comparison of the vertical burning behaviour of the control and the 400 g/L PRE treated cotton fabric in air atmosphere has been represented in Fig. 6. Indeed, it represents the thermo-oxidative decomposition behaviour of the control and the treated cotton fabric. In vertical flammability test, treated sample did not catch flame (after flame: nil). Only afterglow has been observed and it propagates in a very slow rate.
Above mentioned flammability results affirmed the fact that the compounds present in the PRE extracts is solely responsible for the observed flame retardancy effect of the treated material. It was found from the Table 1, that the PRE extract (pH 4.5) contains acid source named nitrogen based carbamic acid, ammonium salt, hexacontanoic acid, different blowing agent like, aminoguanidine, 1,3 di amminoguanidine, asparagines, hydrazine, ethanamine, piperidine, etc., These nitrogen containing alkaloid based bio-molecules expelled (increase in volume) on heating may be assisting in the flame retardancy of the treated cotton fabric. It has been established that the salt of guanidine like guanidine sulphate and guanidine phosphate has intumescent flame retardant effect on the polymeric material (Coquelle et al. 2015; Camino 2009). Moreover, PRE extract also contains few carbon containing compounds like carbonic dihydrazide, nona hexacontanoic acid (aromatic polyphenolic structure) and different large molecular weight aromatic compounds and sugar based material. These carbon based bio-molecules are helping to generate more amount of char mass at higher temperature. Sources of nitrogen present in the PRE extract and the fabric surface (as observed from the EDX analysis) comes from those aforementioned bio-molecules. However, all the nitrogen based bio-molecules present in the PRE extract may not be present in the treated fabric surface. For further confirmation, PRE treated fabric has been washed in methanol solution and the extracted washed solution has been subjected to the GC–MS analysis. It was found that the solution contains monoammonium salt (possibly ammonium nitrate), carbamic acid, amminoguanidine, asparigine like nitrogenous protein material and sulphur based morpholino thiophenol, thiodiazole. These compounds (acid forming agent and the blowing agent) combinedly made a uniform thick coated layer on the cotton fabric surface as observed from the surface morphology analysis, represented in Fig. 8.
Kjeldahl analysis of the PRE treated fabric
Continuing research effort in this direction, nitrogen analysis has been done both for the control and the treated cotton fabrics by using Kjeldahl method. Control cotton fabric having moisture content of 8.2%, registered 0.35% nitrogen and 2.5% protein content whereas high concentrated (400 g/L PRE extract) PRE treated fabric showed 10.2% moisture content and the presence of 1.5% nitrogen with protein content of 9.4% (may comes from the nitrogen containing blowing agent like oxy-bisalanine, asparagines, aminoguanidine, etc. observed from the GC–MS analysis). EDX analysis results, represented in Table 2, also proved the presence of enhanced nitrogen (3.33 weight %) content on the treated fabric surface as compared to the control cotton fabric (0.756 weight % nitrogen).
Volatile species and char morphology analysis
For understanding the mechanism lies behind the fire retardancy imparted by the PRE bio-molecule, GC–MS analysis of the volatile species (by products remained after burning/pyrolysis) of the control and the treated cotton fabric has been carried out and the detail chemicals present has been represented in Table 3 with spectral snapshot at Fig. 7. It was found that the volatile species of the control cotton fabric contains mainly phenolic compounds, different aliphatic and benzoid aromatic based saturated and the unsaturated hydrocarbons (m/z = 41, 50, 51, 53,55, 57, 58, 64, 65, 67, 69, 70, 71, 80, 81, 91), terpenic compounds (m/z = 105, 109, 107, 108, 177, 193, 192, 194), levoglucosan (m/z = 57, 73, 43, 47, 57, 80), carbonyl compound (m/z = 73, 74) (Shen et al. 2013; Pappa et al. 2006). These hydrocarbons, levoglucosan and the carbonyl compounds are very much flammable and also assist in flaming action of the polymer fuel (cotton fabric). As per literature, oil like terpenic compounds are mainly methyl-phenol based and very much prone to catch flame (Ormeno et al. 2009). Pyrolysate of the PRE treated cotton fabric contains more amount (100%) of non flammable water (m/z = 18) compared to the 22% water present in the pyrolysate of the control cotton fabric. In addition, from the spectra of the treated cotton fabric it has been clearly observed that it contains very less amount of the saturated and the unsaturated hydrocarbon products, carbonyl compounds, levoglucosan and the terpenic compounds compared to the control cotton fabric. Moreover, pyrolysate of the treated fabric contains high molecular weight nitrogen, sulphur based aromatic compound presented in Table 3. However, amount of carbon monoxide (m/z = 28) present in the control and the treated fabric is almost similar (15%). Very little amount (4%) of the presence of the carbon dioxide (m/z = 44) and oxygen (m/z = 16) has been observed in the volatile species of the treated fabric. From this result it can be assured that the treated sample does not catch flame because of the absence of the hydrocarbons, terpenic compounds, levoglucosan, etc. Moreover, some dehydrated product based on nitrogen, sulphur based high molecular weight aromatic compound also restrict the heat generation during burning. However, presence of carbon monoxide proves the smouldering (no flame, only with afterglow) of the PRE treated cotton fabric during vertical burning.
Char morphology of the burned PRE extracts, control and the PRE treated cotton fabric has been represented in Fig. 8. Morphology of the burned PRE extract showed foam like voluminous structure with the presence of the small and big porous bubbles on the surface. Actually, during fire exposure, the temperature of the material has been raised (at this point different nucleation sites i.e. small bubbles have been generated randomly in the deeper sites through out the volume) and when the temperature has crossed the degradation temperature of the blowing agent (guanidine, aminoguanidine, piperidine, 1,3 diamino guanidine) present in the extract, the viscosity of the material has been changed and an endothermic gas producing chemical reaction has been triggered. In addition, at this temperature neucleation bubbles begins to grow spherically because of the viscosity change (low Reynold number), surface tension, forces due to gravity, etc. (Liu et al. 2004). According to the DSC figure, depicted in Fig. 8, it has been clearly observed that the total degradation, heat absorption, heat release, all these phenomena have been take place in one endotherm and subsequent exotherm. DSC curve showed change in slope from 60 to 140 °C and showed one heat absorption endotherm at around 160 °C, may be due to the presence of the free acids, aromatic hydrocarbons, nitrogenous alkaloid based blowing agents in the PRE. Heat absorption and the degradation of those components assist non flammable gas formation and generation of more amount of insulating char mass by dehydration techniques. Extentivity of the char mass formation has been observed from the adjacent large heat releasing exothermic peak at around 195 °C. Connected with the curve, area under the exothermic peak denotes the quantity of heat released during burning, which is corroborated with the quantity of the insulating char mass formation. Another research group has observed the same kind of degradation behaviour for the different insect based wax material (Ruguo et al. 2011). The gas produced by the degradation of the blowing agent and the other components present in the PRE, collects in porous bubbles and causes the material to expand its volume and foam formation. However, the size of the bubbles present in the burned PRE extracts (observed from the char morphology, represented in Fig. 8) are different throughout the volume, may be due to the varying local viscosity gradient. This multi-cellular foamy thick material formed act as an insulating layer and reduced the heat transmission rate when it has been applied to a polymeric material. As per literature, thermal conductivity of the bubbles is much lower compared to its surrounding material (Pastorova et al. 1994; Brebu and Spiridon 2011). Char morphology of the control fabric has been distorted completely after burning and discontinuous netlike light weight fragile white colour char mass has been remained. On the contrary, char of the treated fabric showed much voluminous structure and the texture, shape, structure of the treated yarn and the fabric has been maintained even after burning. Indeed, picture showed shaggy or blistery coating wrapped around each fibre with the presence of the small pits on the burned fabric surface. This kind of layer has been appeared due to the formation of gases by the burning degradation or thermal decomposition of the blowing agent present in the PRE treatment. Treated char structure fortified the fact that the flow of the evolved gases and heat transfer has been restricted, discontinued and retards the degradation rate of the underlying polymer material. Proof of the integrity of the char mass has also been observed from the char EDX analysis (data and spectra has been represented in Table 2 and Fig. 9) as it showed that the control cotton produce 49 and 32 weight % carbon and oxygen, respectively whereas 400 g/L PRE (1:4) treated cotton fabric has produced almost 76 and 15 atomic weight % carbon and oxygen, respectively after burning.
FTIR and elemental analysis of the char
FTIR analysis of the char mass of the control and the treated fabric also has been performed (depicted in Fig. 9) to understand the fire retardancy mechanism more precisely. As per literature, control cotton fabric showed clear peak at 1080 cm−1, assigned to the skeletal vibration of the C–O–C pyranose ring. Peaks observed at 1310 to 1360 cm−1 represents the C–C and C–O skeletal vibration. Broad peaks present at 3100–3600 cm−1 and 2800–3000 cm−1 have been attributed with the –OH and –CH stretching vibration, respectively. However, char of the control cotton fabric showed no peaks from 2000–3500 cm−1, proves the depolymerise structure of the cellulose polymer. No clear peaks have been observed at 1080 cm−1 and from 1310 to 1360 cm−1. It proves the fact that most of the glucopyranose structure of the cellulose cotton polymer has been depolymerised and damaged completely. Presence of the broad peaks at 990 cm−1 assigned to the C–O– secondary alcohol skeletal vibration. On the contrary, char mass of the PRE treated cotton fabric showed clear distinct peak at 1080 cm−1, 1620 cm−1 and 1708 cm−1 due to the –C–O–C– skeletal vibration, –C=C– and –C=O– stretching vibration, respectively (Goormaghtigh et al. 2006). Peak at 1620 cm−1 proves the formation of the polyaromatic network into the structure. These phenomena proved the fact that most of the pyranose structures of the cellulose polymer are still preserved even after burning. Formation of the C=O stretching vibration peak denotes the phenomenon of the dehydration of the cellulosic stricture in acidic condition of the PRE molecule. Like EDX analysis no protein peaks are visible in the char structure of the treated cotton, may be due to the volatile non flammable gas (water, carbon dioxide) formation during burning. Curve also showed the presence of the transmission band at around 1000–800 cm−1 region, attributed to the aromatised cellulose structure in the residue remained after burning. Total char analysis supports the dehydration and aromatisation phenomena of the treated cotton. FTIR analysis of the dried PRE extract and the char mass of the PRE extract showed no clear difference of the peaks, is an interesting finding. Indeed, all the peaks present in the dried PRE extract also present in the char mass of the burnt PRE extract, may be attributed with the degradation resistance i.e. the thermal stability of the PRE extract. However, intensity of the amide 1 and the amide 2 peaks are less in the PRE char material compared to the PRE extract, may be due to the degradation of the protein based blowing agent by the formation of non flammable gases during burning.
Cone calorimeter analysis
Cone calorimeter analysis of the three replicates of the control and the treated fabric has been performed to understand the heat release behaviour from the sample during forced combustion process. In practice, the selection of the heat flux level depends on the expected fire scenario (Schartel and Hull 2007; Nazare et al. 2002). Too high heat flux level is difficult to distinguish the sample as the specimen undergo rapid combustion. 35 kW/m2 heat flux level assigned with the mild fire exposure and it is especially used for upholsters furniture, curtain, mattress component, etc.(Schartel and Hull 2007; Morgan and Bundy 2007). Therefore, samples have been exposed to the aforementioned heat flux level (35 kW/m2) and the detail results have been commented in the Table 4. It has been found from the cone calorimeter analysis that the control cotton fabric has been burnt with flame within 45 s (flame in time: 259 s and the flame out time: 304 s) whereas the 400 g/L PRE treated cotton fabric did not showed any flame catch up but the sample has been combusted with afterglow. At the end of the test, peak heat release rate (PHRR) of the control cotton fabric was found 79 kW/m2 whereas the treated cotton fabric showed peak heat release rate of 25 kW/m2 (almost one-third of the control cotton fabric). At the end of the combustion process, treated fabric showed intense black colour char mass whereas the combusted cotton fabric showed light grey colour fragile char mass. Heat release rate (HRR) curve of the control and the treated fabric has been represented in Fig. 10.
As per report, heat release rate has been calculated from the oxygen concentration in the fuel gases (White et al. 2012; Alongi et al. 2014). The heat released from the fabric is proportional to the oxygen consumed during combustion. Lower heat release rate of the PRE treated fabric may be due to the fact that the PRE coating may act as barrier to fuel transport and reradiate the flux from the cone calorimeter heater. Indeed, the concern treatment slowing down the release of flammable volatiles from the decomposed coating to the flame front. Total heat release (THR) for the control cotton fabric is around 1.7 MJ/m2 whereas the heat release value for the treated fabric is around 0.9 MJ/m2. Cone calorimeter analysis also determines the average rate of heat emission (ARHE) which signifies the cumulative heat emission divided by time and its peak value. Indeed, the concern parameter is a good measure of the propensity of the fire development under real scale condition (White et al. 2012; Schartel et al. 2005). Data value in Table 4 indicates that the MARHE value of the control cotton fabric is around 35–38% higher compared to the treated fabric. Maximum smoke production rate has been found out from the curve and it is around 0.03 m2/s for the control fabric whereas the treated fabric showed smoke production rate of 0.04 m2/s. Extent of carbon monoxide generation is almost similar for the control and the treated fabric. However, the amount of carbon di-oxide liberated during the combustion of the treated fabric is around 0.06% which is nearly five times lower compared to the control cotton fabric.
Washing durability of the treated fabric
It has been observed from the washing durability test, that the interaction between the bio molecules [pomegranate rind extract (PRE)], used for the experiment and the cotton fabric is absorption phenomena. 400 g/L PRE treated cotton fabric showed the LOI value of 32. After one single and double cold wash (40 °C, MLR = 1:50, 30 min) the sample showed the oxygen index value of 30 and 29 respectively. It means that the fire retardant components of the PRE are not leaching completely after cold washing cycle. However, one hot wash (more than 60 °C) can turn down the LOI value of the sample from 32 to 25 and lost its fire retardant property by leaching out the component from the treated fabric surface. Therefore, it is recommended to wash the sample in cold (room temperature) condition, if required.
Dyeing with the anionic and cationic dye
Pomegranate rind extract has been used as dye medium for the acid dye (1% shade depth) and the basic dyeing (0.1% shade depth) of the cotton fabric and the glimpse of the dyed fabric has been represented in Fig. 11. It has been observed that the anionic acid dyed cotton fabric from the PRE medium is darker than the cotton fabric dyed from the water medium. This phenomenon may be attributed with the mordanting efficacy of the PRE for anionic dyes. Indeed, light weight positively charged metal ions (K+, Al3+, Ca+, Na+, guanidium ion, carbamic acid, etc.) present in the PRE extract deposited on the cellulosic surface (due to the zeta potential of cellulose in solution) and help to catch the anionic acid dye molecules from the treatment medium. As a result, treated fabric is darker and reddish, yellowish compared to the cotton dyed from the water medium. This phenomenon is more prominent in the acid dyed cotton fabric from the concentrated 200 g/L PRE medium as the colour of the fabric turns more yellowish (due to the presence of natural colouring mater coumarines and betacyanines as shown in Fig. 10), reddish (due to the attachment of the more amount of the anionic dye molecule) and dark (more shade depth) compared to the acid dyed fabric from PRE (as it is) medium (as shown in Table 4). Tonal variation also may be due to the fact that some of the colour groups present in the PRE extract (coumarine, betacyanine, tanin based, etc.) also could be attached to the fabric surface by vander walls force and changed the resultant shade or tone of the treated fabric at elevated dyeing temperature, compared to the cotton fabric dyed with from the water medium. Phytochemical analysis of the plant extract also has been examined in detail by the researchers (Sangeetha and Jayaprakash 2015). Proof of the presence of the natural colouring matter has been confirmed by the phytochemical analysis of the PRE which has shown into the Fig. 11.
On the contrary, cationic basic dyed cotton fabric from the PRE medium is lighter and different shade/colour rather than the dyed cotton fabric from water medium as observed colour value from the Table 5. This phenomenon may be attributed with the fact that the light weight positively charged metal ions deposited on the negatively charged fabric surface in treatment medium. However, picture enlightens us that some of the basic dye molecule also has been attached to the negatively charged cotton fabric surface in the PRE medium that turns the treated cotton fabric into the greenish (bluish + yellowish) tone compared to the bluish tone of the cotton fabric dyed from the water medium. More clearly, most of the light weight positive ions (K+, Al3+), present in the PRE, has been deposited on the negatively charged (zeta potential) cotton fabric and produced yellow tinge. In addition, some of the positively charged basic dye molecule (bluish colour) also attached with the negatively charged cotton surface and combinedly delivered green colour compared to the blue colour shade of the cotton fabric dyed with basic dye from the water medium. In this paper, for dyeing purpose two different category dyes (Methylene Blue and Acid red) has been used by the researchers. As far as the basic Methylene Blue colour is concerned, it is very popular attractive colour and the researchers have found out that it has no detrimental effect in terms of the toxicity and the sensitivity on the human skin (Cleinmensen et al. 1984). Connected to it, they have reported that after dermal application of the 2,000 mg/Kg colour, no toxic effect has been observed on the skin surface. However, further research is needed for the skin irritation test of the Acid Red colour, used for the experiment.
As far as the fire retardancy is concerned, cotton fabric dyed from the PRE medium showed less thermal stability (fabric has burnt with after flame and after-glow with 38 mm flame contact for 6–7 s) compared to the cotton fabric treated with the different concentration of the PRE extract (after flame self extinguished after 12 s flame contact), which has been mentioned in the earlier part of this paper. It may be attributed with the fact that the anionic and the cationic dye molecules deposited on the fabric surface did not assist to improve the fire resistant efficacy. Moreover, dye molecule may also create a hindrance for the deposition of the PRE based bio-molecule which is solely responsible for fire resistant effect. On the contrary, cotton fabric dyed from the water medium has burnt with flashing and flame with just 1–2 s flame contact, again showed lower thermal resistant behaviour of the used anionic and the cationic dyes. Total phenomenon reflects that the dyed fabric from the PRE medium showed more colour depth as well as marginal improvement in the fire resistant behaviour compared to the dyed cotton fabric from the water medium.
Antimicrobial analysis of the PRE and the treated cotton fabric
PRE solution has been dried into solid powder by evaporating the water molecules from it. Thereafter, four different ethanolic solutions of PRE concentration level 5, 10, 15 and 20% (W/V) have been prepared and tested against one Gram positive (S. aureus) and one Gram negative (K. pneumonia) bacteria and represented in Fig. 12. It was found that each and every solution (concentration 5% to 20%) showed zone of inhibition diameter more than 50 mm. It clearly proves the significant antimicrobial effectivity of the extract against the both Gram-positive and the Gram-negative bacteria. Connected to this concept, antimicrobial efficacy test of the PRE treated fabric has also been performed and the resulting colony growing plate is represented in Fig. 12. Figure elucidated that the PRE treated fabric at pH 7.0 showed significant less colonial growth against both the Gram-positive (S. aureus) and the Gram-negative (E. coli) bacteria compared to the control cotton fabric. To this end, treated fabric showed bacterial reduction % of 97.5 and 97.7 against the S. aureus and E. coli, respectively whereas control cotton fabric showed no bacterial reduction (0%) in both the cases. Antibacterial action of the PRE extract may be attributed with the presence of positive metal ions (K+, Al3+, Mg2+, Cr6+, etc. found from the EDX analysis), guanidium ion (CH6N3+) (found from the GC–MS analysis), tannin (found from the phytochemical analysis), phenolic based acid (hexacontanoic acid, caffeic acid), etc. present in it. These positive ions present in the PRE extract may attacks the negatively charged bacterial cell wall and damaged it by complex formation. Khan and Hanee (2011) has reported that the antimicrobial property of the PRE mainly comes from the presence of ellagitanins and the secondary metabolites in it. Very recently Burgos et al. (2017) has reported that highly tannin content material punicalagin present in the extract is responsible for the antimicrobial effect. Researchers also have reported that tannin helps in the precipitation of protein and causing leakage formation in the cell wall of the microorganisms which leads to the cell death. Seshama et al. (2017) has reported that the alcoholic extract of the pomegranate rind significantly reduced the growth of the bacterial cell and also reduced the protein content of the bacterial cell wall after 28 h of incubation. As per their report, polyphenols present in the PRE helps in protein precipitation and enzyme inhibition of the microorganisms. Indeed the mechanism is related to the reaction of the polyphenol with the sulphohydryl group of the bacterial protein. Akiyama et al. (2001) reported that the tannic acid act on the membranes of the bacterial cell walls and damage it from the cell wall. Cowan (1999) has reported that the coumarins present in the plant extract also has detrimental effect on the Gram-positive bacteria. Connected to this area, from the phytochemical analysis it has been proved that PRE contains yellow colour coumarin which is a phenolic based chromophore materials, may be responsible for the antimicrobial property of the extract. Same researcher also reported that the mixture of the different phytochemicals like alkaloids, saponin, tannin, flavonoids, phenol, etc. can show antimicrobial effect. From the GC–MS analysis it was found out that PRE extract contains different nitrogen based alkaloid materials in it, which also may be one of the important responsible factor behind the antimicrobial property of the extract.
Mechanical properties
Tensile strength of the control and the treated cotton fabric have been represented in Fig. 13. Control cotton fabrics, used for the experimental study, have the tensile strength value of 574 N with the extension of 11.5%. On the contrary, PRE extract treated cotton fabric shows an average tensile strength of 693 N (8.7% high compared to the control fabric) with the extension value of 9.2%. Higher the tensile strength of the PRE treated cotton fabric may be attributed to the higher add-on % of the PRE after the treatment. Stiffness of the treated fabric can be improved by the incorporation of softeners into the PRE formulations. With softness the treatment has potential to improve the hand value of the fabric. Further research is going on in this direction in our research laboratory.
Conclusions
In the present work, for the first time, PRE extract has been explored as a novel sustainable green materials for multi value added cotton substrates. The thermal stability of the cotton fabric in air atmosphere has been enhanced by this PRE treatment as evidenced from increased char mass formation with catalysation of pyrolysis phenomena. Treatment has reduced the burning rate of the cotton fabric with significant improvement in flame stopping behaviour. The flame retardancy behaviour of the treated fabric has been further confirmed by the enhanced oxygen consuming index (LOI value) compared to the control cotton fabric. PRE has been proven as a potential flame suppressant agent due to the presence of various acid source (carbamic acid, hexacontanoic acid, ammonium salt), its protein based blowing agent composition (especially guanidium ion and asparagines based protein), carbon source and other nitrogen containing bases. These components combinedly react and catalyse the dehydration process of cotton fabric by liberating more amount of non flammable water and carbon di oxide and the carbon based char mass formation. Cone calorimeter analysis (at heat flux level of 35kw/m2)showed that the peak heat release rate of the PRE treated fabric is more than 50% lower compared to the control cotton fabric. PRE extract also has been used as mordanting material for the anionic acid dye when dyeing has been performed from the PRE medium because of its positive metal ion content. In addition, PRE treated cotton fabric showed significant antimicrobial efficacy against the both Gram-positive and the Gram-negative bacteria because of the presence of tannin, polyphenolic compound, coumarin and other positive metallic constituents which is responsible for protein precipitation and degradation of the bacterial cell wall. The treated fabric can be easily used in the field of making non permanent structures (outdoor applications) like tent, stalls, pandals in book fairs, etc. Moreover, the fabric can also be used for making home textile materials (sofa cover, table cover, curtains, etc.) where much washing is not required. The said technology can be easily adopted by the rural people for making fire retardant textile from the wastage material at low cost. Apart from it, developed technology can also be adopted by the small scale handloom sectors for making different home textile materials, packaging purposes where much washing is not needed.
References
Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K (2001) Antibacterial action of several tannins against staphylococcus aureus. J Antimicro Chemother 48:487–491
Alongi J, Carletto RA, Bosco F, Carosio F, Blasio AD, Cuttica F (2013a) Intrinsic intumescent like properties of the DNA treated cotton fabrics. Carbohydr Polym 96:296–305
Alongi J, Carletto RA, Blasio AD, Carosio F, Bosco F, Malucelli G (2013b) DNA: a novel green natural flame retardant and suppressant for cotton. J Mater Chem A 1:4779–4785
Alongi J, Cuttica F, Blasio AD, Carosio F, Malucelli G (2014) Intumescent features of neuclic acids and proteins. Thermochim Acta 591:31–39
Alongi J, Blasio AD, Milnes J, Malucelli G (2015) Thermal degradation of DNA, an all in one natural intumescent flame retardant. Polym Degrad Stabil 113:110–118
Basak S, Ali WS (2016) Sustainable fire retardancy of textiles using bio-macromolecules. Polym Degrad Stabil 133:47–64
Basak S, Ali SW (2017) Leveraging flame retardant efficacy of the pomegranate rind extract: a novel biomolecule on the lingo-cellulosic material. Polym Degrad Stabil 144:83–92
Basak S, Samanta KK, Chattopadhyay SK, Narkar R (2015) Thermally stable cellulosic paper made using banana pseudostem sap, a wasted by product. Cellulose 22:2767–2776
Basak S, Patil PG, Shaikh AJ, Samanta KK (2016) Green coconut shell extract and boric acid: new formulation for making thermally stable cellulosic paper. J Chem Technol Biotechnol 91:2871–2881
Bourbigot S, Chlebicki S, Mamleev V (2002) Thermal degradation of cotton under linear heating. Polym Degrad Stabil 78:57–62
Brebu M, Spiridon I (2011) Thermal degradation of keratin waste. J Anal Appl Pyrolysis 91:288–295
Burgos ECR, Hernender AB, Artiaga LN, Kacanlova M, Garcia FH, Lopez JLC, Barrachiana LLC (2017) Antimicrobial activity of the pomegranate peel extracts as effected by cultivation. J Sci Food Agri 97:525–532
Camino G (2009) Intumescent materials, 2nd edn. Woodhead Publishing Limited, Cambridge
Carosio F, Blasio AD, Cuttica F, Alongi A, Malucelli G (2014) Flame retardancy of polyester and polyester cotton blends treated with caseins. Indus Engg Chem Res 53:3917–3923
Cleinmensen S, Jensen JC, Jensen NJ, Meyer O, Olsen P, Wurtzen G (1984) Toxicological studies on malachite green: a triphenyl methane dye. Arch Toxicol 56:43–45
Coquelle M, Duquensene S, Casetta M, Sun J, Gu X, Zhang S, Bourbigot S (2015) Flame retardancy of PA6 using a guanidine sulfamate/melamine phosphate mixture. Polymers 7:316–332
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Fawde OA, Makunga NP, Opara UL (2012) Antibacterial antioxidant and tyrosinase inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern Med 12:200–210
Goormaghtigh E, Ruysschaert JM, Raussens V (2006) Evaluation of the information content in infrared spectra for protein secondary structure deformation. Biophys J 90:2946–2957
Horrocks AR (2009) Textiles, 2nd ed; Woodhead Publishing Limited, Cambridge England. http://law.resource.org/pub/in/bis/S12/is.13501.1992.pdf. http://law.resource.org/pub/in/bis/S12/is.11871.1986pdf
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab 96:377–392
Khan JA, Hanee S (2011) Antimicrobial activity of the punica granatum peels. Int J Appl Biol Pharmaceol Technol 2:23–27
Liu Q, Lu C, Yang Y, He F, Ling L (2004) Investigation on the effects of fire retardant on the thermal decomposition of wood derive rayon fibre in an inert atmosphere by TG-MS analysis. Thermochimic Acta 419:205–209
Malucelli G, Bosco F, Alongi J, Carosio F, Blasio AD, Mollea C, Cuttica F, Casale A (2014) Biomacromolecules as novel green fire retardant systems for textiles: an overview. RSC Adv 4:46024–46039
Mohammed GJ, Al-Jassani MJ, Hameed IH (2016) Antibacterial, Antifungal activity and chemical analysis of punica granatum (pomegranate peel) using GC-MS and FTIR spectroscopy. Int J Pharmacogonsy Phytochem Res 8:480–494
Morgan AB, Bundy M (2007) Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater 31:257–283
Nazare S, Kandola B, Horrocks R (2002) Use of cone calorimetry to quantify the burning hazard of apparel fabrics. Fire Mater 26:191–199
Ormeno E, Cespedes B, Sanchez IA, Garcia AV, Moreno JM, Fernandez C, Baldy V (2009) The relationship between terpenes and flammability of leaf litter. Forest Eco Management 257:471–482
Pappa A, Mikedi K, Tzamtzis N, Stathoropoules M (2006) TG-MS analysis for studying the effects of fire retardant on the pyrolysis of pine needles and their components. J Therm Anal Calorim 84:655–661
Pastorova I, Botto RE, Arisz PW, Boon JJ (1994) Cellulose char structure: a combined analysis Py-GC-MS, FTIR study. Carbohydr Res 262:27–47
Ruguo Z, Hua Z, Hong Z, Ying F, Kun L (2011) Thermal analysis of four insect waxes based on differential scanning calorimetry (DSC). Procedia Eng 18:101–106
Sangeetha R, Jayaprakash A (2015) Phytochemical screening of the punica granatum linn. Peel Extract J Acad Indust Res 4:160–162
Schartel B, Hull JR (2007) Development of fire retardant materials: interpretation of cone calorimeter data. Fire Mater 31:327–354
Schartel B, Bartholmai M, Knoll U (2005) Some comments on the use of cone calorimeter data. Polym Degrad Stabil 88:540–547
Seshama M, Khatri H, Suther M, Basak S, Ali SW (2017) Bulk versus nano ZnO: influence of fire retardant behaviour on sisal fibre yarn. Carbohydr Polym 175:257–264
Shafizadeh F, Bradbury AGW (1979) Thermal degradation of cellulose in air and nitrogen at low temperature. J Appl Polym Sci 23:1431–1442
Shen D, Ye J, Xiao R, Zhang H (2013) TG-MS analysis for thermal decomposition of cellulose under different atmosphere. Carbohydr Polym 98:514–521
White RH, Nam S, Parikh DY (2012). Cone calorimeter evaluation of two flame retardant cotton fabrics. Fire Mater. https://doi.org/10.1002/fam.2111
Acknowledgments
Authors would like to thank Central Institute of Research on Cotton Technology, Indian Council of Agricultural Research, Mumbai, Govt. of India for sponsoring doctoral research work of the one of the author (S. Basak) of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basak, S., Wazed Ali, S. Wastage pomegranate rind extracts (PRE): a one step green solution for bioactive and naturally dyed cotton substrate with special emphasis on its fire protection efficacy. Cellulose 26, 3601–3623 (2019). https://doi.org/10.1007/s10570-019-02327-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02327-x